3. Materials and Methods
3.1. General Procedures
Melting points were determined in open capillary tubes and were uncorrected. Reactions were monitored by thin-layer chromatography (TLC) on silica gel plates. 1H-NMR and 13C-NMR spectra were measured on an AV-300 (Bruker BioSpin, Fällanden, Switzerland) and AV-500 (Bruker BioSpin, Fällanden, Switzerland) and all chemical shifts were given in ppm relative to tetramethylsilane (TMS). High-resolution mass spectra were measured using a matrix-assisted laser desorption/ionization (MALDI)-time of flight (TOF)/TOF mass spectrometer (Bruker Daltonik, Bremen, Germany). The major chemicals were purchased from Aldrich Chemical Corporation (Milwaukee, WI, USA). All other chemicals were of analytical grade.
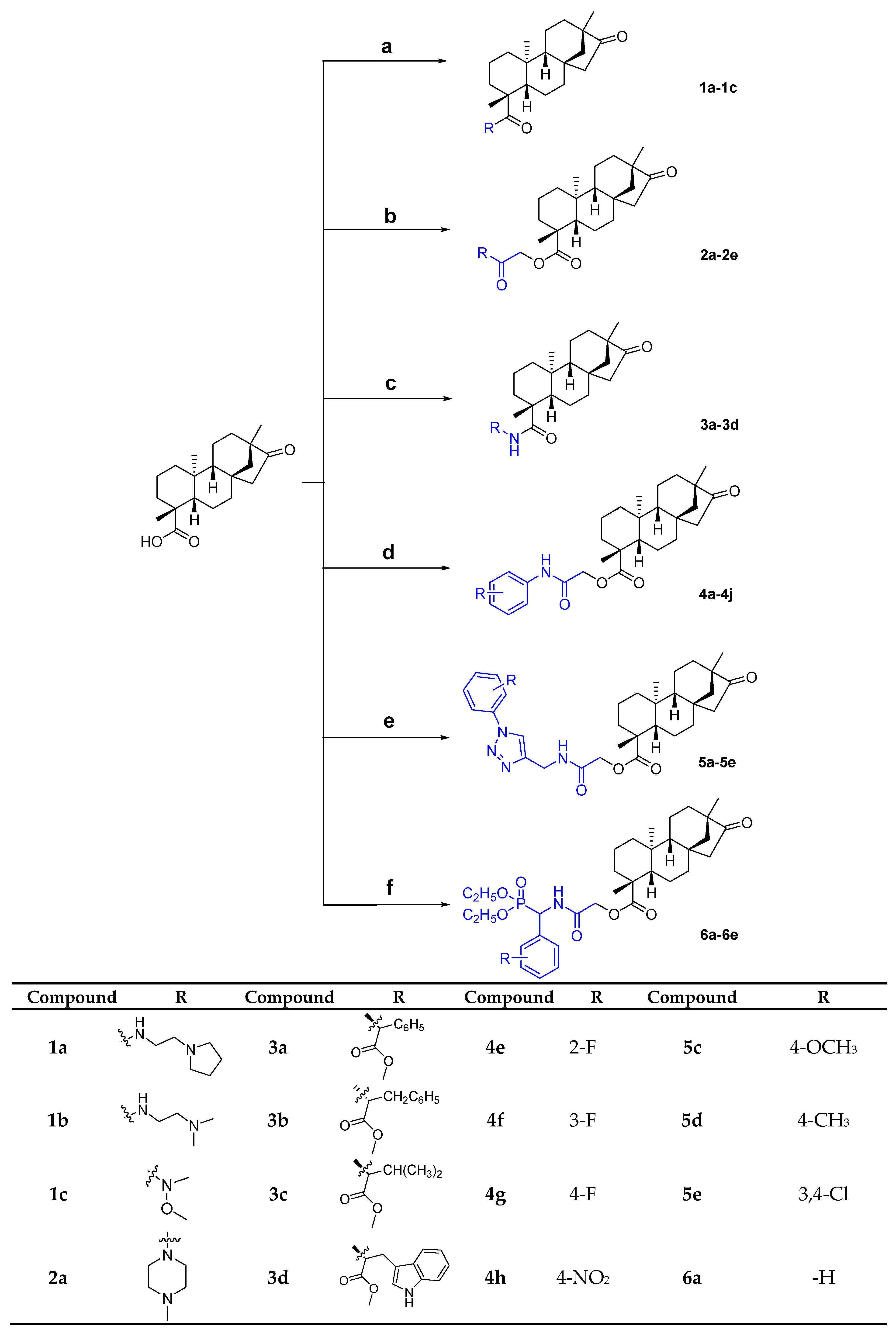
3.2. General Procedure for the Synthesis of Compound (1a–1c)
Isosteviol (63.6 mg, 0.20 mmol) was placed in CHCl3 (10 mL), oxalyl chloride (38.1 mg, 0.30 mmol) was added and the mixture was stirred at 60 °C for 2 h. Upon completion, the solvent was evaporated in vacuo to obtain the crude product, a colorless crystal, which was used in the next step without further purification. Next, the crude product and different amines (0.21 mmol) and Et3N (22.2 mg, 0.22 mmol) were added to CH2Cl2 (10 mL) and the resulting mixture was stirred at 30 °C for 2 h. Then, the solvent was evaporated in vacuo, water was added (10 mL), the mixture was filtered and the residue was washed with water to obtain the target compounds (1a–1c) as white powders.
(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxo-N-(2-(pyrrolidin-1-yl)ethyl)tetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxamide (1a). White powder; yield, 71%; m.p. 136–137 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 6.34 (brs, 1H, -CO-NH-), 3.31 (q, J = 6 Hz, 2H, -CO-NH-CH2-), 2.70–2.51 (m, 7H, N(CH2)3, 15-He), 2.10 (d, J = 12 Hz, 1H, 3-He), 2.00–1.95 (m, 1H), 1.89–1.86 (m, 1H), 1.83–1.75 (m, 8H), 1.71–1.64 (m, 2H), 1.60–1.59 (m, 1H), 1.55 (s, 1H), 1.50–1.49 (m, 1H), 1.45–1.44 (m, 1H), 1.41–1.38 (m, 1H), 1.35–1.23 (m, 2H), 1.19 (s, 3H, 18-CH3), 1.16–1.07 (m, 2H), 0.99 (s, 3H, 17-CH3), 0.95–0.89 (m, 1H), 0.79 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 222.40, 176.70, 57.49, 54.75, 54.26, 53.78, 53.61(2C), 48.71, 48.38, 43.61, 41.80, 40.26, 39.50, 38.11(2C), 37.92, 37.31, 30.21, 23.63(2C), 22.01, 20.33, 19.86, 19.04, 13.41. HRMS (m/z): calcd for C26H43N2O2+ [M + H]+: 415.3319, found: 415.3316.
(4R,4aS,6aR,9S,11aR,11bS)-N-(2-(dimethylamino)ethyl)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxamide (1b). White powder; yield, 66%; m.p. 102–103 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 6.30 (brs, 1H, -CO-NH-), 2.29 (q, J = 6 Hz, 2H, -CO-NH-CH2-), 2.67 (dd, J = 18, 3 Hz, 1H, 15-He), 2.41 (t, J = 6 Hz, 2H, -CO-NH-CH2-), 2.29–2.19 (m, 6H, N(CH2)3), 2.09 (d, J = 12 Hz, 1H, 3-He), 2.02–1.94 (m, 1H, 6-He), 1.88–1.78 (m, 3H), 1.76–1.68 (m, 3H), 1.64–1.59 (m, 2H), 1.55–1.48 (m, 2H), 1.45–1.38 (m, 3H), 1.36–1.31 (m, 1H), 1.27 (s, 3H, 18-CH3), 1.16–1.09 (m, 2H), 0.99 (s, 3H, 17-CH3), 0.95–0.90 (m, 1H), 0.79 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 222.43, 176.71, 57.55(2C), 54.75, 54.28, 48.71, 48.37, 45.09(2C), 43.62, 41.79, 40.25, 39.51, 38.09(2C), 37.32, 36.73, 30.18, 22.04, 20.34, 19.86, 19.11, 13.44. HRMS (m/z): calcd for C24H41N2O2+ [M + H]+: 389.3163, found: 389.3166.
(4R,4aS,6aR,9S,11aR,11bS)-N-methoxy-N,4,9,11b-tetramethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxamide (1c). White powder; yield, 68%; m.p. 144–145 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 3.66 (s, 3H, -O-CH3), 3.12 (s, 3H, -N-CH3), 2.73 (dd, J = 18, 3 Hz, 1H, 15-He), 2.53 (d, J = 12 Hz, 1H, 3-He), 2.16–2.02 (m, 1H, 6-He), 1.90–1.78 (m, 2H), 1.74–1.58 (m, 6H), 1.54–1.34 (m, 5H), 1.28 (s, 3H, 18-CH3), 1.22–1.17 (m, 1H), 1.13–1.04 (m, 2H), 0.99 (s, 3H, 17-CH3), 0.96–0.90 (m, 1H), 0.83 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 222.81, 178.30, 60.94, 60.46, 55.71, 54.43, 48.72, 48.51, 46.21, 42.36, 40.91, 39.66, 38.55, 38.44, 37.40, 34.38, 27.22, 22.31, 20.43, 20.13, 19.90, 15.48. HRMS (m/z): calcd for C22H36NO3+ [M + H]+: 362.2690, found: 362.2987.
3.3. General Procedure for the Synthesis of Compound (2a–2e)
A mixture of isosteviol (63.6 mg, 0.20 mmol), K2CO3 (41.5 mg, 0.30 mmol) and chlorinated derivatives (0.21 mmol) in CH3CN (10 mL) was stirred at 80 °C for 4 h. After confirming the reaction progress by thin-layer chromatography, the solvent was evaporated in vacuo, the mixture was dissolved with 15 mL ethyl acetate and then washed with saline (5 mL × 3). The mixture was then purified using silica gel column chromatography and eluted with petroleum ether:ethyl acetate (5:1) to obtain the target compound 2a–2e.
