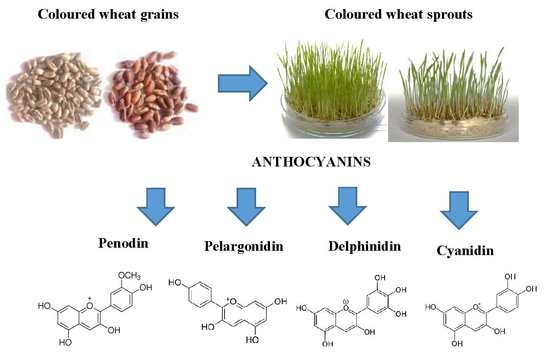
Bioactive Phytochemicals and Antioxidant Properties of the Grains and Sprouts of Colored Wheat Genotypes
Abstract
:1. Introduction
2. Results
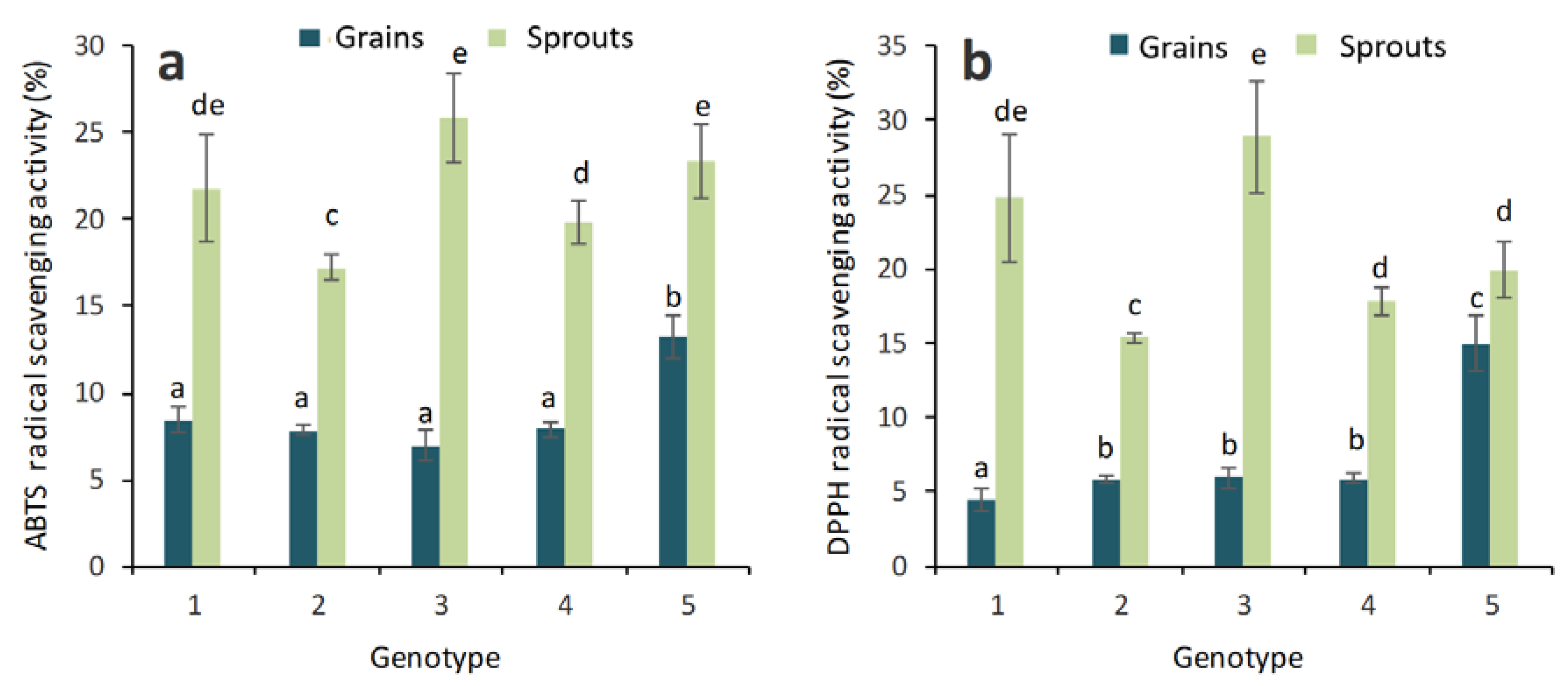
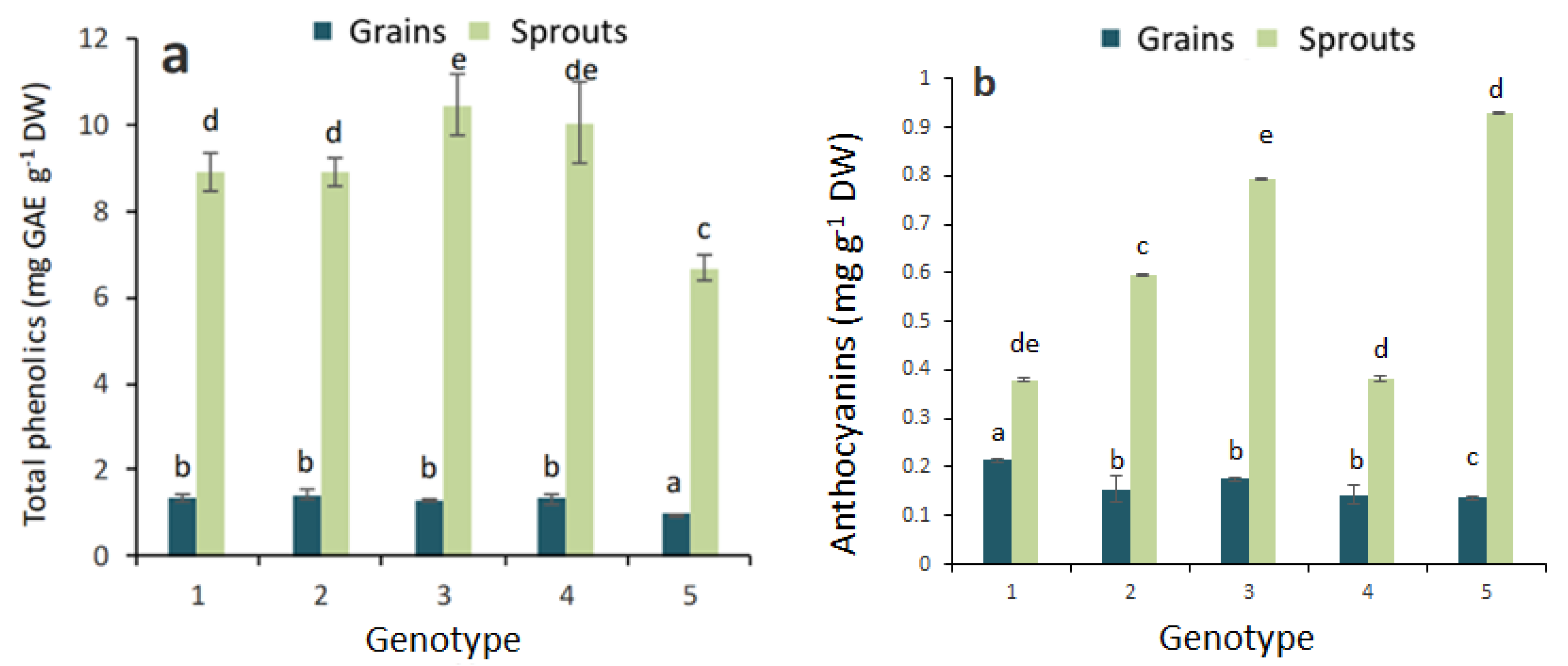
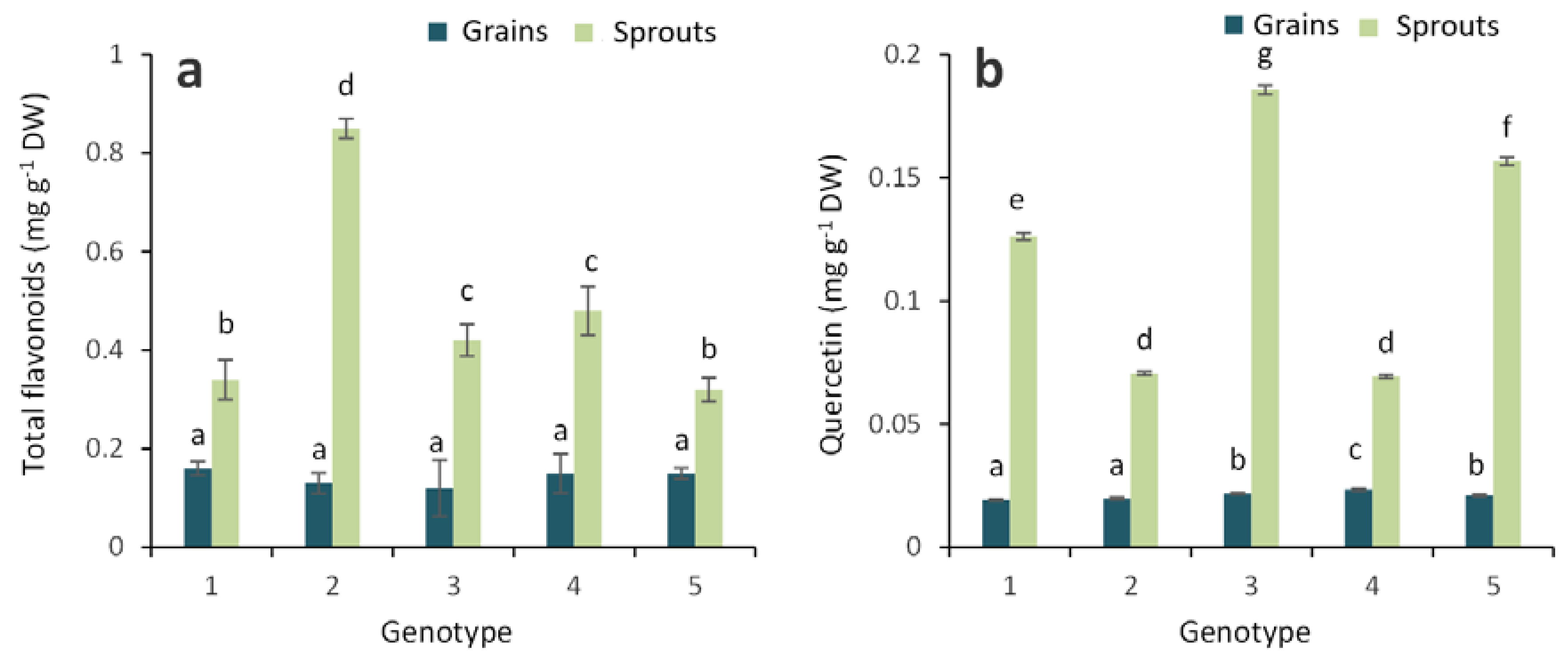
2.1. Antioxidant Activity and Total Phenolic, Flavonoid and Anthocyanin Contents in Grains and Sprouts
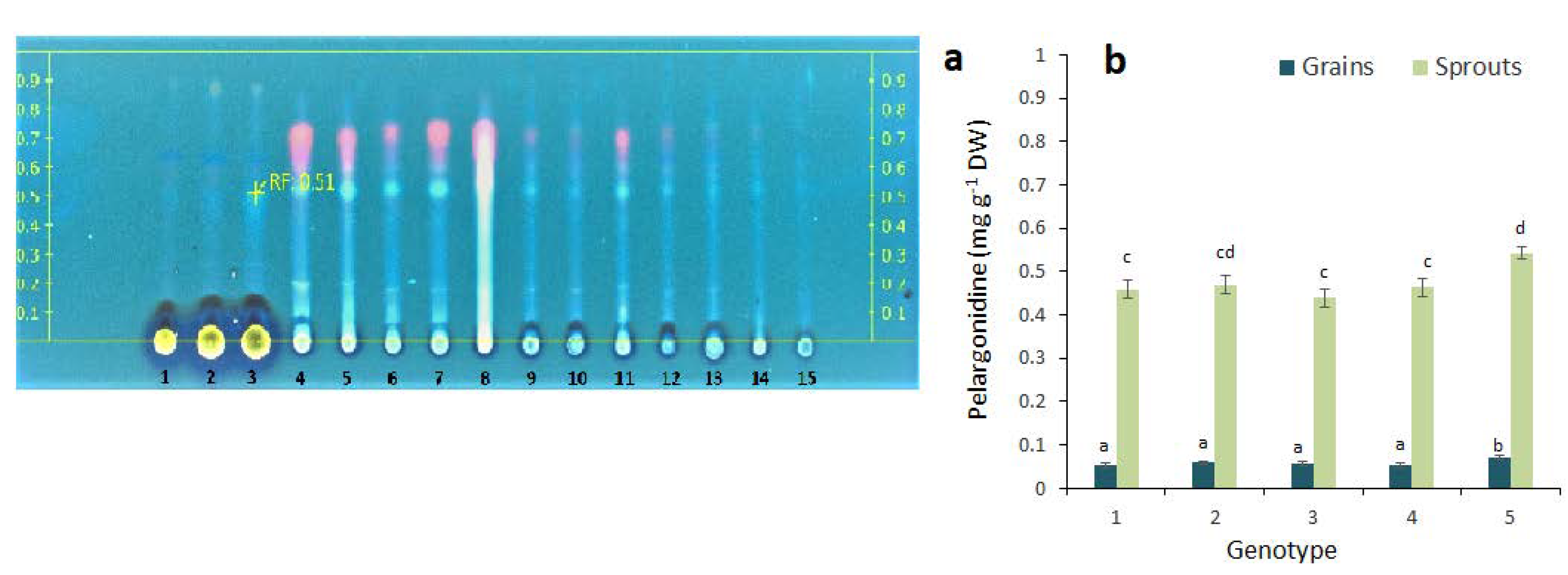
2.2. Anthocyanin Composition in the Grains and Sprouts of Colored Wheat Genotypes
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Plant Objects
4.3. Determination of DPPH Radical Scavenging Capacity
4.4. ABTS Radical Scavenging Assay
4.5. Determination of Total Phenolics
4.6. Detection of Total Flavonoid Content
4.7. Estimation of Anthocyanins
4.8. Stock Solutions and Sample Preparation for HPTLC
4.9. TLC Conditions
4.10. High-Performance Thin-Layer Chromatography
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lao, F.; Sigurdson, G.T.; Giusti, M.M. Health benefits of purple corn (Zea mays L.) phenolic compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 234–246. [Google Scholar] [CrossRef]
- Havrlentová, M.; Pšenáková, I.; Žofajová, A.; Rückschloss, l.; Kraic, J. Anthocyanins in wheat seed—A mini review. Nova Biotechnol. Chim. 2014, 13, 1–12. [Google Scholar] [CrossRef]
- Zykin, P.A.; Andreeva, E.A.; Lykholay, A.N.; Tsvetkova, N.V.; Voylokov, A.V. Anthocyanin composition and content in rye plants with different grain color. Molecules 2018, 23, 948. [Google Scholar] [CrossRef] [PubMed]
- Lao, F.; Giusti, M.M. Quantification of purple corn (Zea mays L.) anthocyanins using spectrophotometric and HPLC approaches: Method comparison and correlation. Food Anal. Methods 2016, 9, 1367. [Google Scholar] [CrossRef]
