Prunetin Relaxed Isolated Rat Aortic Rings by Blocking Calcium Channels
Abstract
:1. Introduction
2. Results
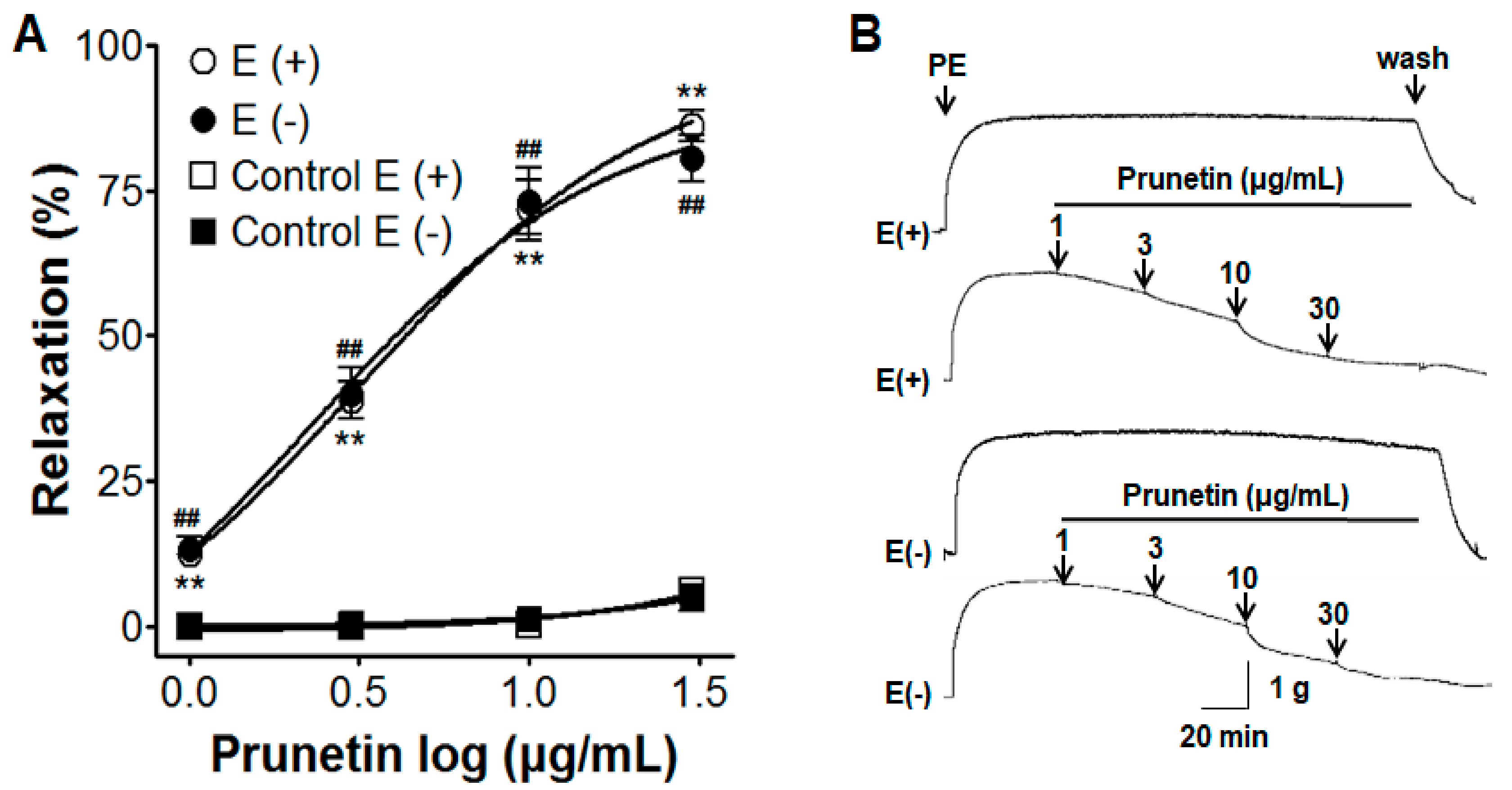
2.1. Vasorelaxant Effects of Prunetin in Presence or Absence of Vascular Endothelium
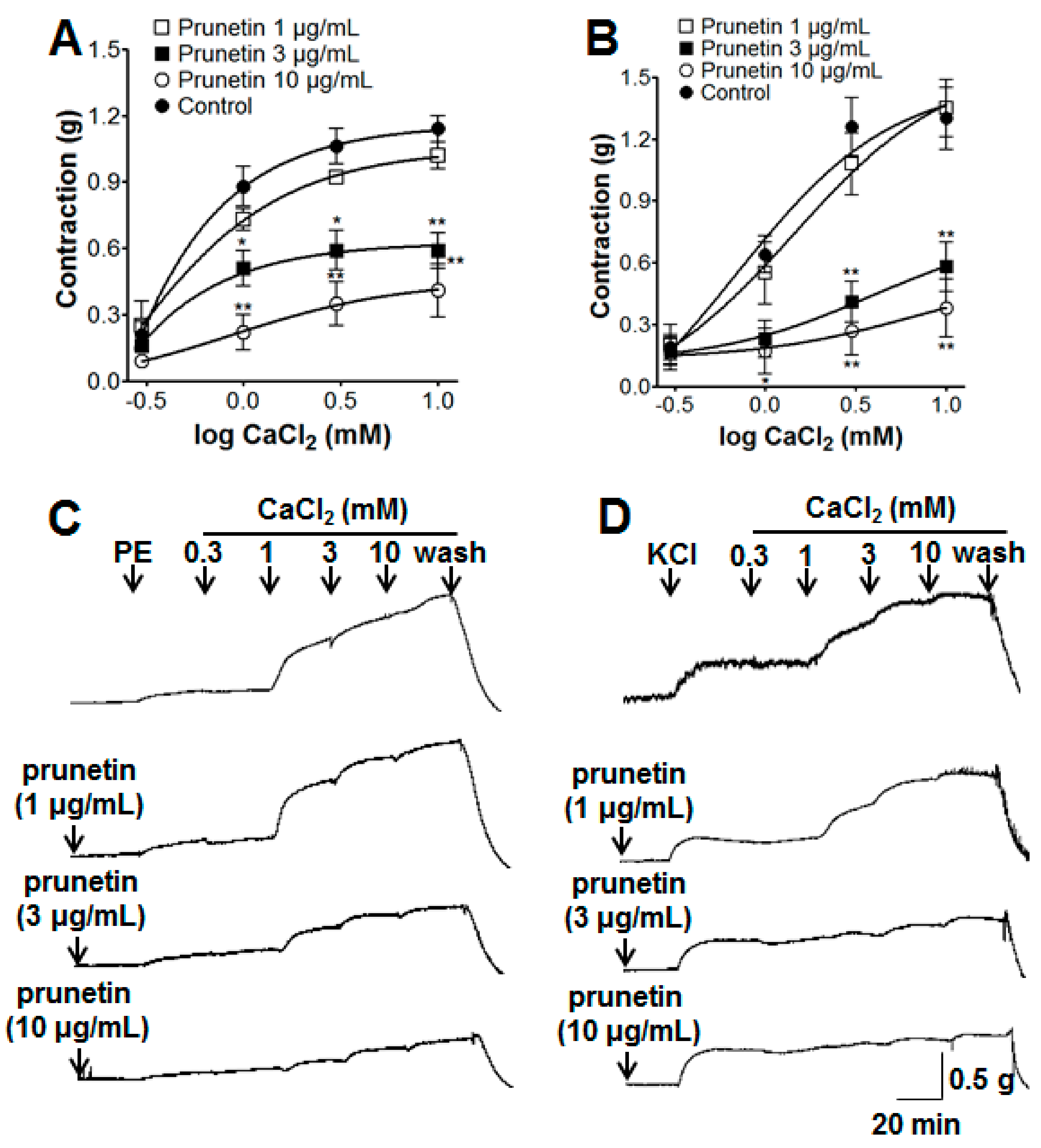
2.2. Vasorelaxant Effects of Prunetin on Extracellular Ca2+-Induced Contraction
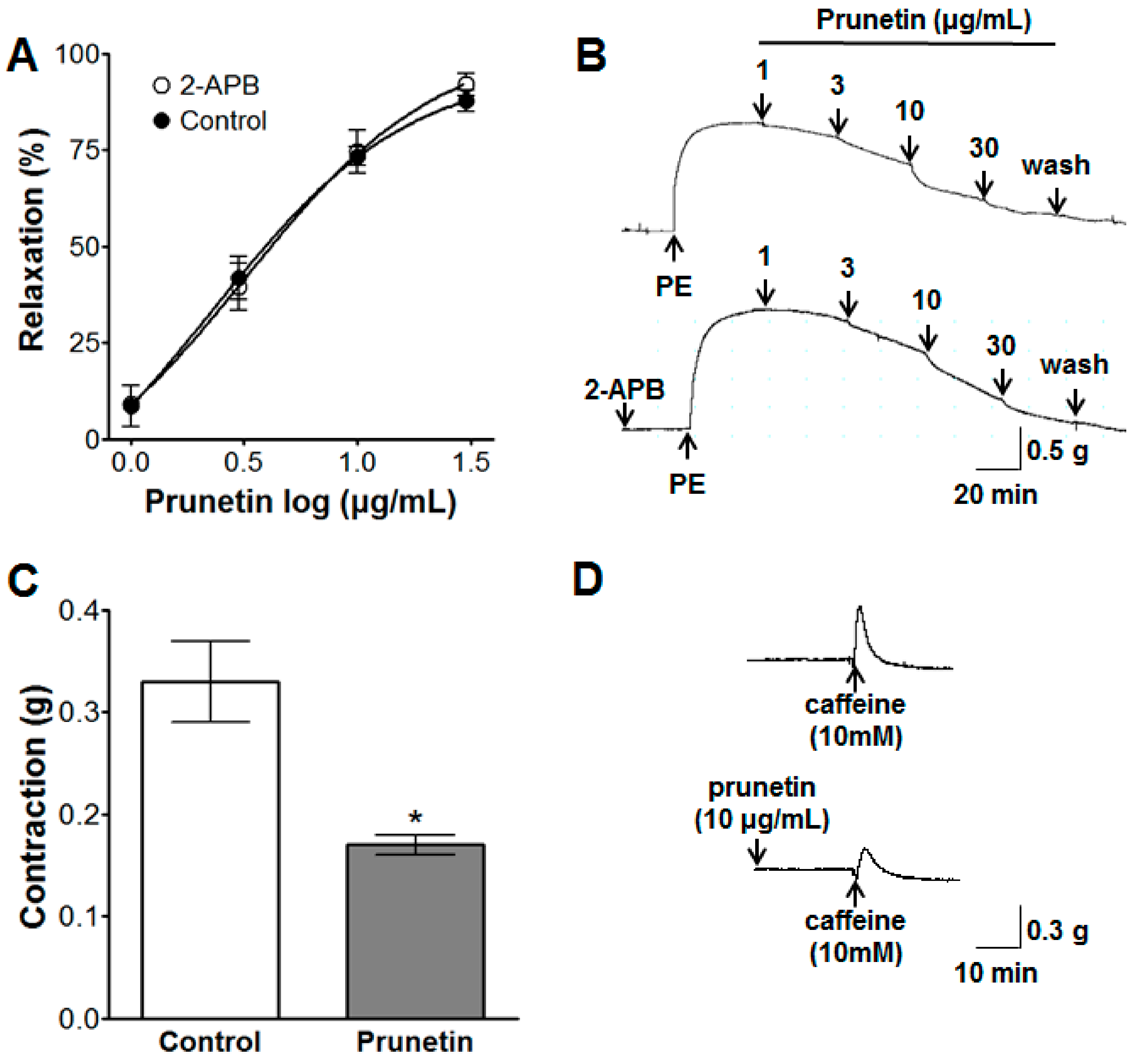
2.3. Vasorelaxant Effects of Prunetin on Intracellular Ca2+ Release
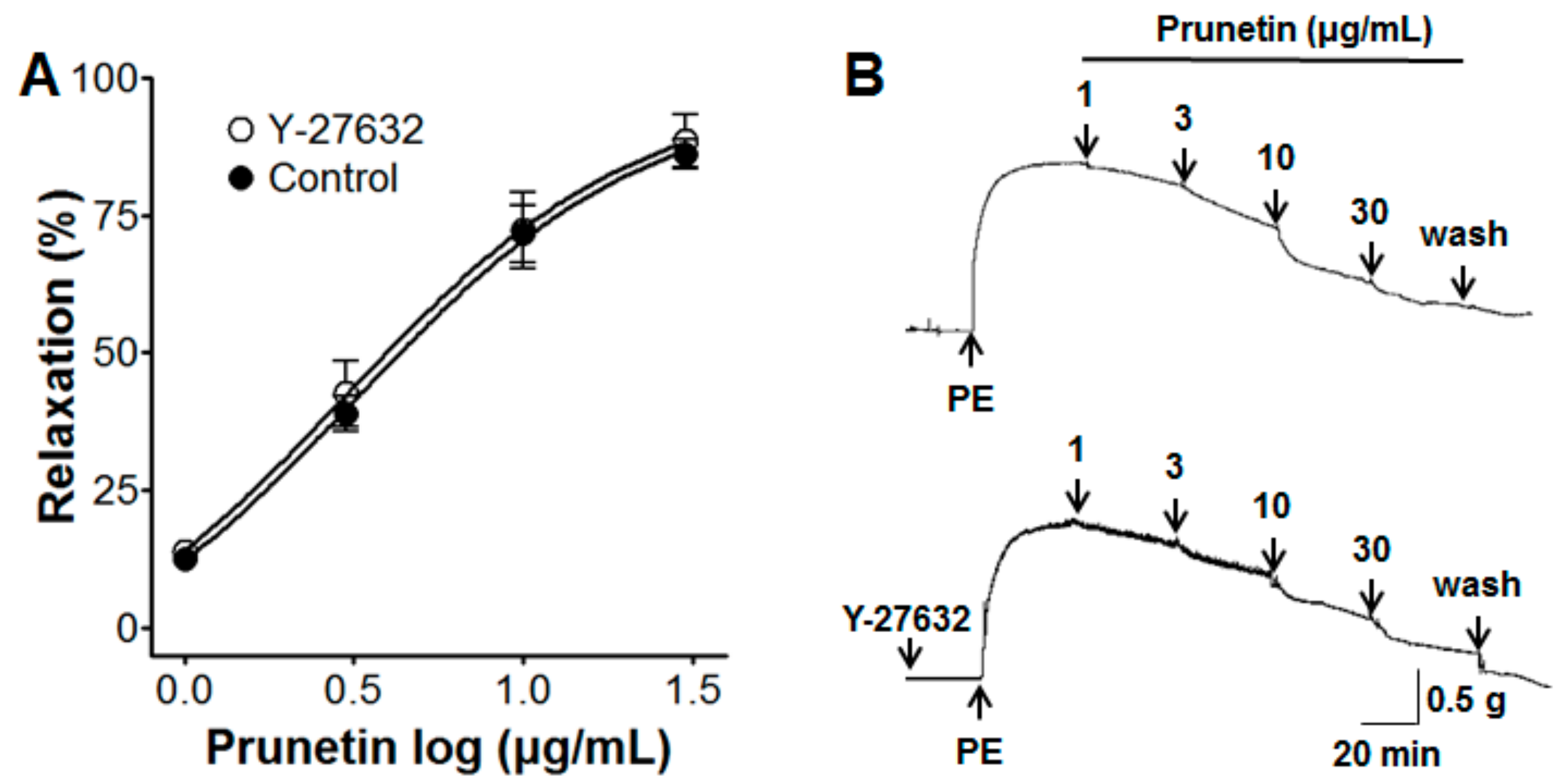
2.4. Vasorelaxant Effects of Prunetin on the Rho-Kinase Pathway
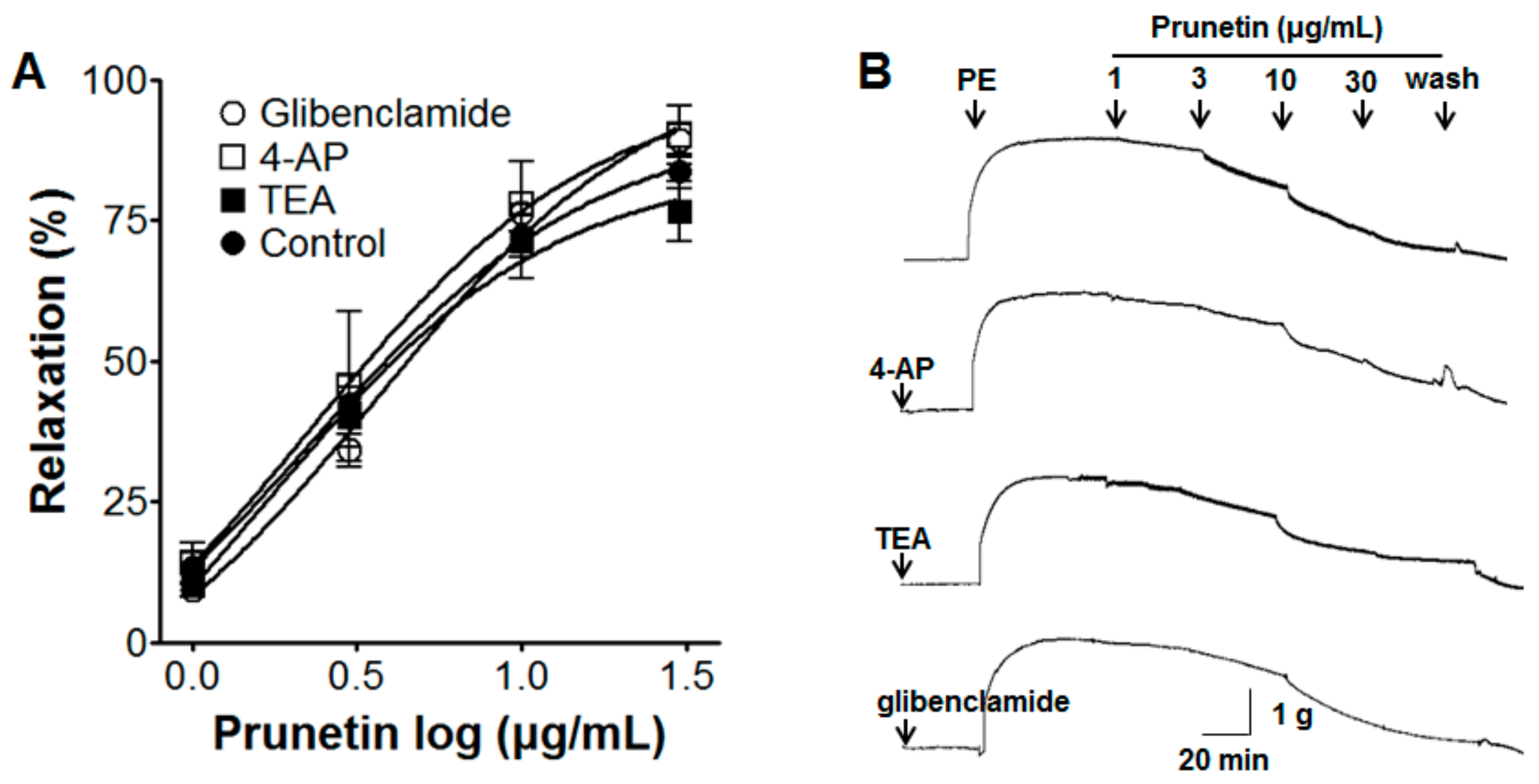
2.5. Vasorelaxant Effects of Prunetin on Aortic Rings with Vascular Endothelium Pre-Treated by Various K+ Channel Blockers
2.6. Hypotensive Effects of Prunetin on SBP in SHR
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Animals and Preparation of Rat Aortic Rings
3.3. Experimental Protocols
3.3.1. Effects of Prunetin with or without the Vascular Endothelium
3.3.2. Effects of Prunetin on Extracellular Ca2+-Induced Contraction
3.3.3. Effects of Prunetin on Intracellular Ca2+ Release
3.3.4. Effects of Prunetin on the Rho-kinase Pathway
3.3.5. Effects of Prunetin on Aortic Rings with Vascular Endothelium Pre-Treated by Various K+ Channel Blockers
3.3.6. Effects of Prunetin on Blood Pressure in SHR
3.4. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO|Blood Pressure. Available online: http://www.who.int/gho/ncd/risk_factors/blood_pressure_prevalence/en/ (accessed on 11 July 2018).