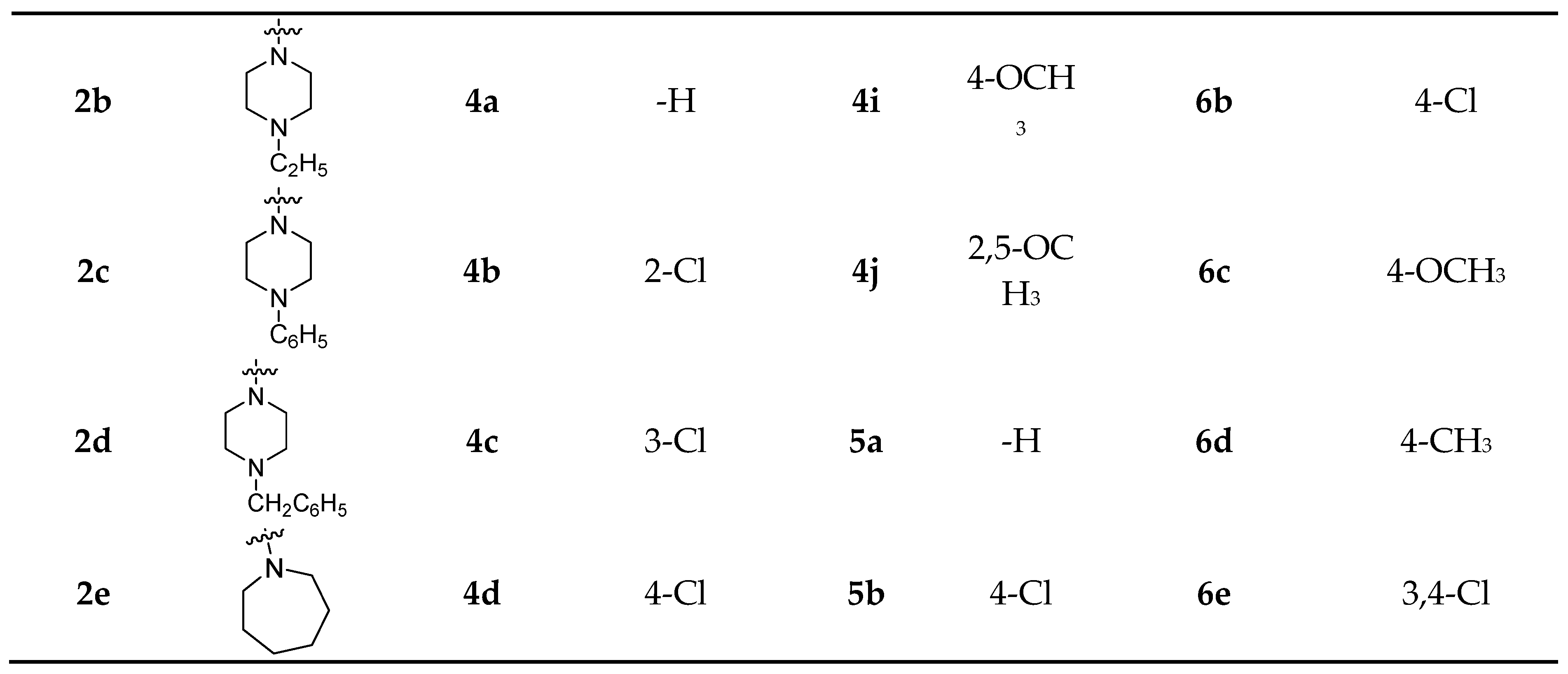
2-(4-methylpiperazin-1-yl)-2-oxoethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (2a). White powder; yield, 87%; m.p. 112–113 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 4.82 (d, J = 15 Hz, 1H, -O-CHe-CO-), 4.63 (d, J = 15 Hz, 1H, -O-CHa-CO-), 3.65 (s, 2H, -CO-N-CH2-), 3.45 (s, 2H, -CO-N-CH2-), 2.65 (dd, J = 12, 3 Hz, 1H, 15-He), 2.45–2.26 (m, 8H, 3-He, -(CH2)2-N-CH3), 1.97–1.84 (m, 3H), 1.78–1.65 (m, 4H), 1.60–1.55 (m, 2H), 1.50–1.40 (m, 4H), 1.32 (s, 3H, 18-CH3), 1.28–1.21 (m, 3H), 1.16–1.07 (m, 1H), 0.99 (s, 3H, 17-CH3), 0.95–0.88 (m, 1H), 0.75 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 125 MHz, ppm): δ 222.50, 176.89, 165.02, 60.69, 57.16, 54.77, 54.70, 54.38, 54.33, 50.90, 48.73, 48.51, 45.85, 44.24, 44.08, 41.54, 39.82, 39.48, 38.11, 37.98, 37.33, 29.06, 21.65, 20.34, 19.87, 18.99, 13.57. HRMS (m/z): calcd for C27H43N2O4+ [M + H]+: 459.3217, found: 459.3221.
2-(4-ethylpiperazin-1-yl)-2-oxoethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (2b).White crystal; yield, 84%; m.p. 115–117 °C; 1H-NMR (CDCl3, 500 MHz, ppm): δ 4.81 (d, J = 9 Hz, 1H, -O-CHe-CO-), 4.62 (d, J = 9 Hz, 1H, -O-CHa-CO-), 3.64 (s, 2H, -CO-N-CH2-), 3.42 (s, 2H, -CO-N-CH2-), 2.63 (d, J = 12 Hz, 1H, 15-He), 2.45–2.43 (m, 6H, -(CH2)2-N-CH2-), 2.27 (d, J = 9 Hz, 1H, 3-He), 1.95–1.92 (m, 1H), 1.87–1.78 (m, 3H), 1.73–1.65 (m, 3H), 1.61 (d, J = 9 Hz, 1H), 1.55 (d, J = 6 Hz, 1H), 1.51–1.34 (m, 4H), 1.31 (s, 3H, 18-CH3), 1.26–1.15 (m, 4H), 1.11–1.06 (m, 3H, -NCH2CH3), 0.97 (s, 3H, 17-CH3), 0.94–0.89 (m, 1H), 0.74 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 222.57, 176.85, 164.93, 60.72, 57.13, 54.74, 54.30, 52.62, 52.20, 52.13, 48.70, 48.48, 44.40, 44.05, 41.72, 41.51, 39.80, 39.46, 38.08, 37.96, 37.31, 29.04, 21.63, 20.32, 19.85, 18.97, 13.55, 11.77. HRMS (m/z): calcd for C28H45N2O4+ [M + H]+: 473.3374, found: 473.3379.
2-oxo-2-(4-phenylpiperazin-1-yl)ethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (2c). White crystal; yield, 81%; m.p. 143–144 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 7.34–7.30 (m, 2H, Ar-H), 6.96–6.92 (m, 3H, Ar-H), 4.88 (d, J = 15 Hz, 1H, -O-CHe-CO-), 4.70 (d, J = 15 Hz, 1H, -O-CHa-CO-), 3.80 (s, 2H, -CO-N-CH2-), 3.59 (s, 2H, -CO-N-CH2-), 3.21 (s, 4H, ph-N-(CH2)2-), 2.67 (dd, J = 18, 3 Hz, 1H, 15-He), 2.30 (d, J = 12 Hz, 1H, 3-He), 1.99–1.94 (m, 1H), 1.90–1.77 (m, 3H), 1.74–1.66 (m, 3H), 1.56–1.50 (m, 2H), 1.46–1.39 (m, 2H), 1.34 (s, 3H, 18-CH3), 1.27 (s, 2H), 1.23–1.04 (m, 4H), 1.00 (s, 3H, 17-CH3), 0.95–0.89 (m, 1H), 0.77 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 222.44, 176.85, 165.09, 150.82, 129.28(2C), 120.77, 116.80(2C), 60.73, 57.16, 54.77, 54.32, 49.66, 49.39, 48.71, 48.50, 44.56, 44.08, 41.80, 41.53, 39.81, 39.47, 38.11, 37.99, 37.32, 29.07, 21.65, 20.34, 19.87, 18.99, 13.58. HRMS (m/z): calcd for C32H45N2O4+ [M + H]+: 521.3374, found: 521.3370.
2-(4-benzylpiperazin-1-yl)-2-oxoethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (2d). White powder; yield, 83%; m.p. 149–150 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 7.38–7.31 (m, 5H, Ar-H), 4.82 (d, J = 15 Hz, 1H, -O-CHe-CO-), 4.63 (d, J = 15 Hz, 1H, -O-CHa-CO-), 3.64 (s, 2H, -CO-N-CH2-), 3.54 (s, 2H, -N-CH2-ph), 3.41 (s, 2H, -CO-N-CH2-), 2.66 (dd, J = 18, 3 Hz, 1H, 15-He), 2.46 (s, 4H, -N-(CH2)2-), 2.29 (d, J = 12 Hz, 1H, 3-He), 1.97–1.92 (m, 1H), 1.85 (s, 1H), 1.78–1.70 (m, 4H), 1.66–1.59 (m, 3H), 1.55 (s, 1H), 1.50–1.40 (m, 3H), 1.27 (s, 3H, 18-CH3), 1.22–1.16 (m, 3H), 1.13–1.07 (m, 1H), 0.99 (s, 3H, 17-CH3), 0.94–0.85 (m, 1H), 0.76 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 222.57, 176.85, 164.94, 137.47, 129.10(2C), 128.37(2C), 127.35, 62.84, 60.74, 57.15, 54.76, 54.32, 52.84, 52.53, 48.72, 48.49, 44.53, 44.06, 41.90, 41.53, 39.81, 39.47, 38.09, 37.97, 37.32, 29.06, 21.63, 20.33, 19.86, 18.99, 13.56. HRMS (m/z): calcd for C33H47N2O4+ [M + H]+: 535.3530, found: 535.3525.
2-(azepan-1-yl)-2-oxoethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (2e). White powder; yield, 83%; m.p. 110–112 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 4.84 (d, J = 15 Hz, 1H, -O-CHe-CO-), 4.62 (d, J = 15 Hz, 1H, -O-CHa-CO-), 3.55 (t, J = 6 Hz, 2H, -CO-N-CH2-), 3.39 (t, J = 6 Hz, 2H, -CO-N-CH2-), 2.67 (dd, J = 18, 3 Hz, 1H, 15-He), 2.31 (d, J = 12 Hz, 1H, 3-He), 1.99–1.82 (m, 3H), 1.79–1.71 (m, 7H), 1.66–1.59 (m, 7H), 1.55 (s, 1H), 1.50–1.38 (m, 4H), 1.34 (s, 3H, 18-CH3), 1.12–1.07 (m, 1H,), 0.99 (s, 3H, 17-CH3), 0.94–0.84 (m, 3H), 0.76 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 222.60, 176.92, 166.05, 60.69, 57.18, 54.76, 54.32, 48.71, 48.50, 46.46, 45.99, 44.02, 41.55, 39.85, 39.47, 38.10, 37.98, 37.33, 29.08, 28.93, 27.47, 27.22, 26.89, 21.64, 20.32, 19.87, 19.01, 13.56. HRMS (m/z): calcd for C28H44NO4+ [M + H]+: 458.3265, found: 458.3261.
3.4. General Procedure for the Synthesis of Compound (3a–3d)
A mixture of isosteviol (63.6 mg, 0.20 mmol), EDC∙HCl (42.2 mg, 0.22 mmol), HOBt (29.7 mg, 0.22 mmol), Et3N (102 µL, 0.30 mmol) and various amino acid esters (0.30 mmol) in CHCl3 (10 mL) was stirred at 60 °C for 8 h. After confirming the reaction progress by thin-layer chromatography, the solvent was evaporated in vacuo, the mixture was dissolved with 15 mL ethyl acetate and then washed with saline (5 mL × 3). The mixture was then purified using silica gel column chromatography and eluted with a gradient of petroleum ether:ethyl acetate (10:1–5:1) to obtain the target compound 3a–3d.