- Yu, L.; Beta, T. Identification and antioxidant properties of phenolic compounds during production of bread from purple wheat grains. Molecules 2015, 20, 15525–15549. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Giusti, M.M. Contribution of berry anthocyanins to their chemopreventive properties. In Berries and Cancer Prevention; Seeram, N., Stoner, G., Eds.; Springer: New York, NY, USA, 2011; pp. 3–40. [Google Scholar]
- Putta, S.; Yarla, N.S.; Kumar, E.K.; Lakkappa, D.B.; Kamal, M.A.; Scotti, L.; Scotti, M.T.; Ashraf, G.M.; Barreto, G.E.; Rao, B.S.B.; et al. Preventive and therapeutic potentials of anthocyanins in diabetes and associated complications. Curr. Med. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Martinek, P.; Jirsa, O.; Vaculová, K.; Chrpová, J.; Watanabe, N.; Burešová, V.; Kopecký, D.; Štiasna, K.; Vyhnánek, T.; Trojan, V. Use of wheat gene resources with different grain colour in breeding. In Proceedings of the Tagungsband der 64. Jahrestagung der Vereinigung der Pflanzenzüchter und Saatgutkaufleute Österreichs, Raumberg-Gumpenstein, Austria, 25–26 November 2013; pp. 75–78. [Google Scholar]
- Giordano, D.; Locatelli, M.; Travaglia, F.; Bordiga, M.; Reyneri, A.; Coïsson, J.D.; Blandino, M. Bioactive compound and antioxidant activity distribution in roller-milled and pearled fractions of conventional and pigmented wheat varieties. Food Chem. 2017, 233, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Mamoucha, S.; Tsafantakis, N.; Fokialakis, N.; Christodoulakis, N.S. A two-season impact study on Globularia alypum: Adaptive leaf structures and secondary metabolite variations. Plant Biosyst. 2018, 152, 1118–1127. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Hucl, P. Composition and stability of anthocyanins in blue-grained wheat. J. Agric. Food Chem. 2003, 51, 2174–2180. [Google Scholar] [CrossRef] [PubMed]