- Sanae, M.; Yasuo, A. Green asparagus (Asparagus officinalis) prevented hypertension by an inhibitory effect on angiotensin-converting enzyme activity in the kidney of spontaneously hypertensive rats. J. Agric. Food Chem. 2013, 61, 5520–5525. [Google Scholar] [CrossRef] [PubMed]
- Dianat, M.; Veisi, A.; Ahangarpour, A.; Fathi Moghaddam, H. The effect of hydro-alcoholic celery (Apium graveolens) leaf extract on cardiovascular parameters and lipid profile in animal model of hypertension induced by fructose. Avicenna J. Phytomed. 2015, 5, 203–209. [Google Scholar] [PubMed]
- Figueiredo, E.A.; Alves, N.F.; Monteiro, M.M.; Cavalcanti, C.D.; Silva, T.M.; Silva, T.M.; Braga, V.D.; Oliveira, E.D. Antioxidant and Antihypertensive Effects of a Chemically Defined Fraction of Syrah Red Wine on Spontaneously Hypertensive Rats. Nutrients 2017, 9, 574. [Google Scholar] [CrossRef] [PubMed]
- Maneesai, P.; Prasarttong, P.; Bunbupha, S.; Kukongviriyapan, U.; Kukongviriyapan, V.; Tangsucharit, P.; Prachaney, P.; Pakdeechote, P. Synergistic Antihypertensive Effect of Carthamus tinctorius L. Extract and Captopril in L-NAME-Induced Hypertensive Rats via Restoration of eNOS and AT1 R Expression. Nutrients 2016, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Ham, I.; Choi, H.-Y. Anti-inflammatory effect of prunetin via the suppression of NF-κB pathway. Food Chem. Toxicol. 