Methyl(S)-2-phenyl-2-((4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxamido)acetate (3a). White crystal; yield, 70%; m.p. 143–145 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 7.40–7.33 (m, 5H, Ar-H), 6.67 (d, J = 9 Hz, 1H, -CO-NH-), 5.56 (d, J = 9 Hz, 1H, -NH-CH-ph), 3.75 (s, 3H, -OCH3), 2.59 (dd, J = 18, 3 Hz, 1H, 15-He), 2.13 (d, J = 15 Hz, 1H, 3-He), 1.99–1.85 (m, 2H), 1.81–1.66 (m, 5H), 1.61 (s, 4H), 1.54–1.51 (m, 1H), 1.48–1.39 (m, 2H), 1.24–1.20 (m, 5H), 1.15–1.13 (m, 1H), 0.99 (s, 3H, 17-CH3), 0.94–0.87 (m, 1H), 0.65 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 222.40, 175.88, 171.76, 136.77, 128.94(2C), 128.47, 127.30(2C), 57.49, 56.36, 54.73, 54.25, 52.77, 48.70, 48.32, 43.70, 41.67, 40.14, 39.47, 38.10, 38.08, 37.28, 29.93, 22.11, 20.30, 19.85, 19.04, 13.40. HRMS (m/z): calcd for C29H40NO4+ [M + H]+: 466.2952, found: 466.2947.
Methyl((4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carbonyl)-D-phenylalaninate (3b). White crystal; yield, 76%; m.p. 147–148 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 7.33–7.25 (m, 3H, Ar-H), 7.15–7.13 (m, 2H, Ar-H), 5.97 (d, J = 6 Hz, 1H, -CO-NH-), 4.84 (dd, J = 15, 9 Hz, 1H, -NH-CH-ph), 3.75 (s, 3H, -OCH3), 3.19 (dd, J = 15, 6 Hz, 1H, ph-CHe-), 3.06 (dd, J = 15, 6 Hz, 1H, ph-CHa-), 2.63 (dd, J = 18, 3 Hz, 1H, 15-He), 2.05 (d, J = 15 Hz, 1H, 3-He), 1.82 (s, 2H), 1.76–1.73 (m, 2H), 1.68–1.64 (m, 5H), 1.59–1.56 (m, 2H), 1.53–1.48 (m, 1H), 1.45–1.33 (m, 4H), 1.27 (s, 3H, 18-CH3), 0.98 (s, 3H, 17-CH3), 0.92–0.81 (m, 2H), 0.61 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75MHz, ppm): δ 222.44, 176.48, 172.43, 136.09, 129.14(2C), 128.65(2C), 127.17, 57.33, 54.71, 54.20, 53.00, 52.29, 48.70, 48.34, 43.74, 41.66, 40.16, 39.49, 38.17, 38.08, 37.82, 37.29, 29.84, 21.88, 20.32, 19.84, 18.89, 13.38. HRMS (m/z): calcd for C30H42NO4+ [M + H]+: 480.3108, found: 480.3100.
Methyl((4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carbonyl)-L-valinate (3c). White powder; yield, 71%; m.p. 96–98 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 5.99 (d, J = 6 Hz, 1H, -CO-NH-), 4.68–4.61 (m, 1H, -NH-CH-), 3.74 (s, 3H, -OCH3), 2.66 (dd, J = 18, 3 Hz, 1H, 15-He), 2.10–1.96 (m, 2H), 1.89–1.84 (m, 2H), 1.80–1.75 (m, 2H), 1.72–1.58 (m, 7H), 1.55–1.48 (m, 3H), 1.45–1.38 (m, 2H), 1.27 (s, 2H), 1.25–1.20 (m, 5H), 1.18–1.14 (m, 1H), 0.99 (s, 3H, 17-CH3), 0.97-0.92 (m, 5H), 0.77 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 222.39, 176.41, 173.84, 57.54, 54.68, 54.25, 52.16, 50.36, 48.69, 48.34, 43.76, 41.70, 41.61, 40.17, 39.48, 38.08(2C), 37.28, 30.11, 25.06, 22.85, 22.17, 21.91, 20.31, 19.84, 19.00, 13.53. HRMS (m/z): calcd for C27H44NO4+ [M + H]+: 446.3265, found: 446.3260.
Methyl((4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carbonyl)-L-tryptophanate (3d). White crystal; yield, 74%; m.p. 165–166 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 8.36 (s, 1H, -NH-), 7.59 (d, J = 6 Hz, 1H, Ar-H), 7.40 (d, J = 9 Hz, 1H, Ar-H), 7.26–7.12 (m, 2H, Ar-H), 7.05 (s, 1H, -NH-CH=), 6.03 (d, J = 6 Hz, 1H, -CO-NH-), 4.85 (q, J = 6 Hz, 1H, -NH-CH-), 3.75 (s, 3H, -OCH3), 3.38–3.20 (m, 2H, Ar-CH2-), 2.40 (dd, J = 18, 3 Hz, 1H, 15-He), 1.93 (d, J = 15 Hz, 1H, 3-He), 1.75–1.67 (m, 5H), 1.63–1.56 (m, 3H), 1.51–1.43 (m, 2H), 1.37–1.31 (m, 5H), 1.28 (s, 3H, 18-CH3), 1.10–1.08 (m, 1H), 0.97 (s, 3H, 17-CH3), 0.92–0.77 (m, 2H), 0.52 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 222.60, 176.60, 172.94, 136.12, 127.60, 122.48(2C), 119.84, 118.50, 111.43, 110.41, 57.35, 54.62, 54.18, 53.15, 52.29, 48.69, 48.26, 43.53, 41.61, 40.07, 39.32, 37.96, 37.92, 37.24, 29.85, 27.23, 21.80, 20.24, 19.83, 18.84, 13.08. HRMS (m/z): calcd for C32H43N2O4+ [M + H]+: 519.3217, found: 519.3221.
3.5. General Procedure for the Synthesis of Compound (4a–4j)
A mixture of isosteviol (63.6 mg, 0.20 mmol), K2CO3 (41.5 mg, 0.30 mmol) and different chloroacetanilides (0.21 mmol) in CH3CN (10 mL) was stirred at 80 °C for 2 h. After confirming the reaction progress by thin-layer chromatography, the solvent was evaporated in vacuo, the mixture was dissolved with 15 mL ethyl acetate and then washed with saline (5 mL × 3). The mixture was then purified using silica gel column chromatography and eluted with a gradient of petroleum ether:ethyl acetate (4:1–5:1) to obtain the target compound 4a–4j.
2-oxo-2-(phenylamino)ethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (4a). White powder; yield, 86%; m.p. 154–156 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 7.92 (s, 1H, -CO-NH-), 7.51 (d, J = 6 Hz, 2H, Ar-H), 7.35 (t, J = 6 Hz, 2H, Ar-H), 7.15 (t, J = 6 Hz, 1H, Ar-H), 4.75 (d, J = 15 Hz, 1H, -O-CHe-CO-), 4.58 (d, J = 15 Hz, 1H, -O-CHa-CO-), 2.62 (dd, J = 18, 3 Hz, 1H, 15-He), 2.28 (d, J = 12 Hz, 1H, 3-He), 2.02 (d, J = 15 Hz, 1H, 6-He), 1.84 (s, 1H), 1.79–1.70 (m, 5H), 1.64–1.54 (m, 4H), 1.51–1.40 (m, 3H), 1.34 (s, 3H, 18-CH3), 1.23–1.20 (m, 3H), 1.17–1.11 (m, 1H), 0.99 (s, 3H, 17-CH3), 0.74 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 221.95, 175.76, 165.03, 136.83, 129.22(2C), 124.94, 119.74(2C), 63.36, 57.00, 54.71, 54.19, 48.68, 48.39, 44.03, 41.39, 39.61, 39.43, 38.06, 37.99, 37.20, 28.99, 22.01, 20.34, 19.83, 18.93, 13.50. HRMS (m/z): calcd for C28H38NO4+ [M + H]+: 452.2795, found: 452.2790.
2-((2-chlorophenyl)amino)-2-oxoethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (4b). White powder; yield, 83%; m.p. 107–108 °C; 1H-NMR (CDCl3, 500 MHz, ppm): δ 8.46–8.43 (m, 1H, -CO-NH-), 7.39 (d, J = 10 Hz, 1H, Ar-H), 7.33–7.29 (m, 2H, Ar-H), 7.09 (t, J = 10 Hz, 1H, Ar-H), 4.79 (d, J = 15 Hz, 1H, -O-CHe-CO-), 4.62 (d, J = 15 Hz, 1H, -O-CHa-CO-), 2.61 (dd, J = 20, 5 Hz, 1H, 15-He), 2.31 (d, J = 15 Hz, 1H, 3-He), 2.02 (d, J = 15 Hz, 1H, 6-He), 1.91–1.85 (m, 1H), 1.82–1.68 (m, 4H), 1.63–1.60 (m, 3H), 1.55–1.49 (m, 4H), 1.44–1.40 (m,1H), 1.35 (s, 3H, 18-CH3), 1.27–1.22 (m, 3H), 1.20–1.13 (m, 1H), 0.98 (s, 3H, 17-CH3), 0.73 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 222.03, 175.62, 165.27, 133.74, 129.12, 127.95, 125.25, 122.65, 121.67, 63.41, 57.10, 54.73, 54.23, 48.67, 48.39, 44.06, 41.31, 39.59, 39.40, 38.02, 37.98, 37.22, 29.01, 21.97, 20.33, 19.83, 19.08, 13.43. HRMS (m/z): calcd for C28H37ClNO4+ [M + H]+: 486.2406, found: 486.2401.
2-((3-chlorophenyl)amino)-2-oxoethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (4c). White powder; yield, 80%; m.p. 120–122 °C; 1H-NMR (CDCl3, 500 MHz, ppm): δ 7.80 (s, 1H, -CO-NH-), 7.60 (s, 1H, Ar-H), 7.36 (d, J = 5 Hz, 1H, Ar-H), 7.31–7.28 (m, 1H, Ar-H), 7.14 (t, J = 5 Hz, 1H, Ar-H), 4.74 (d, J = 15 Hz, 1H, -O-CHe-CO-), 4.57 (d, J = 15 Hz, 1H, -O-CHa-CO-), 2.61 (dd, J = 20, 5 Hz, 1H, 15-He), 2.27 (d, J = 15 Hz, 1H, 3-He), 2.00 (d, J = 10 Hz, 1H, 6-He), 1.87–1.71 (m, 5H), 1.63–1.53 (m, 5H), 1.45–1.38 (m, 2H), 1.33 (s, 3H, 18-CH3), 1.26–1.21 (m, 3H), 1.20–1.13 (m, 1H), 0.98 (s, 3H, 17-CH3), 0.95–0.84 (m, 1H), 0.73 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 222.02, 175.84, 165.17, 137.98, 134.86, 130.21, 124.98, 119.83, 117.67, 63.28, 56.99, 54.70, 54.18, 48.69, 48.40, 44.04, 41.36, 39.59, 39.43, 38.06, 37.97, 37.20, 28.99, 21.99, 20.33, 19.81, 18.92, 13.50. HRMS (m/z): calcd for C28H37ClNO4+ [M + H]+: 486.2406, found: 486.2403.