- Knievel, D.C.; Abdel-Aal, E.S.M.; Rabalski, I.; Nakamura, T.; Hucl, P. Grain color development and the inheritance of high anthocyanin blue aleurone and purple pericarp in spring wheat (Triticum aestivum L.). J. Cereal Sci. 2009, 50, 113–120. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Hucl, P.; Rabalski, I. Compositional and antioxidant properties of anthocyanin-rich products prepared from purple wheat. Food Chem. 2018, 254, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Pasqualone, A.; Bianco, A.M.; Paradiso, V.M.; Summo, C.; Gambacorta, G.; Caponio, F.; Blanco, A. Production and characterization of functional biscuits obtained from purple wheat. Food Chem. 2015, 180, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.; Stojanovska, L.; Vasiljevic, T.; Mathai, M. Germinated grains: A superior whole grain functional food? Can. J. Physiol. Pharmacol. 2015, 91, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Perni, S.; Calzuola, I.; Caprara, G.A.; Gianfranceschi, G.L.; Marsili, V. Natural antioxidants in wheat sprout extracts. Curr. Org. Chem. 2014, 18, 2950–2960. [Google Scholar] [CrossRef]
- Farasat, M.; Khavari-Nejad, R.-A.; Nabavi, S.M.B.; Namjooyan, F. antioxidant activity, total phenolics and flavonoid contents of some edible green seaweeds from northern coasts of the persian gulf. IJPR 2014, 13, 163–170. [Google Scholar] [PubMed]
- Ravikumar, P.; Shalini, G.; Jeyam, M. Wheat seedlings as food supplement to combat free radicals: An in vitro approach. Indian J. Pharm. Sci. 2015, 77, 592–598. [Google Scholar] [PubMed]
- Abdel-Aal, E.S.M.; Hucl, P. A rapid method for quantifying total anthocyanins in blue aleurone and purple pericarp wheats. Cereal Chem. 1999, 76, 350–354. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Impact of germination on phenolic content and antioxidant activity of 13 edible seed species. Food Chem. 2010, 119, 1485–1490. [Google Scholar] [CrossRef]
