The Response of IL-17-Producing B Cells to ArtinM Is Independent of Its Interaction with TLR2 and CD14
Abstract
:1. Introduction
2. Results
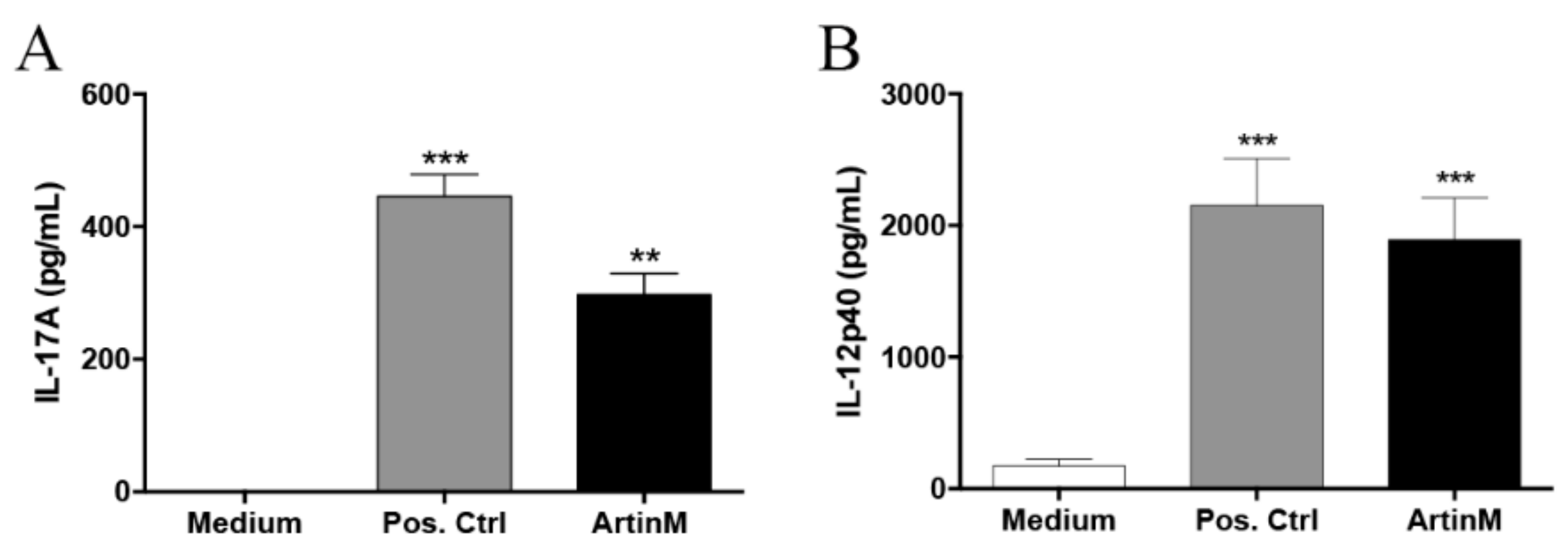
2.1. Response of IL-17-Producing B Cells in the Presence of ArtinM
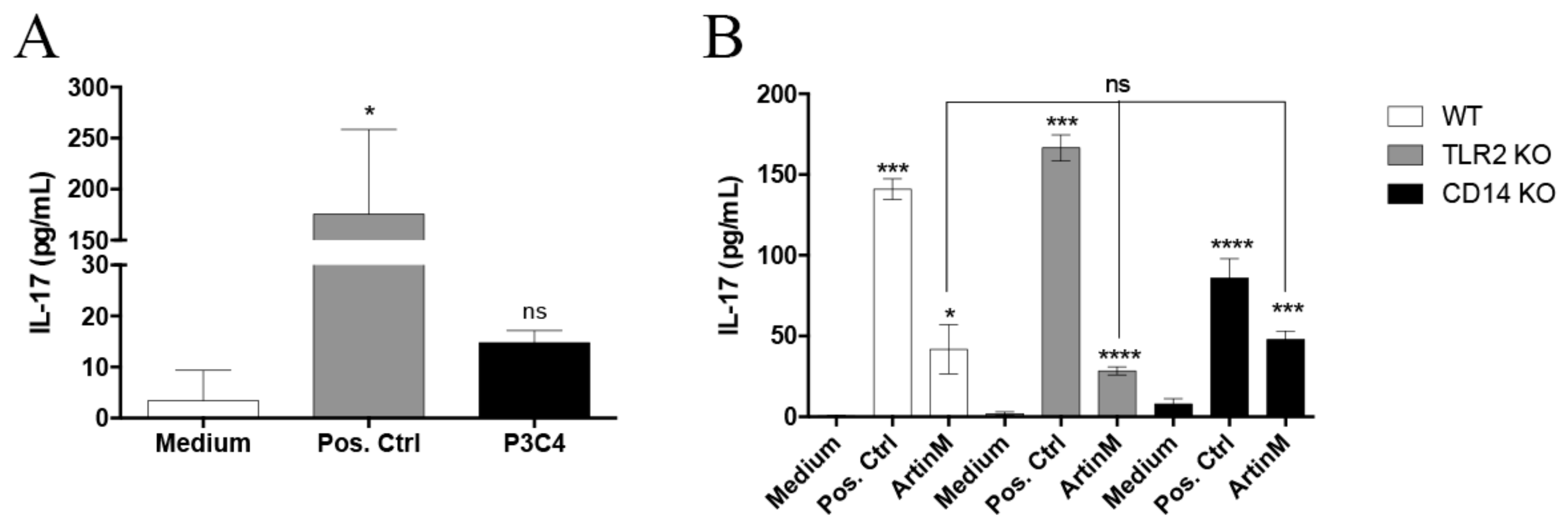
2.2. IL-17 Production Induced by ArtinM in B Cells Is Not Dependent on TLR2 and CD14 Recognition
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals
4.3. ArtinM Lectin and B-Cell Isolation
4.4. Measurement of the Cytokines
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kawaguchi, M.; Adachi, M.; Oda, N.; Kokubu, F.; Huang, S.K. Il-17 cytokine family. J. Allergy Clin. Immun. 2004, 114, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Durant, L.; Watford, W.T.; Ramos, H.L.; Laurence, A.; Vahedi, G.; Wei, L.; Takahashi, H.; Sun, H.W.; Kanno, Y.; Powrie, F.; et al. Diverse targets of the transcription factor stat3 contribute to t cell pathogenicity and homeostasis. Immunity 2010, 32, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Ghoreschi, K.; Laurence, A.; Yang, X.P.; Tato, C.M.; McGeachy, M.J.; Konkel, J.E.; Ramos, H.L.; Wei, L.; Davidson, T.S.; Bouladoux, N.; et al. Generation of pathogenic t(h)17 cells in the absence of tgf-beta signalling. Nature 2010, 467, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Ciric, B.; El-behi, M.; Cabrera, R.; Zhang, G.X.; Rostami, A. Il-23 drives pathogenic il-17-producing cd8(+) t cells. J. Immunol. 2009, 182, 5296–5305. [Google Scholar] [CrossRef] [PubMed]
- Guillot-Delost, M.; Le Gouvello, S.; Mesel-Lemoine, M.; Cherai, M.; Baillou, C.; Simon, A.; Levy, Y.; Weiss, L.; Louafi, S.; Chaput, N.; et al. Human cd90 identifies th17/tc17 t cell subsets that are depleted in hiv-infected patients. J. Immunol. 2012, 188, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Stark, M.A.; Huo, Y.Q.; Burcin, T.L.; Morris, M.A.; Olson, T.S.; Ley, K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via il-23 and il-17. Immunity 2005, 22, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Umemura, M.; Kawabe, T.; Shudo, K.; Kidoya, H.; Fukui, M.; Asano, M.; Iwakura, Y.; Matsuzaki, G.; Imamura, R.; Suda, T. Involvement of il-17 in fas ligand-induced inflammation. Int. Immunol. 2004, 16, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.L.; Keller, A.C.; Paget, C.; Fujio, M.; Trottein, F.; Savage, P.B.; Wong, C.H.; Schneider, E.; Dy, M.; Leite-De-Moraes, M.C. Identification of an il-17-producing nk1.1(neg) inkt cell population involved in airway neutrophilia. J. Exp. Med. 2007, 204, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.L.; Mendes-Da-Cruz, D.; Keller, A.C.; Lochner, M.; Schneider, E.; Dy, M.; Eberl, G.; Leite-De-Moraes, M.C. Critical role of ror-gamma t in a new thymic pathway leading to il-17-producing invariant nkt cell differentiation. Proc. Natl. Acad. Sci. USA 2008, 105, 19845–19850. [Google Scholar] [CrossRef] [PubMed]