2-((4-chlorophenyl)amino)-2-oxoethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (4d). White powder; yield, 84%; m.p. 140–142 °C; 1H-NMR (CDCl3, 500 MHz, ppm): δ 7.80 (s, 1H, -CO-NH-), 7.46 (d, J = 5 Hz, 2H, Ar-H), 7.32-7.30 (m, 2H, Ar-H), 4.73 (d, J = 15 Hz, 1H, -O-CHe-CO-), 4.57 (d, J = 15 Hz, 1H, -O-CHa-CO-), 2.61 (dd, J = 20, 5 Hz, 1H, 18-He), 2.27 (d, J = 15 Hz, 1H, 3-He), 2.00 (d, J = 15 Hz, 1H, 6-He), 1.84–1.69 (m, 6H), 1.63–1.62 (m, 2H), 1.56–1.52 (m, 2H), 1.49–1.38 (m, 2H), 1.32 (s, 3H, 18-CH3), 1.26–1.23 (m, 3H), 1.20–1.13 (m, 1H), 0.98–0.88 (m, 4H), 0.73 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 221.95, 175.84, 165.08, 135.42, 129.95, 129.25(2C), 120.94(2C), 63.33, 56.99, 54.69, 54.18, 48.69, 48.39, 44.03, 41.37, 39.58, 39.42, 38.06, 37.96, 37.19, 28.99, 21.97, 20.33, 19.82, 18.92, 13.51. HRMS (m/z): calcd for C28H37ClNO4+ [M + H]+: 486.2406, found: 486.2401.
2-((2-fluorophenyl)amino)-2-oxoethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (4e). White crystal; yield, 81%; m.p. 168–169 °C; 1H-NMR (CDCl3, 500 MHz, ppm): δ 8.42–8.39 (m, 1H, Ar-H), 8.18 (s, 1H, -CO-NH-), 7.18–7.10 (m, 3H, Ar-H), 4.78 (d, J = 15 Hz, 1H, -O-CHe-CO-), 4.60 (d, J = 15 Hz, 1H, -O-CHa-CO-), 2.61 (d, J = 20 Hz, 1H, 15-He), 2.28 (d, J = 15 Hz, 1H, 3-He), 2.03 (d, J = 10 Hz, 1H, 6-He), 1.89–1.71 (m, 5H), 1.63–1.59 (m, 2H), 1.54–1.51 (m, 2H), 1.44–1.38 (m, 2H), 1.33 (s, 3H, 18-CH3), 1.26–1.23 (m, 4H), 1.18–1.13 (m, 1H), 0.99 (s, 3H, 17-CH3), 0.94–0.84 (m, 1H), 0.74 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 222.03, 175.64, 165.16, 125.58, 124.92, 124.84, 121.43, 114.95, 114.70, 63.28, 56.95, 54.74, 54.21, 48.68, 48.36, 44.05, 41.35, 39.64, 39.43, 38.05(2C), 37.21, 28.04, 22.00, 20.34, 19.83, 18.84, 13.40. HRMS (m/z): calcd for C28H37FNO4+ [M + H]+: 470.2701, found: 470.2704.
2-((3-fluorophenyl)amino)-2-oxoethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (4f). White powder; yield, 80%; m.p. 144–145 °C; 1H-NMR (CDCl3, 500 MHz, ppm): δ 7.84 (s, 1H, -CO-NH-), 7.47 (d, J = 10 Hz, 1H, Ar-H), 7.31–7.28 (m, 1H, Ar-H), 7.13 (d, J = 5 Hz, 1H, Ar-H), 6.88–6.84 (m, 1H, Ar-H), 4.74 (d, J = 15 Hz, 1H, -O-CHe-CO-), 4.58 (d, J = 15 Hz, 1H, -O-CHa-CO-), 2.61 (dd, J = 20, 5 Hz, 1H, 18-He), 2.27 (d, J = 15 Hz, 1H, 3-He), 2.01 (d, J = 15 Hz, 1H, 6-He), 1.87–1.69 (m, 5H), 1.63–1.61 (m, 1H), 1.54-1.52 (m, 5H), 1.43 (dd, J = 9, 3 Hz, 1H), 1.40–1.35 (m, 1H), 1.33 (s, 2H), 1.27–1.20 (m, 3H), 1.19–1.13 (m, 1H), 0.98 (s, 3H, 17-CH3), 0.95–0.86 (m, 1H), 0.73 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 221.98, 175.81, 165.14, 164.65, 138.32, 130.31, 114.84, 111.67, 107.31, 63.30, 56.98, 54.69, 54.17, 48.69, 48.39, 44.03, 41.36, 39.58, 39.42, 38.06, 37.96, 37.19, 28.98, 21.99, 20.32, 19.81, 18.92, 13.50. HRMS (m/z): calcd for C28H37FNO4+ [M + H]+: 470.2701, found: 470.2699.
2-((4-fluorophenyl)amino)-2-oxoethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (4g). White powder; yield, 90%; m.p. 140–141 °C; 1H-NMR (CDCl3, 500 MHz, ppm): δ 7.76 (s, 1H, -CO-NH-), 7.48–7.45 (m, 2H, Ar-H), 7.05 (t, J = 10 Hz, 2H, Ar-H), 4.74 (d, J = 15 Hz, 1H, -O-CHe-CO-), 4.58 (d, J = 15 Hz, 1H, -O-CHa-CO-), 2.61 (d, J = 20 Hz, 1H, 18-He), 2.27 (d, J = 15 Hz, 1H, 3-He), 2.00 (d, J = 15 Hz, 1H, 6-He), 1.87–1.70 (m, 5H), 1.63–1.61 (m, 2H), 1.55–1.52 (m, 3H), 1.44–1.36 (m, 2H), 1.33 (s, 3H), 1.26–1.23 (m, 3H), 1.19–1.13 (m, 1H), 0.98 (s, 3H), 0.95–0.86 (m, 1H), 0.73 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 222.01, 175.86, 165.06, 161.29, 132.87, 121.63, 121.53, 116.06, 115.75, 63.29, 56.99, 54.69, 54.18, 48.69, 48.40, 44.02, 41.37, 39.59, 39.42, 38.06, 37.96, 37.19, 28.99, 21.96, 20.32, 19.82, 18.92, 13.51. HRMS (m/z): calcd for C28H37FNO4+ [M + H]+: 470.2701, found: 470.2707.
2-((4-nitrophenyl)amino)-2-oxoethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (4h). Pale yellow solid; yield, 78%; m.p. 189–190 °C; 1H-NMR (CDCl3, 500 MHz, ppm): δ 8.24 (d, J = 10 Hz, 2H, Ar-H), 8.08 (s, 1H, -CO-NH-), 7.69 (d, J = 10 Hz, 2H, Ar-H), 4.77 (d, J = 15 Hz, 1H, -O-CHe-CO-), 4.62 (d, J = 15 Hz, 1H, -O-CHa-CO-), 2.60 (d, J = 20 Hz, 1H, 18-He), 2.27 (d, J = 10 Hz, 1H, 3-He), 2.00 (d, J = 15 Hz, 1H, 6-He), 1.83–1.76 (m, 3H), 1.74–1.70 (m, 2H), 1.63–1.59 (m, 2H), 1.45–1.37 (m, 3H), 1.34 (s, 3H), 1.30 (s, 1H), 1.23-1.10 (m, 4H), 0.98 (s, 3H), 0.95–0.84 (m, 2H), 0.73 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 125 MHz, ppm): δ 222.00, 175.99, 165.48, 144.11, 142.50, 125.27(2C), 119.17(2C), 63.42, 56.99, 54.69, 54.18, 48.70, 48.41, 44.10, 41.36, 39.55, 39.44, 38.08, 37.97, 37.19, 29.01, 21.98, 20.34, 19.81, 18.94, 13.55. HRMS (m/z): calcd for C28H37N2O6+ [M + H]+: 497.2646, found: 497.2640.
2-((4-methoxyphenyl)amino)-2-oxoethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (4i). White powder; yield, 81%; m.p. 86–88 °C; 1H-NMR (CDCl3, 500 MHz, ppm): δ 7.70 (s, 1H, -CO-NH-), 7.40 (d, J = 15 Hz, 2H, Ar-H), 6.88 (d, J = 10 Hz, 2H, Ar-H), 4.73 (d, J = 15 Hz, 1H, -O-CHe-CO-), 4.57 (d, J = 15 Hz, 1H, -O-CHa-CO-), 3.80 (s, 3H, ph-O-CH3), 2.62 (d, J = 15 Hz, 1H, 18-He), 2.27 (d, J = 15 Hz, 1H, 3-He), 2.00 (d, J = 10 Hz, 1H, 6-He), 1.88–1.70 (m, 5H), 1.63–1.58 (m, 3H), 1.56–1.52 (m, 2H), 1.44–1.39 (m, 2H), 1.32 (s, 3H, 18-CH3), 1.25–1.23 (m, 3H), 1.18–1.12 (m, 1H), 0.98 (s, 3H), 0.94–0.88 (m, 1H), 0.74 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 222.01, 175.81, 164.88, 156.85, 129.90, 121.60(2C), 114.35(2C), 63.35, 57.01, 55.52, 54.71, 54.20, 48.70, 48.39, 44.02, 41.39, 39.61, 39.42, 38.05, 37.98, 37.20, 28.99, 21.98, 20.33, 19.81, 18.93, 13.50. HRMS (m/z): calcd for C29H40NO5+ [M + H]+: 482.2901, found: 482.2905.
2-((2,5-dimethoxyphenyl)amino)-2-oxoethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (4j). White powder; yield, 79%; m.p. 166–168 °C; 1H-NMR (CDCl3, 500 MHz, ppm): δ 8.55 (s, 1H, -CO-NH-), 8.14 (d, J = 3 Hz, 1H, Ar-H), 6.81 (d, J = 5 Hz, 1H, Ar-H), 6.61 (dd, J = 10, 5 Hz, 1H, Ar-H), 4.80 (d, J = 15 Hz, 1H, -O-CHe-CO-), 4.54 (d, J = 15 Hz, 1H, -O-CHa-CO-), 3.83 (s, 3H, ph-O-CH3), 3.79 (s, 3H, ph-O-CH3), 2.61 (dd, J = 15, 5 Hz, 1H, 18-He), 2.31 (d, J = 15 Hz, 1H, 3-He), 2.01 (d, J = 15 Hz, 1H, 6-He), 1.88 (qt, J = 9, 3Hz, 1H) 1.82–1.74 (m, 3H), 1.71–1.67 (m, 2H), 1.63–1.60 (m, 1H), 1.55–1.47 (m, 3H), 1.44–1.37 (m, 2H), 1.35 (s, 3H, 18-CH3), 1.27–1.22 (m, 3H), 1.18–1.10 (m, 1H), 0.98–0.93 (m, 4H), 0.73 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 222.00, 175.50, 164.97, 153.94, 142.05, 127.36, 110.68, 109.07, 106.08, 63.14, 57.09, 56.04, 55.83, 54.74, 54.24, 48.66, 48.39, 43.99, 41.39, 39.63, 39.41, 38.02, 37.88, 37.22, 28.83, 21.76, 20.32, 19.83, 18.96, 13.38. HRMS (m/z): calcd for C30H42NO6+ [M + H]+: 512.3007, found: 512.3001.