- Benincasa, P.; Galieni, A.; Manetta, A.C.; Pace, R.; Guiducci, M.; Pisante, M.; Stagnari, F. Phenolic compounds in grains, sprouts and wheatgrass of hulled and non-hulled wheat species. J. Sci. Food Agric. 2015, 95, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; Tinarelli, C.; Schulz, R.J.; Polidori, M.C. Nutraceuticals in cognitive impairment and Alzheimer’s disease. Front. Pharmacol. 2014, 5, 147. [Google Scholar] [CrossRef] [PubMed]
- Marsili, V.; Calzuola, I.; Gianfranceschi, G.L. Nutritional relevance of wheat sprouts containing high levels of organic phosphates and antioxidant compounds. J. Clin. Gastroenterol. 2004, 38, 123–126. [Google Scholar] [CrossRef]
- Hanlon, P.R.; Barnes, D.M. Phytochemical composition and biological activity of 8 varieties of radish (Raphanus sativus L.) sprouts and mature taproots. Food Sci. 2011, 76, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Howard, H.W.; Kukimura, H.; Whitmore, E.T. The anthocyanin pigments of the tubers and sprouts of Tuberosum potatoes. Potato Res. 1970, 13, 142–145. [Google Scholar] [CrossRef]
- Amini, A.M.; Muzs, K.; Spencer, J.P.; Yaqoob, P. Pelargonidin-3-O-glucoside and its metabolites have modest anti-inflammatory effects in human whole blood cultures. Nutr. Res. 2017, 46, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB Activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 45673. [Google Scholar]
- Cherian, S.; Kumar, R.V.; Augusti, K.T.; Kidwai, J.R. Antidiabetic effect of a glycoside of pelargonidin isolated from the bark of Ficus bengalensis Linn. Indian J. Biochem. Biophys. 1992, 29, 380–382. [Google Scholar] [PubMed]
- Kim, S.J.; Maeda, T.; Sarker, M.Z.; Takigawa, S.; Matsuura-Endo, C.; Yamauchi, H.; Mukasa, Y.; Saito, K.; Hashimoto, N.; Noda, T.; et al. Identification of anthocyanins in the sprouts of buckwheat. J. Agric. Food Chem. 2007, 55, 6314–6318. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Oroudjev, E.; Wilson, L.; Ayoub, G. Delphinidin and cyanidin exhibit antiproliferative and apoptotic effects in MCF7 human breast cancer cells. Integr. Cancer Sci. Ther. 2015, 2, 82–86. [Google Scholar]