- Pandya, A.D.; Al-Jaderi, Z.; Hoglund, R.A.; Holmoy, T.; Harbo, H.F.; Norgauer, J.; Maghazachi, A.A. Identification of human nk17/nk1 cells. PLoS ONE 2011, 6, e26780. [Google Scholar] [CrossRef] [PubMed]
- Passos, S.T.; Silver, J.S.; O’Hara, A.C.; Sehy, D.; Stumhofer, J.S.; Hunter, C.A. Il-6 promotes nk cell production of il-17 during toxoplasmosis. J. Immunol. 2010, 184, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Cupedo, T.; Crellin, N.K.; Papazian, N.; Rombouts, E.J.; Weijer, K.; Grogan, J.L.; Fibbe, W.E.; Cornelissen, J.J.; Spits, H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to rorc+ cd127(+) natural killer-like cells. Nat. Immunol. 2009, 10, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Takatori, H.; Kanno, Y.; Watford, W.T.; Tato, C.M.; Weiss, G.; Ivanov, I.I.; Littman, D.R.; O’Shea, J.J. Lymphoid tissue inducer-like cells are an innate source of il-17 and il-22. J. Exp. Med. 2009, 206, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, D.A.; Jackson, S.W.; Gorosito-Serran, M.; Acosta-Rodriguez, E.V.; Amezcua-Vesely, M.C.; Sather, B.D.; Singh, A.K.; Khim, S.; Mucci, J.; Liggitt, D.; et al. Trypanosoma cruzi trans-sialidase initiates a program independent of the transcription factors ror gamma t and ahr that leads to il-17 production by activated b cells. Nat. Immunol. 2013, 14, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Leon, B.; Lund, F.E. Il-17-producing b cells combat parasites. Nat. Immunol. 2013, 14, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Santos-de-Oliveira, R.; Dias-Baruffi, M.; Thomaz, S.M.; Beltramini, L.M.; Roque-Barreira, M.C. A neutrophil migration-inducing lectin from artocarpus integrifolia. J. Immunol. 1994, 153, 1798–1807. [Google Scholar] [PubMed]
- Carvalho, F.C.; Soares, S.G.; Tamarozzi, M.B.; Rego, E.M.; Roque-Barreira, M.C. The recognition of N-glycans by the lectin artinm mediates cell death of a human myeloid leukemia cell line. PLoS ONE 2011, 6, e27892. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cecilio, N.T.; Carvalho, F.C.; Roque-Barreira, M.C.; Feizi, T. Glycan microarray analysis of the carbohydrate-recognition specificity of native and recombinant forms of the lectin artinm. Data Brief 2015, 5, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, T.A.; Zorzetto-Fernandes, A.L.V.; Cecilio, N.T.; Sardinha-Silva, A.; Fernandes, F.F.; Roque-Barreira, M.C. Cd14 is critical for tlr2-mediated m1 macrophage activation triggered by N-glycan recognition. Sci. Rep. 2017, 7, 7083. [Google Scholar] [CrossRef] [PubMed]