3.6. General Procedure for the Synthesis of Compound (5a–5e)
A mixture of isosteviol (63.6 mg, 0.20 mmol), K2CO3 (41.5 mg, 0.30 mmol) and different phenyl 1,2,3-triazole chloroacetamides (0.21 mmol) in CH3CN (10 mL) was stirred at 80 °C for 2 h. After confirming the reaction progress by thin-layer chromatography, the solvent was evaporated in vacuo, the mixture was dissolved with 15 mL ethyl acetate and then washed with saline (5 mL × 3). The mixture was then purified using silica gel column chromatography and eluted with petroleum ether:ethyl acetate (1:1) to obtain the target compound 5a–5e.
2-oxo-2-(((1-phenyl-1H-1,2,3-triazol-4-yl)methyl)amino)ethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (5a). White solid; yield, 81%; m.p. 83–84 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 8.01 (s, 1H, triazole-H), 7.74-7.71 (m, 2H, Ar-H), 7.56–7.42 (m, 3H, Ar-H), 6.91 (brs, 1H, -NH-CO-), 4.74–4.46 (m, 4H, -O-CH2-, -CH2-NH-), 2.56 (dd, J = 18, 3 Hz, 1H, 15-He), 2.22 (d, J = 12 Hz, 1H, 3-He), 1.95 (d, J = 12 Hz, 1H, 6-He), 1.85–1.66 (m, 6H), 1.62–1.47 (m, 4H), 1.42–1.37 (m, 2H), 1.27 (s, 3H, 18-CH3), 1.20–1.13 (m, 3H), 1.10–1.04 (m, 1H), 0.96 (s, 3H, 17-CH3), 0.93–0.88 (m, 1H), 0.67 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 222.09, 176.00, 167.37, 144.64, 136.87, 129.81(2C), 128.93, 120.52(2C), 120.44, 62.98, 56.98, 54.66, 54.17, 48.64, 48.31, 43.95, 41.28, 39.61, 39.39, 37.99, 37.94, 37.20, 34.69, 28.91, 21.90, 20.29, 19.82, 18.87, 13.42. HRMS (m/z): calcd for C31H41N4O4+ [M + H]+: 533.3122, found: 533.3119.
2-(((1-(4-chlorophenyl)-1H-1,2,3-triazol-4-yl)methyl)amino)-2-oxoethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (5b). White solid; yield, 82%; m.p. 80–82 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 8.00 (s, 1H, triazole-H), 7.71–7.68 (m, 2H, Ar-H), 7.54–7.51 (m, 2H, Ar-H), 6.81 (brs, 1H, -NH-CO-), 4.74–4.47 (m, 4H, -O-CH2-, -CH2-NH-), 2.57 (dd, J = 18, 3 Hz, 1H, 15-He), 2.23 (d, J = 12 Hz, 1H, 3-He), 1.96 (d, J = 12 Hz, 1H, 6-He), 1.82–1.68 (m, 6H), 1.60–1.39 (m, 6H), 1.28 (s, 3H, 18-CH3), 1.22–1.15 (m, 3H), 1.12–1.06 (m, 1H), 0.99 (s, 3H, 17-CH3), 0.95–0.87 (m, 1H), 0.68 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 222.09, 176.02, 167.44, 144.90, 135.35, 134.76, 130.01(2C), 121.69(2C), 120.44, 62.99, 56.98, 54.67, 54.18, 48.66, 48.33, 43.96, 41.30, 39.61, 39.40, 38.00, 37.94, 37.21, 34.64, 28.91, 21.91, 20.30, 19.82, 18.87, 13.42. HRMS (m/z): calcd for C31H40ClN4O4+ [M + H]+: 567.2733, found: 567.2729.
2-(((1-(4-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methyl)amino)-2-oxoethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (5c). White solid; yield, 83%; m.p. 71–72 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 7.92 (s, 1H, triazole-H), 7.62 (d, J = 6 Hz, 2H, Ar-H), 7.04 (d, J = 6 Hz, 2H, Ar-H), 6.87 (brs, 1H, -NH-CO-), 4.74–4.47 (m, 4H, -O-CH2-, -CH2-NH-), 3.88 (s, 3H, -O-CH3), 2.57 (dd, J = 18, 3 Hz, 1H, 15-He), 2.23 (d, J = 12 Hz, 1H, 3-He), 1.96 (d, J = 12 Hz, 1H, 6-He), 1.80–1.68 (m, 6H), 1.62–1.48 (m, 4H), 1.45–1.38 (m, 3H), 1.23–1.19 (m, 3H), 1.16–1.05 (m, 2H), 0.98 (s, 3H, 17-CH3), 0.94–0.87 (m, 2H), 0.68 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 222.11, 175.97, 167.33, 159.95, 144.37, 130.30, 122.18(2C), 120.55, 114.84(2C), 63.00, 56.98, 55.64, 54.67, 54.17, 48.65, 48.30, 43.95, 41.28, 39.61, 39.39, 38.00, 37.94, 37.21, 34.70, 28.91, 21.92, 20.29, 19.82, 18.87, 13.41. HRMS (m/z): calcd for C32H43N4O5+ [M + H]+: 563.3228, found: 563.3225.
2-oxo-2-(((1-(p-tolyl)-1H-1,2,3-triazol-4-yl)methyl)amino)ethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (5d). White solid; yield, 83%; m.p. 66–68 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 7.96 (s, 1H, triazole-H), 7.60 (d, J = 9 Hz, 2H, Ar-H), 7.33 (d, J = 9 Hz, 2H, Ar-H), 6.84 (brs, 1H, -NH-CO-), 4.74–4.47 (m, 4H, -O-CH2-, -CH2-NH-), 2.56 (dd, J = 21, 3 Hz, 1H, 15-He), 2.43 (s, 3H, Ar-CH3), 2.23 (d, J = 15 Hz, 1H, 3-He), 1.95 (d, J = 12 Hz, 1H, 6-He), 1.80–1.66 (m, 6H), 1.62–1.48 (m, 4H), 1.43–1.38 (m, 2H), 1.28 (s, 3H, 18-CH3), 1.21–1.15 (m, 3H), 1.10–1.05 (m, 1H), 0.97 (s, 3H, 17-CH3), 0.93–0.88 (m, 1H), 0.68 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 222.15, 175.97, 167.34, 144.42, 139.10, 134.58, 130.31(2C), 120.44(2C), 120.41, 62.99, 56.97, 54.66, 54.16, 48.65, 48.30, 43.94, 41.27, 39.60, 39.38, 37.99, 37.94, 37.20, 34.71, 28.92, 21.91, 21.11, 20.29, 19.83, 18.87, 13.41. HRMS (m/z): calcd for C32H43N4O4+ [M + H]+: 547.3279, found: 547.3276.
2-(((1-(3,4-dichlorophenyl)-1H-1,2,3-triazol-4-yl)methyl)amino)-2-oxoethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (5e). White solid; yield, 79%; m.p. 96–97 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 8.02 (s, 1H, triazole-H), 7.92–7.91 (m, 1H, Ar-H), 7.65–7.59 (m, 2H, Ar-H), 6.81 (brs, 1H, -NH-CO-), 4.73–4.47 (m, 4H, -O-CH2-, -CH2-NH-), 2.56 (dd, J = 18, 3 Hz, 1H, 15-He), 2.23 (d, J = 15 Hz, 1H, 3-He), 1.98–1.93 (m, 1H, 6-He), 1.82-1.67 (m, 6H), 1.63–1.47 (m, 4H), 1.45–1.35 (m, 2H), 1.28 (s, 3H, 18-CH3), 1.22–1.15 (m, 3H), 1.12–1.06 (m, 1H), 0.98 (s, 3H, 17-CH3), 0.95–0.89 (m, 1H), 0.69 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 222.08, 176.06, 167.50, 145.20, 135.87, 134.08, 133.07, 131.55, 122.26, 120.43, 119.39, 63.00, 56.98, 54.66, 54.19, 48.66, 48.34, 43.96, 41.31, 39.60, 39.40, 38.01, 37.94, 37.21, 34.64, 28.92, 21.89, 20.31, 19.82, 18.88, 13.44. HRMS (m/z): calcd for C31H39Cl2N4O4+ [M + H]+: 601.2343, found: 601.2340.
3.7. General Procedure for the Synthesis of Compound (6a–6e)
A mixture of isosteviol (63.6 mg, 0.20 mmol), K2CO3 (41.5 mg, 0.30 mmol) and different phosphonic acid [[(chloroacetyl) amino] phenylmethyl]-diethyl esters (0.21 mmol) in CH3CN (10 mL) was stirred at 80 °C for 3 h. After confirming the reaction progress by thin-layer chromatography, the solvent was evaporated in vacuo, the mixture was dissolved with 15 mL ethyl acetate and then washed with saline (5 mL ×3). The mixture was then purified using silica gel column chromatography and eluted with a gradient of petroleum ether:ethyl acetate (3:1-1:1) to obtain the target compound 6a–6e.
2-(((diethoxyphosphoryl)(phenyl)methyl)amino)-2-oxoethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (6a). White powder; yield, 81%; m.p. 156–157 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 7.41–7.35 (m, 5H, Ar-H), 7.20–7.12 (m, 1H, -NH-CO-), 5.48 (dd, J = 18, 9 Hz, 1H, -P-CH-NH-), 4.76–4.64 (m, 1H, -O-CHe-CO-), 4.53–4.40 (m, 1H, -O-CHa-CO-), 4.19–4.07 (m, 2H, -O-CHe-), 4.00–3.87 (m, 1H, -O-CHa-), 3.77–3.62 (m, 1H, -O-CHa-), 2.65–2.56 (m, 1H, 15-He), 2.27 (d, J = 15 Hz, 1H, 3-He), 2.09–1.99 (m, 1H, 6-He), 1.89–1.69 (m, 6H), 1.65–1.50 (m, 4H), 1.45–1.41 (m, 2H), 1.35–1.29 (m, 6H), 1.27–1.19 (m, 3H), 1.15–1.07 (m, 4H), 1.00–0.99 (m, 3H), 0.95–0.87 (m, 1H), 0.70 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 221.94, 175.72, 166.43, 134.77, 128.78(2C), 128.38, 127.91(2C), 63.60, 63.12, 62.74, 56.91, 54.73, 54.21, 50.82, 48.68, 48.40, 43.97, 41.41, 39.64, 39.41, 38.02(2C), 37.23, 28.86, 21.92, 20.32, 19.82, 18.93, 16.42, 16.10, 13.38. HRMS (m/z): calcd for C33H49NO7P+ [M + H]+: 602.3241, found: 602.3238.