- Cretu, G.C.; Morlock, G.E. Analysis of anthocyanins in powdered berry extracts by planar chromatography linked with bioassay and mass spectrometry. Food Chem. 2014, 146, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Thwe, A.A.; Park, N.I.; Suzuki, T.; Kim, S.J.; Park, S.U. Accumulation of phenylpropanoids and correlated gene expression during the development of tartary buckwheat sprouts. J. Agric. Food Chem. 2012, 60, 5629–5635. [Google Scholar] [CrossRef] [PubMed]
- Sigurdson, G.T.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Effects of hydroxycinnamic acids on blue color expression of cyanidin derivatives and their metal chelates. Food Chem. 2017, 234, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Calzuola, I.; Marsili, V.; Gianfranceschi, G.L. Synthesis of antioxidants in wheat sprouts. J. Agric. Food Chem. 2004, 52, 5201–5206. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Mbarki, S.; Sytar, O.; Zivcak, M.; Abdelly, C.; Cerda, A.; Brestic, M. Anthocyanins of Coloured Wheat Genotypes in Specific Response to Salt Stress. Molecules 2018, 23, 1518. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E.; Bloom, A.J. Mineral Nutrition of Plants: Principles and Perspectives; Sinauer Associates: Sunderland, MA, USA, 1972; 412p. [Google Scholar]
- Molyneux, P. The use of the stable free radical diphenylpicryl-hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimentry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins with UV-visible spectroscopy. In Current Protocols in Food Analytical Chemistry Banner; Wiley: New York, NY, USA, 2001; pp. Fl.2.1–F1.2.13. [Google Scholar]