2013, 58, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.-L.; Chang, H.-S.; Wang, G.-J.; Chiang, M.Y.; Huang, H.-Y.; Chen, C.-H.; Tsai, S.-C.; Lin, C.-H.; Chen, I.-S. Secondary metabolites from the roots of Neolitsea daibuensis and their anti-inflammatory activity. J. Nat. Prod. 2011, 74, 2489–2496. [Google Scholar] [CrossRef] [PubMed]
- Ahn, T.-G.; Yang, G.; Lee, H.-M.; Kim, M.-D.; Choi, H.-Y.; Park, K.-S.; Lee, S.-D.; Kook, Y.-B.; An, H.-J. Molecular mechanisms underlying the anti-obesity potential of prunetin, an O-methylated isoflavone. Biochem. Pharmacol. 2013, 85, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.C.; Kim, B.K.; Lee, H.J.; Shin, H.-D.; Lee, C.J.; Hwang, S.-C. Effects of prunetin on the proteolytic activity, secretion and gene expression of MMP-3 in vitro and production of MMP-3 in vivo. Korean J. Physiol. Pharmacol. 2016, 20, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Pal, S.; Yadav, M.; Maurya, R.; Trivedi, A.K.; Sanyal, S.; Chattopadhyay, N. Prunetin signals via G-protein-coupled receptor, GPR30(GPER1): Stimulation of adenylyl cyclase and cAMP-mediated activation of MAPK signaling induces Runx2 expression in osteoblasts to promote bone regeneration. J. Nutr. Biochem. 2015, 26, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, T.A.; Ramalho, R.M.; Luo, X.; Ramalhete, C.; Rodrigues, C.M.P.; Ferreira, M.