2-(((4-chlorophenyl)(diethoxyphosphoryl)methyl)amino)-2-oxoethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (6b). White powder; yield, 83%; m.p. 149–150 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 7.46–7.33 (m, 4H, Ar-H), 7.27–7.16 (m, 1H, -NH-CO-), 5.44 (ddd, J = 21, 9, 3 Hz, 1H, -P-CH-NH-), 4.68 (t, J = 15 Hz, 1H, -O-CHe-CO-), 4.46 (t, J = 15 Hz, 1H, -O-CHa-CO-), 4.18–4.09 (m, 2H, -O-CHe-), 4.03–3.90 (m, 1H, -O-CHa-), 3.84–3.70 (m, 1H, -O-CHa-), 2.60 (d, J = 18 Hz, 1H, 15-He), 2.25 (d, J = 12 Hz, 1H, 3-He), 2.05–1.98 (m, 1H, 6-He), 1.89–1.65 (m, 7H), 1.60–1.53 (m, 3H), 1.45–1.40 (m, 2H), 1.35–1.29 (m, 6H), 1.26–1.22 (m, 4H), 1.16–1.11 (m, 3H), 1.00–0.99 (m, 3H), 0.95–0.91 (m, 1H), 0.70–0.69 (m, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 221.99, 175.77, 166.59, 134.39, 133.40, 129.30, 129.19, 128.99(2C), 63.72, 63.30, 62.78, 56.96, 54.72, 54.21, 50.31, 48.68, 48.41, 43.97, 41.36, 39.64, 39.41, 38.02, 37.98, 37.22, 28.89, 21.99, 20.32, 19.82, 18.92, 16.45, 16.16, 13.38. HRMS (m/z): calcd for C33H48ClNO7P+ [M + H]+: 636.2851, found: 636.2846.
2-(((diethoxyphosphoryl)(4-methoxyphenyl)methyl)amino)-2-oxoethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (6c). White powder; yield, 81%; m.p. 134–135 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 7.37–7.34 (m, 2H, Ar-H), 7.17–7.15 (m, 1H, -NH-CO-), 6.91–6.87 (m, 2H, Ar-H), 5.48 (dd, J = 21, 9 Hz, 1H, -P-CH-NH-), 4.72–4.62 (m, 1H, -O-CHe-CO-), 4.51–4.39 (m, 1H, -O-CHe-CO-), 4.18–4.07 (m, 2H, -O-CHe-), 4.00–3.90 (m, 1H, -O-CHa-), 3.80 (s, 3H, ph-OCH3), 3.76–3.66 (m, 1H, -O-CHa-), 2.60 (d, J = 18 Hz, 1H, 15-He), 2.25 (d, J = 12 Hz, 1H, 3-He), 2.05–1.98 (m, 1H, 6-He), 1.88–1.73 (m, 5H), 1.68–1.53 (m, 5H), 1.44–1.40 (m, 2H), 1.35–1.29 (m, 6H), 1.26–1.17 (m, 4H), 1.13–1.08 (m, 3H), 0.99–0.98 (m, 3H), 0.96–0.90 (m, 1H), 0.69 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 221.86, 175.71, 166.29, 159.63, 129.24(2C), 126.75, 114.19(2C), 63.57, 62.98, 62.75, 56.92, 55.31, 54.73, 54.21, 50.14, 48.67, 48.40, 43.96, 41.41, 39.64, 39.42, 38.02(2C), 37.21, 28.86, 21.95, 20.32, 19.82, 18.93, 16.39, 16.24, 13.31. HRMS (m/z): calcd for C34H51NO8P+ [M + H]+: 632.3347, found: 632.3344.
2-(((diethoxyphosphoryl)(p-tolyl)methyl)amino)-2-oxoethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (6d). White powder; yield, 84%; m.p. 119–120 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 7.32–7.29 (m, 2H, Ar-H), 7.18–7.11 (m, 3H, Ar-H, -NH-CO-), 5.44 (dd, J = 21, 9 Hz, 1H, -P-CH-NH-), 4.74–4.62 (m, 1H, -O-CHe-CO-), 4.51–4.38 (m, 1H, -O-CHe-CO-), 4.18–4.06 (m, 2H, -O-CHe-), 4.00–3.87 (m, 1H, -O-CHa-), 3.78–3.64 (m, 1H, -O-CHa-), 2.60 (dq, J = 18, 3 Hz, 1H, 15-He), 2.34 (s, 3H, ph-CH3), 2.26 (d, J = 15 Hz, 1H, 3-He), 2.07–1.99 (m, 1H, 6-He), 1.89–1.78 (m, 3H), 1.73–1.69 (m, 3H), 1.64–1.54 (m, 4H), 1.49–1.40 (m, 3H), 1.35–1.29 (m, 6H), 1.26–1.22 (m, 3H), 1.13–1.08 (m, 3H), 1.00–0.99 (m, 3H), 0.95–0.91 (m, 1H), 0.69 (s, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 221.98, 175.68, 166.28, 138.26, 131.68, 129.48(2C), 127.86(2C), 63.48, 63.08, 62.79, 57.00, 54.74, 54.24, 50.53, 48.68, 48.41, 43.97, 41.36, 39.65, 39.42, 38.02(2C), 37.24, 28.96, 22.16, 21.14, 20.32, 19.82, 18.95, 16.42, 16.13, 13.30. HRMS (m/z): calcd for C34H51NO7P+ [M + H]+: 616.3398, found: 616.3393.
2-(((3,4-dichlorophenyl)(diethoxyphosphoryl)methyl)amino)-2-oxoethyl(4R,4aS,6aR,9S,11aR,11bS)-4,9,11b-trimethyl-8-oxotetradecahydro-6a,9-methanocyclohepta[a]naphthalene-4-carboxylate (6e). White powder; yield, 80%; m.p. 168–169 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 7.50–7.44 (m, 2H, Ar-H), 7.26–7.24 (m, 1H, Ar-H), 7.08–7.03 (m, 1H, -NH-CO-), 5.40 (ddd, J = 21, 9, 3 Hz, 1H, -P-CH-NH-), 4.78–4.65 (m, 1H, -O-CHe-CO-), 4.54–4.41 (m, 1H, -O-CHa-CO-), 4.18–4.10 (m, 2H, -O-CHe-), 4.07–3.99 (m, 1H, -O-CHa-), 3.92–3.81 (m, 1H, -O-CHa-), 2.66–2.58 (m, 1H, 15-He), 2.27 (d, J = 15 Hz, 1H, 3-He), 2.06–1.97 (m, 1H, 6-He), 1.89–1.66 (m, 6H), 1.61 (s, 2H), 1.58–1.50 (m, 2H), 1.46–1.41 (m, 2H), 1.36–1.30 (m, 6H), 1.27–1.11 (m, 7H), 1.01–1.00 (m, 3H), 0.98–0.92 (m, 1H), 0.72–0.71 (m, 3H, 20-CH3). 13C-NMR (CDCl3, 75 MHz, ppm): δ 221.98, 175.83, 166.68, 135.15, 132.96, 132.62, 130.75, 129.67, 127.25, 63.81, 63.50, 62.81, 56.95, 54.78, 54.27, 50.01, 48.67, 48.39, 44.05, 41.40, 39.70, 39.48, 38.02, 37.91, 37.29, 28.92, 21.99, 20.41, 19.91, 18.90, 16.42, 16.24, 13.39. HRMS (m/z): calcd for C33H47Cl2NO7P+ [M + H]+: 670.2462, found: 670.2458.
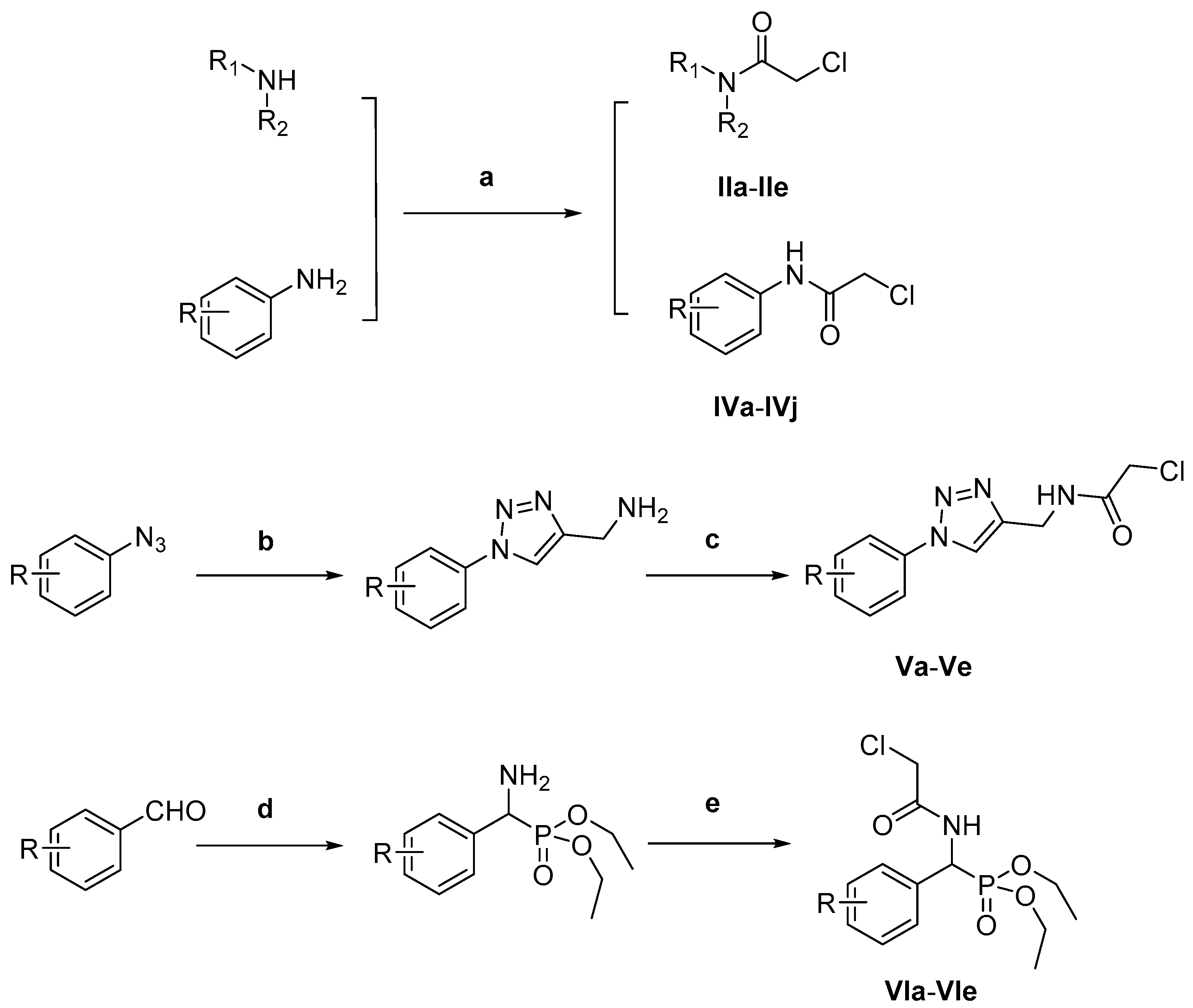
3.8. General Procedure for the Synthesis of Intermediates (IIa-IIe, IVa-IVj, Va-Ve, VIa-VIe)
Chloroacetyl chloride (1.23 g, 11 mmol), Et
3N (1.11 g, 11 mmol) and different amines (10 mmol) were added to CH
2Cl
2 (20 mL) and the resulting mixture was stirred at 30 °C for 2–8 h. Then, the solvent was evaporated in vacuo, water was added (15 mL), the mixture was filtered and the residue was washed with water to obtain the different intermediates (
Scheme 2).