- Mirecki, R.M.; Teramura, A.H. Effects of ultraviolet-B irradiance on soybean. V. The dependence of plant sensitivity on the photosynthetic photon flux density during and after leaf expansion. Plant Physiol. 1984, 74, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Krüger, S.; Urmann, O.; Morlock, G. Development of a planar chromatographic method for quantitation of anthocyanins in pomace, feed, juice and wine. J. Chromatogr. A 2013, 1289, 105–118. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds pelargonidin, peonidin, delphinidin, cyanidin are not available from the authors. |


| Sample | Antiox Activ. | Total Phenolics | Total Anthocyan | Total Flavonoids | Quercetin | Pelargonidin | Cyanidin | Delphinidin |
|---|---|---|---|---|---|---|---|---|
| Grains | ABTS | 0.51 * | 0.29 | 0.43 * | 0.08 | 0.68 * | 0.96 * | 0.04 |
| DPPH | 0.62 * | 0.38 | 0.22 | 0.20 | 0.59 * | 0.96 * | 0.09 | |
| Sprouts | ABTS | 0.34 | 0.48 | 0.16 | 0.69 * | 0.55 * | 0.63 * | 0.22 |
| DPPH | 0.48 | 0.21 | 0.14 | 0.67 * | 0.26 | 0.60 * | 0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sytar, O.; Bośko, P.; Živčák, M.; Brestic, M.; Smetanska, I. Bioactive Phytochemicals and Antioxidant Properties of the Grains and Sprouts of Colored Wheat Genotypes. Molecules 2018, 23, 2282. https://doi.org/10.3390/molecules23092282
Sytar O, Bośko P, Živčák M, Brestic M, Smetanska I. Bioactive Phytochemicals and Antioxidant Properties of the Grains and Sprouts of Colored Wheat Genotypes. Molecules. 2018; 23(9):2282. https://doi.org/10.3390/molecules23092282
Chicago/Turabian StyleSytar, Oksana, Paulina Bośko, Marek Živčák, Marian Brestic, and Iryna Smetanska. 2018. "Bioactive Phytochemicals and Antioxidant Properties of the Grains and Sprouts of Colored Wheat Genotypes" Molecules 23, no. 9: 2282. https://doi.org/10.3390/molecules23092282
APA StyleSytar, O., Bośko, P., Živčák, M., Brestic, M., & Smetanska, I. (2018). Bioactive Phytochemicals and Antioxidant Properties of the Grains and Sprouts of Colored Wheat Genotypes. Molecules, 23(9), 2282. https://doi.org/10.3390/molecules23092282