-J. U. Isoflavones as apoptosis inducers in human hepatoma HuH-7 cells. Phytother. Res. 2011, 25, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Şöhretoğlu, D.; Sari, S.; Özel, A.; Barut, B. α-Glucosidase inhibitory effect of Potentilla astracanica and some isoflavones: Inhibition kinetics and mechanistic insights through in vitro and in silico studies. Int. J. Biol. Macromol. 2017, 105, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Piegholdt, S.; Rimbach, G.; Wagner, A.E. The phytoestrogen prunetin affects body composition and improves fitness and lifespan in male Drosophila melanogaster. FASEB J. 2016, 30, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Yokosawa, R.; Kuninaga, S.; Sekizaki, H. Aphanomyces euteiches zoospore attractant isolated from pea root: Prunetin. Jpn. J. Phytopathol. 1986, 52, 809–816. [Google Scholar] [CrossRef]
- Raturi, R.; Sati, S.C.; Badoni, P.P.; Singh, H.; Sati, M.D. Chemical Constituents of Prunus persica Stem Bark. J. Sci. Res. 2012, 4, 769–774. [Google Scholar] [CrossRef]
- Shriner, R.L.; Hull, C.J. Isoflavones. III. The Structure of Prunetin And A New Synthesis of Genistein1. J. Org. Chem. 1945, 10, 288–291. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Herrera-Herrera, A.V.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á. Multiresidue determination of estrogens in different dairy products by ultra-high-performance liquid chromatography triple quadrupole mass spectrometry. J. Chromatogr. A 2017, 1496, 58–67. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, J.G.L.; Silveira, E.R.; Pessoa, O.D.L. NMR spectral assignments of a new [C-O-C] isoflavone dimer from Andira surinamensis. Magn. Reson. Chem. 2008, 46, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Ngamrojanavanich, N.; Loontaisong, A.; Pengpreecha, S.; Cherdshewasart, W.; Pornpakakul, S.; Pudhom, K.; Roengsumran, S.; Petsom, A. Cytotoxic constituents from Butea superba Roxb. J. Ethnopharmacol. 2007, 109, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, N.S.; Sethuraman, M.G.; Manoj, C.N.; Priya Rao, R. Dalsympathetin—A new isoflavone gentiobioside from Dalbergia sympathetica (Dennst.). Nat. Prod. Res. 2006, 20, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-W.; Cheng, M.-J.; Peng, C.-F.; Chen, I.-S. Secondary metabolites and antimycobacterial activities from the roots of Ficus nervosa. Chem. Biodivers. 2010, 7, 1814–1821. [Google Scholar] [CrossRef] [PubMed]
- Vitor, R.F.; Mota-Filipe, H.; Teixeira, G.; Borges, C.; Rodrigues, A.I.; Teixeira, A.; Paulo, A. Flavonoids of an extract of Pterospartum tridentatum showing endothelial protection against oxidative injury. J. Ethnopharmacol. 2004, 93, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Nono, E.C.N.; Mkounga, P.; Marat, K.; Hultin, P.G.; Nkengfack, A.E. Antimicrobial activities of the CH2Cl2-CH3OH (1:1) extracts and compounds from the roots and fruits of Pycnanthus angolensis (Myristicaceae). Nat. Prod. Res. 2011, 25, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Ham, I.; Yang, G.; Lee, M.; Bu, Y.; Kim, H.; Choi, H.