3.9. Biological Evaluation Materials
3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT) was purchased from Sigma Chemical Co. (St. Louis, MO, USA). The propidium iodide (PI) and Annexin V-FITC apoptosis detection kit was purchased from BD Pharmingen (San Diego, CA, USA).
3.10. In Vitro Antiproliferative Activity
The antiproliferative activity of the target compounds against a normal L02 cell line and the three different human cancer cell lines viz. colorectal (HCT-116), liver (BEL-7402) and liver (HepG2) were evaluated using a standard MTT-based colorimetric assay. All cell lines were obtained from the Key Laboratory of Natural Resources and Functional Molecules of the Changbai Mountain (Yanbian University, Jilin, China) and maintained in Dulbecco’s modified Eagle’s medium (DMEM) and RPMI Media 1640 (RPMI1640), supplemented with 10% fetal bovine serum (FBS) at 37 °C in a humidified atmosphere containing 5% CO2.
Cells were plated in 96-well plates at appropriate densities to ensure exponential growth throughout the experimental period (9 × 10
3 cells per well) and then allowed to adhere for 24 h. Cells were then treated for 48 h with four serial concentrations (1, 10, 50 and 100 µM) of each compound. 5-fluorouracil (5-FU) was used as a positive control. After 48 h of incubation, 10 µL of MTT solution was added to each well to a final concentration of 2 mg/mL. Plates were then incubated for a further 4 h. After incubation, the MTT solution was removed and 150 µL of DMSO was added to each well for coloration. The plates were shaken vigorously for 10 min at room temperature to ensure complete solubilization. The optical density (OD) was read on a microplate reader (ELx800, BioTek, Highland Park, Winooski, VT, USA) at a wavelength of 492 nm and the data were subsequently analyzed. The percentage of cell growth inhibition was calculated from the following equation:
3.11. Colony Formation Assay
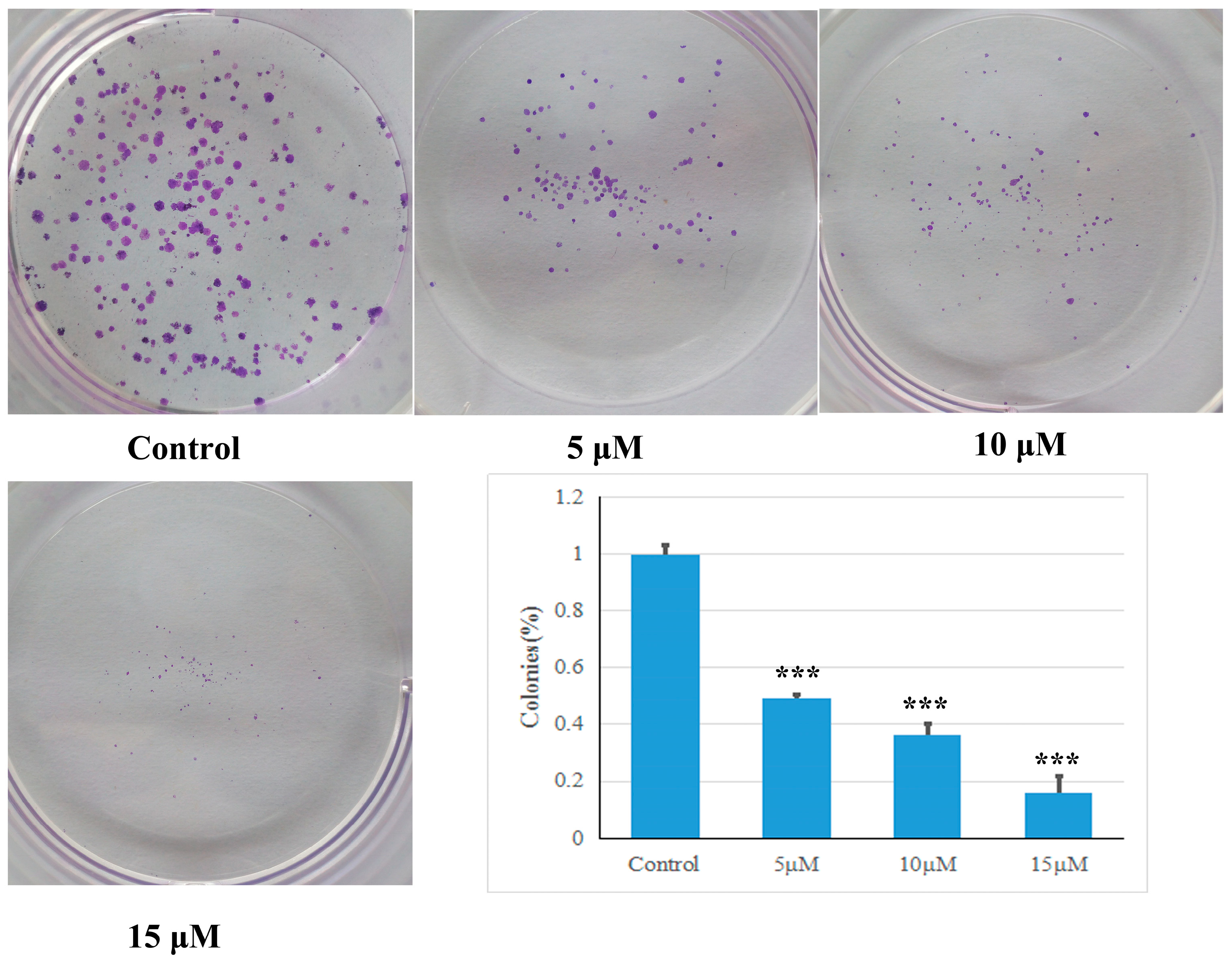
Exponentially growing determined HCT-116 cells (6 × 102 per well) were plated in 6-well plates with the Roswell Park Memorial Institute (RPMI) 1640 culture medium (Gibco, USA) containing with 10% fetal bovine serum (FBS) (Gibco, Waltham, MA, USA). After an overnight incubation, the culture medium was removed and replaced fresh medium. Then, the cells were treated with various concentrations of compound 5d (5, 10 and 15 μM) dissolved in DMSO. Some cells were treated with DMSO only as a negative control. The cells were then incubated for another 7 days. Finally, the cells were fixed with 4% paraformaldehyde for 30 min and stained with 0.1% crystal violet for 15 min at room temperature, after which, the staining was washed with PBS until the colonies were totally cleared.
3.12. Analysis for Cell Cycle and Apoptosis by Flow Cytometry
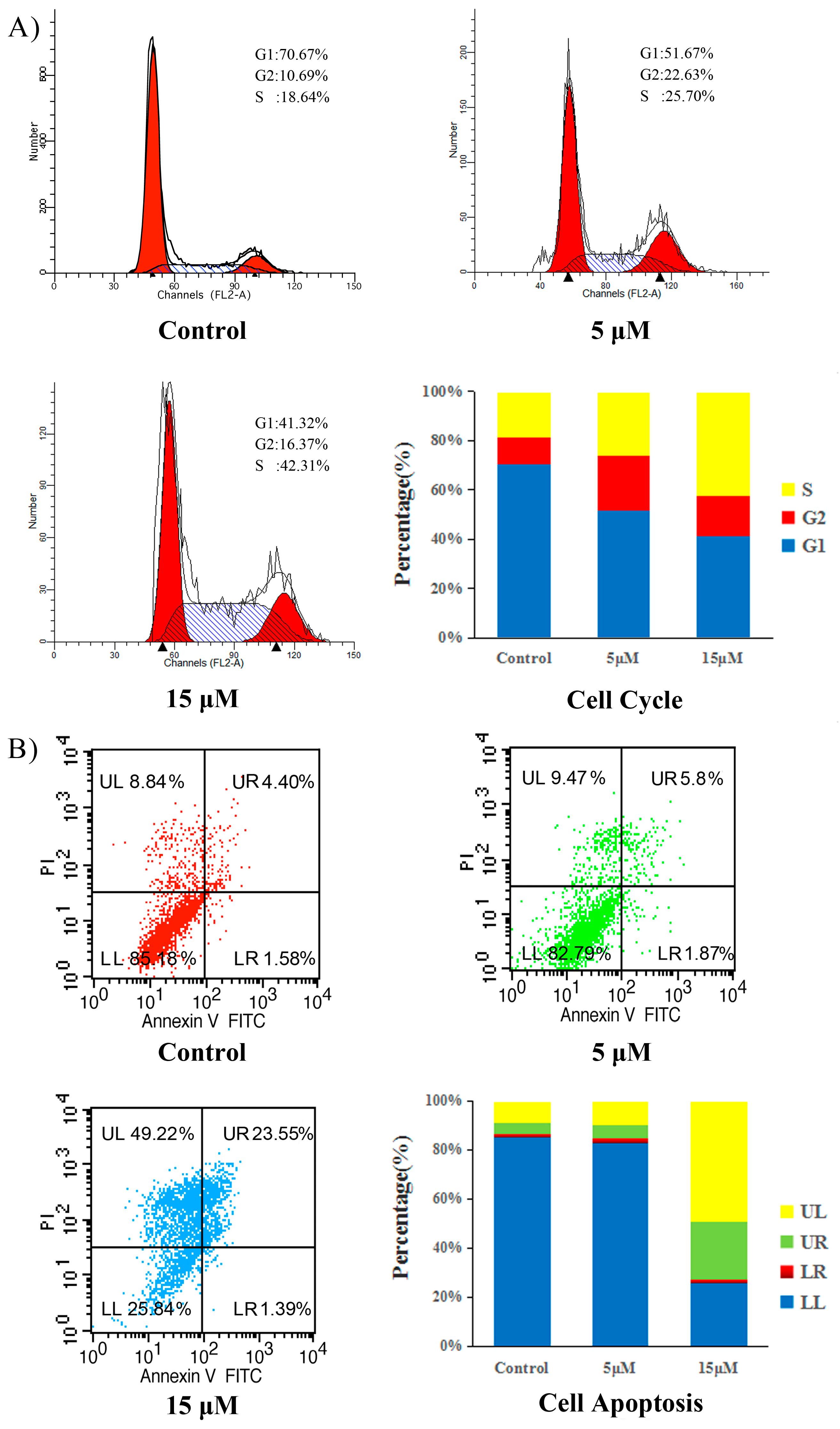
HCT-116 cells were plated in 6-well plates (5.0 × 105 cells per well) and incubated at 37 °C for 24 h. Exponentially growing cells were then incubated with compound 5d at 5 and 15 µM. After 24 h, untreated cells (control) or cells treated with compound 5d were centrifuged at 1000 rpm for 10 min and then fixed in 70% ethanol at −20 °C for at least 24 h. The cells were subsequently resuspended in phosphate-buffered saline (PBS) containing 0.1 mg/mL of RNase A and 5 µg/mL propidium iodide (PI). The cellular DNA content for the cell cycle distribution analysis was measured by flow cytometry using a FACSCalibur flow cytometer with Cell Quest software (Becton-Dickinson, Franklin Lakes, NJ, USA), plotting at least 30,000 events per sample. The percentage of cells in the G1, S and G2 phases of the cell cycle were determined using the ModFit LT version 4.0 software package (Verity Software, Topsham, ME, USA).