- Mariano, V.S.; Zorzetto-Fernandes, A.L.; da Silva, T.A.; Ruas, L.P.; Nohara, L.L.; Almeida, I.C.; Roque-Barreira, M.C. Recognition of tlr2 N-glycans: Critical role in artinm immunomodulatory activity. PLoS ONE 2014, 9, e98512. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, T.A.; Mariano, V.S.; Sardinha-Silva, A.; de Souza, M.A.; Mineo, T.W.P.; Roque-Barreira, M.C. Il-17 induction by artinm is due to stimulation of il-23 and il-1 release and/or interaction with cd3 in cd4(+) t cells. PLoS ONE 2016, 11, e0149721. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, T.A.; Fernandes, F.F.; Roque-Barreira, M.C. Data on il-17 production induced by plant lectins. Data Brief 2016, 7, 1584–1587. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.R.; Mota, C.M.; Ribeiro, D.P.; Santiago, F.M.; Carvalho, J.V.; Araujo, E.C.; Silva, N.M.; Mineo, T.W.; Roque-Barreira, M.C.; Mineo, J.R.; et al. Artinm, a d-mannose-binding lectin from artocarpus integrifolia, plays a potent adjuvant and immunostimulatory role in immunization against neospora caninum. Vaccine 2011, 29, 9183–9193. [Google Scholar] [CrossRef] [PubMed]
- Coltri, K.C.; Oliveira, L.L.; Pinzan, C.F.; Vendruscolo, P.E.; Martinez, R.; Goldman, M.H.; Panunto-Castelo, A.; Roque-Barreira, M.C. Therapeutic administration of km+ lectin protects mice against paracoccidioides brasiliensis infection via interleukin-12 production in a toll-like receptor 2-dependent mechanism. Am. J. Pathol. 2008, 173, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Coltri, K.C.; Oliveira, L.L.; Ruas, L.P.; Vendruscolo, P.E.; Goldman, M.H.; Panunto-Castelo, A.; Roque-Barreira, M.C. Protection against paracoccidioides brasiliensis infection conferred by the prophylactic administration of native and recombinant artinm. Med. Mycol. 2010, 48, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Custodio, L.A.; Loyola, W.; Conchon-Costa, I.; da Silva Quirino, G.F.; Felipe, I. Protective effect of artin m from extract of artocarpus integrifolia seeds by th1 and th17 immune response on the course of infection by candida albicans. Int. Immunopharmacol. 2011, 11, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Panunto-Castelo, A.; Souza, M.A.; Roque-Barreira, M.C.; Silva, J.S. Km(+), a lectin from artocarpus integrifolia, induces il-12 p40 production by macrophages and switches from type 2 to type 1 cell-mediated immunity against leishmania major antigens, resulting in balb/c mice resistance to infection. Glycobiology 2001, 11, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.R.; Cavassani, K.A.; Gomes, R.B.; Teixeira, M.J.; Roque-Barreira, M.C.; Cavada, B.S.; da Silva, J.S.; Barral, A.; Barral-Netto, M. Potential of km+ lectin in immunization against leishmania amazonensis infection. Vaccine 2006, 24, 3001–3008. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Casanova, J.L.; Puel, A. Mucocutaneous il-17 immunity in mice and humans: Host defense vs. Excessive inflammation. Mucosal Immunol. 2018, 11, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, T.A.; de Souza, M.A.; Cecilio, N.T.; Roque-Barreira, M.C. Activation of spleen cells by artinm may account for its immunomodulatory properties. Cell Tissue Res. 2014, 357, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, T.A.; Oliveira-Brito, P.K.M.; Goncalves, T.E.; Vendruscolo, P.E.; Roque-Barreira, M.C. Artinm mediates murine t cell activation and induces cell death in jurkat human leukemic t cells. Int. J. Mol. Sci. 2017, 18, 1400. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Brito, P.K.M.; Goncalves, T.E.; Fernandes, F.F.; Miguel, C.B.; Rodrigues, W.F.; Lazo Chica, J.E.; Roque-Barreira, M.C.; da Silva, T.A. Systemic effects in naive mice injected with immunomodulatory lectin artinm. PLoS ONE 2017, 12, e0187151. [Google Scholar] [CrossRef] [PubMed]
- Rudd, P.M.; Wormald, M.R.; Stanfield, R.L.; Huang, M.D.; Mattsson, N.; Speir, J.A.; DiGennaro, J.A.; Fetrow, J.S.; Dwek, R.A.; Wilson, I.A. Roles for glycosylation of cell surface receptors involved in cellular immune recognition. J. Mol. Biol. 1999, 293, 351–366. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira-Brito, P.K.M.; Roque-Barreira, M.C.; Da Silva, T.A. The Response of IL-17-Producing B Cells to ArtinM Is Independent of Its Interaction with TLR2 and CD14. Molecules 2018, 23, 2339. https://doi.org/10.3390/molecules23092339
Oliveira-Brito PKM, Roque-Barreira MC, Da Silva TA. The Response of IL-17-Producing B Cells to ArtinM Is Independent of Its Interaction with TLR2 and CD14. Molecules. 2018; 23(9):2339. https://doi.org/10.3390/molecules23092339
Chicago/Turabian StyleOliveira-Brito, Patrícia Kellen Martins, Maria Cristina Roque-Barreira, and Thiago Aparecido Da Silva. 2018. "The Response of IL-17-Producing B Cells to ArtinM Is Independent of Its Interaction with TLR2 and CD14" Molecules 23, no. 9: 2339. https://doi.org/10.3390/molecules23092339
APA StyleOliveira-Brito, P. K. M., Roque-Barreira, M. C., & Da Silva, T. A. (2018). The Response of IL-17-Producing B Cells to ArtinM Is Independent of Its Interaction with TLR2 and CD14. Molecules, 23(9), 2339. https://doi.org/10.3390/molecules23092339