-Y. Vasorelaxant effect of Prunus yedoensis bark. BMC Complement. Altern. Med. 2013, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, K.; Chinannai, K.S.; Ham, I.; Bu, Y.; Kim, H.; Choi, H.-Y. Endothelium-Independent Vasorelaxant Effect of Ligusticum jeholense Root and Rhizoma on Rat Thoracic Aorta. Molecules 2015, 20, 10721–10733. [Google Scholar] [CrossRef] [PubMed]
- Stankevicius, E.; Kevelaitis, E.; Vainorius, E.; Simonsen, U. Role of nitric oxide and other endothelium-derived factors. Medicina (Kaunas) 2003, 39, 333–341. [Google Scholar] [PubMed]
- Mitchell, J.A.; Ali, F.; Bailey, L.; Moreno, L.; Harrington, L.S. Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Exp. Physiol. 2008, 93, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; He, Y.; Xu, L.; Yan, X.; Dai, H. Trends and patterns of five antihypertensive drug classes between 2007 and 2012 in China using hospital prescription data. Int. J. Clin. Pharmacol. Ther. 2015, 53, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-Q.; Xiong, Z.-Z.; Chen, T.-T.; Gao, X.-Y.; Yu, H.; Zhang, S.-Q.; Cao, Y.-X. Vasodilation effect of 2-benzyl-5-hydroxy-6-methoxy-3,4-dihydroisoquinolin-1-one. Arch. Pharm. Res. 2012, 35, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, H.; Mori, Y.; Hara, Y.; Uchida, K.; Zhou, H.; Mikoshiba, K. 2-Aminoethoxydiphenyl borate (2-APB) inhibits capacitative calcium entry independently of the function of inositol 1,4,5-trisphosphate receptors. Recept. Channels 2001, 7, 429–439. [Google Scholar] [PubMed]
- Bootman, M.D.; Collins, T.J.; Mackenzie, L.; Roderick, H.L.; Berridge, M.J.; Peppiatt, C.M. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002, 16, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Bilmen, J.G.; Wootton, L.L.; Godfrey, R.E.; Smart, O.S.; Michelangeli, F. Inhibition of SERCA Ca2+ pumps by 2-aminoethoxydiphenyl borate (2-APB). 2-APB reduces both Ca2+ binding and phosphoryl transfer from ATP, by interfering with the pathway leading to the Ca2+-binding sites. Eur. J. Biochem. 2002, 269, 3678–3687. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Jones, P.P.; Koop, A.; Zhang, L.; Duff, H.J.; Chen, S.R.W. Caffeine induces Ca2+ release by reducing the threshold for luminal Ca2+ activation of the ryanodine receptor. Biochem. J. 2008, 414, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Somlyo, A.P.; Somlyo, A.V. Signal transduction and regulation in smooth muscle. Nature 1994, 372, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Uehata, M.; Ishizaki, T.; Satoh, H.; Ono, T.; Kawahara, T.; Morishita, T.; Tamakawa, H.; Yamagami, K.; Inui, J.; Maekawa, M.; et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 1997, 389, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Stott, J. B.; Jepps, T. A.; Greenwood, I. A. KV7 potassium channels: A new therapeutic target in smooth muscle disorders. Drug Discov. Today 2014, 19, 413–424. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
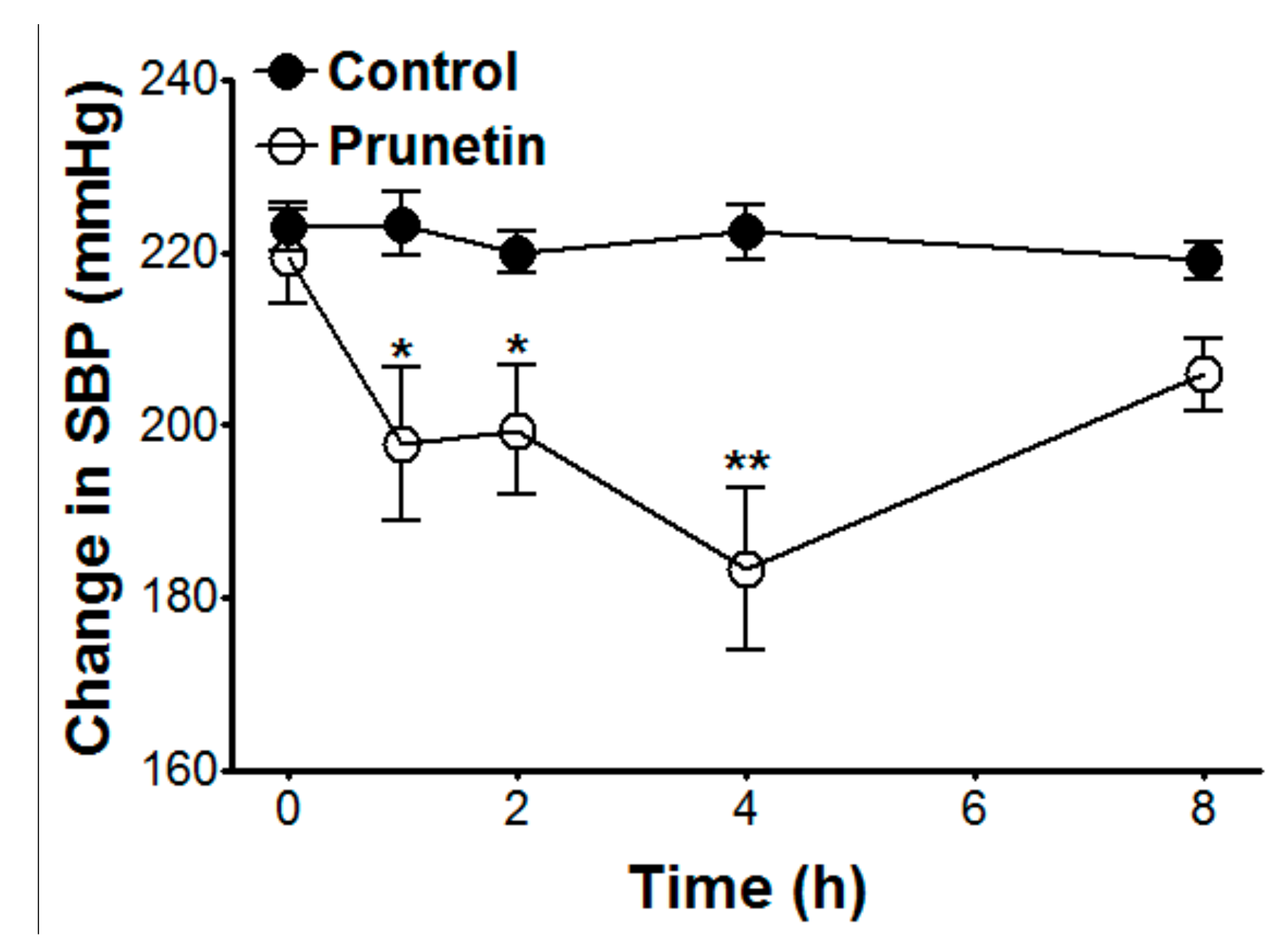
| Groups | Control | Prunetin (25 mg/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time (hour) | 0 | 1 | 2 | 4 | 8 | 0 | 1 | 2 | 4 | 8 |
| SBP (mmHg) | 223.0 ± 2.8 | 223.3 ± 3.7 | 220.0 ± 2.5 | 222.3 ± 3.3 | 219.0 ± 2.2 | 219.5 ± 5.4 | 197.8 ± 8.9 * | 199.4 ± 7.4 * | 183 ± 9.4 ** | 205.8 ± 4.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.; Jo, C.; Choi, H.-Y.; Lee, K. Prunetin Relaxed Isolated Rat Aortic Rings by Blocking Calcium Channels. Molecules 2018, 23, 2372. https://doi.org/10.3390/molecules23092372
Kim B, Jo C, Choi H-Y, Lee K. Prunetin Relaxed Isolated Rat Aortic Rings by Blocking Calcium Channels. Molecules. 2018; 23(9):2372. https://doi.org/10.3390/molecules23092372
Chicago/Turabian StyleKim, Bumjung, Cheolmin Jo, Ho-Young Choi, and Kyungjin Lee. 2018. "Prunetin Relaxed Isolated Rat Aortic Rings by Blocking Calcium Channels" Molecules 23, no. 9: 2372. https://doi.org/10.3390/molecules23092372
APA StyleKim, B., Jo, C., Choi, H.-Y., & Lee, K. (2018). Prunetin Relaxed Isolated Rat Aortic Rings by Blocking Calcium Channels. Molecules, 23(9), 2372. https://doi.org/10.3390/molecules23092372