Apoptosis was detected using an Apoptosis Detection Kit (Invitrogen, Eugene, OR, USA). In brief, cells were cultured in 6-well plates (5.0 × 105 cells per well) and incubated at 37 °C for 24 h. Cells with exponential growth were then incubated with compound 5d at 5 and 15 µM. Following 24 h of incubation, the cells were collected, washed twice with PBS and once with 1 × binding buffer and then stained with 5 µM of annexin V-FITC and 2.5 µM of PI 5mg/mL in 1 × binding buffer for 30 min at room temperature in the dark. Apoptotic cells were enumerated using a FACSCalibur flow cytometer with Cell Quest software (Becton–Dickinson, Franklin Lakes, NJ, USA).
3.13. Western Blotting
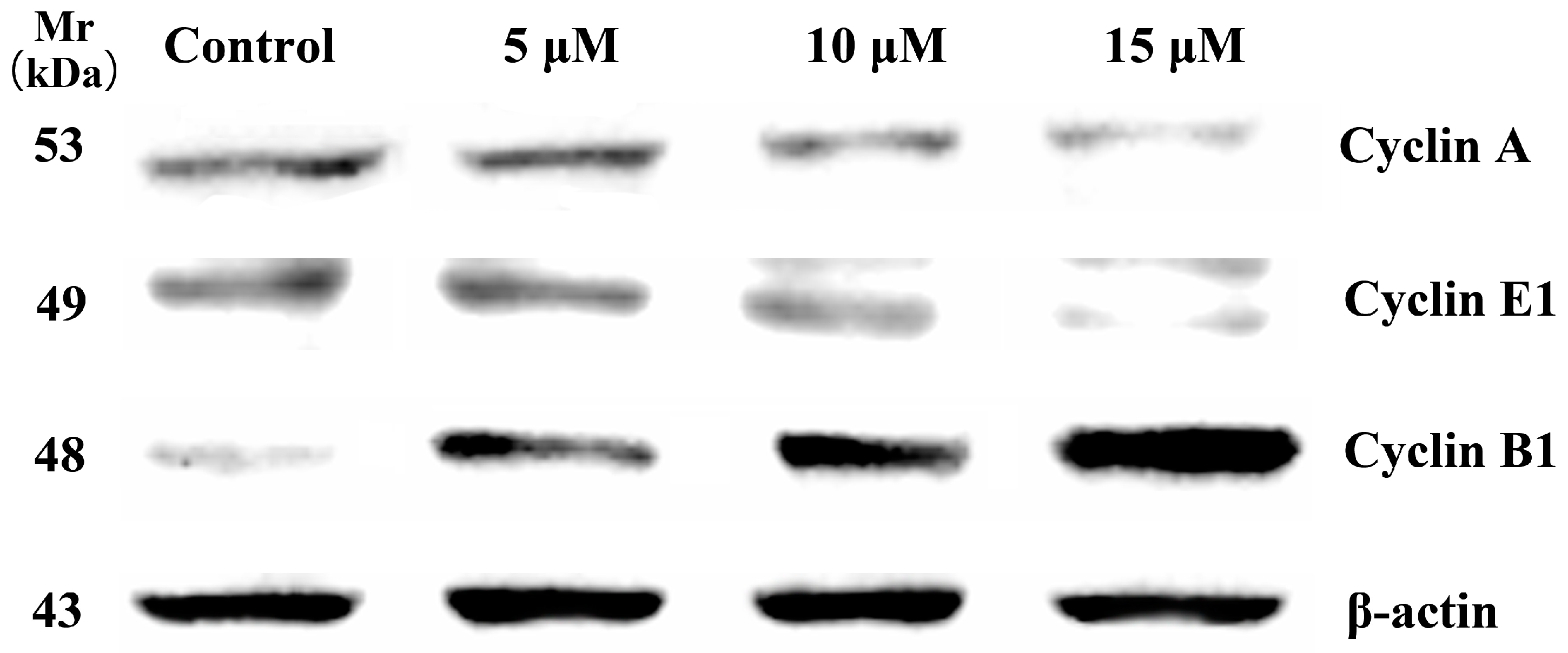
HCT-116 cells were cultured with different concentrations of compound 5d for 24 h. Then, the floating cells was collected and washed two times with ice cold PBS. The pellet was resuspended in lysis buffer. After the cells were lysed on ice for 20 min, lysates were centrifuged at 12,000 rpm at 4 °C for 15 min. The protein concentration in the supernatant was determined using BCA protein assay reagents. Equal amounts of protein (50 μg) were resolved using sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) (8−12% acrylamide gels) and transferred to a PVDF Hybond-P membrane. Membranes were blocked with PBS containing 5% non-fat milk for 1 h at room temperature. Membranes were then incubated with primary antibodies against Cyclin A (ENT1167, Elabscience, Wuhan, China), Cyclin E1 (ENT1176, Elabscience, Wuhan, China), Cyclin B1 (ENT1169, Elabscience, Wuhan, China) and β-actin (ENM0028, Elabscience, Wuhan, China), with gentle rotation overnight at 4 °C. Membranes were next incubated with fluorescent secondary antibodies for 2 h. Proteins were detected by electrochemiluminescence (Bio-Rad, Hercules, CA, USA).
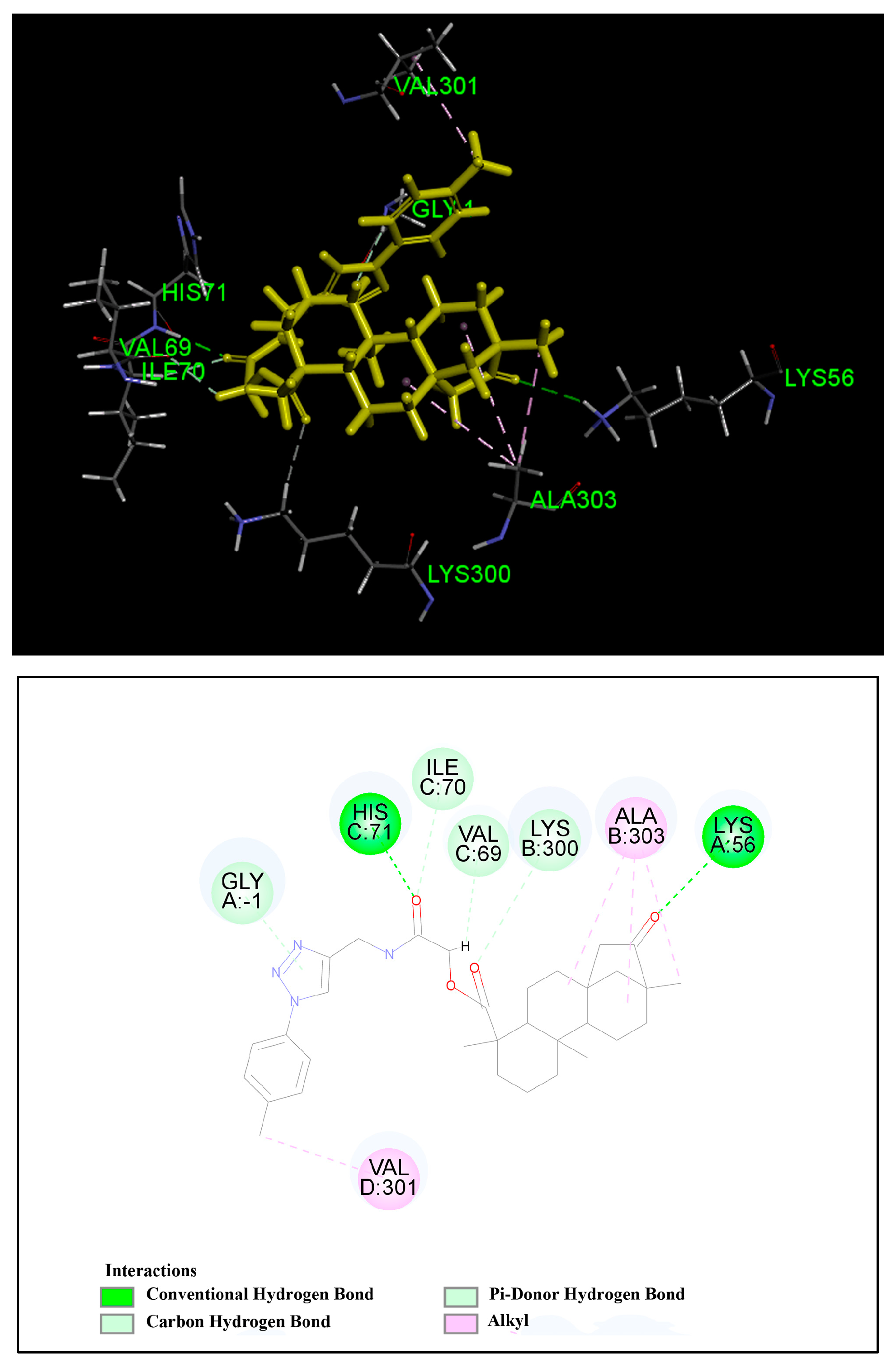
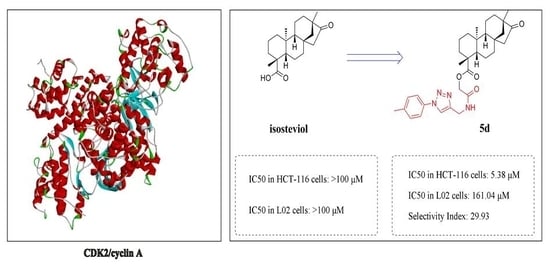
3.14. Molecular Docking Study
The molecular docking study was performed using Discovery Studio (DS) 2017. The protein and ligand were prepared, water molecules were deleted and a DS Server added hydrogen. The docking result was treated with DS Client. In this study, the crystal structure of the CDK2/cyclin A complex (3my5) was chosen for docking. The xyz coordinates (−25.8518, 4.7751, −23.0309, radius 34.6 Å) of CDK2/cyclin A complex residues were defined as the binding site sphere. The protocol, CDOCKER was used to perform the docking. The output poses of the ligands generated were analyzed based on the LibDockScore function.