Application of Centrifugal Partition Chromatography for Bioactivity-Guided Purification of Antioxidant-Response-Element-Inducing Constituents from Atractylodis Rhizoma Alba
Abstract
1. Introduction
2. Results and Discussion
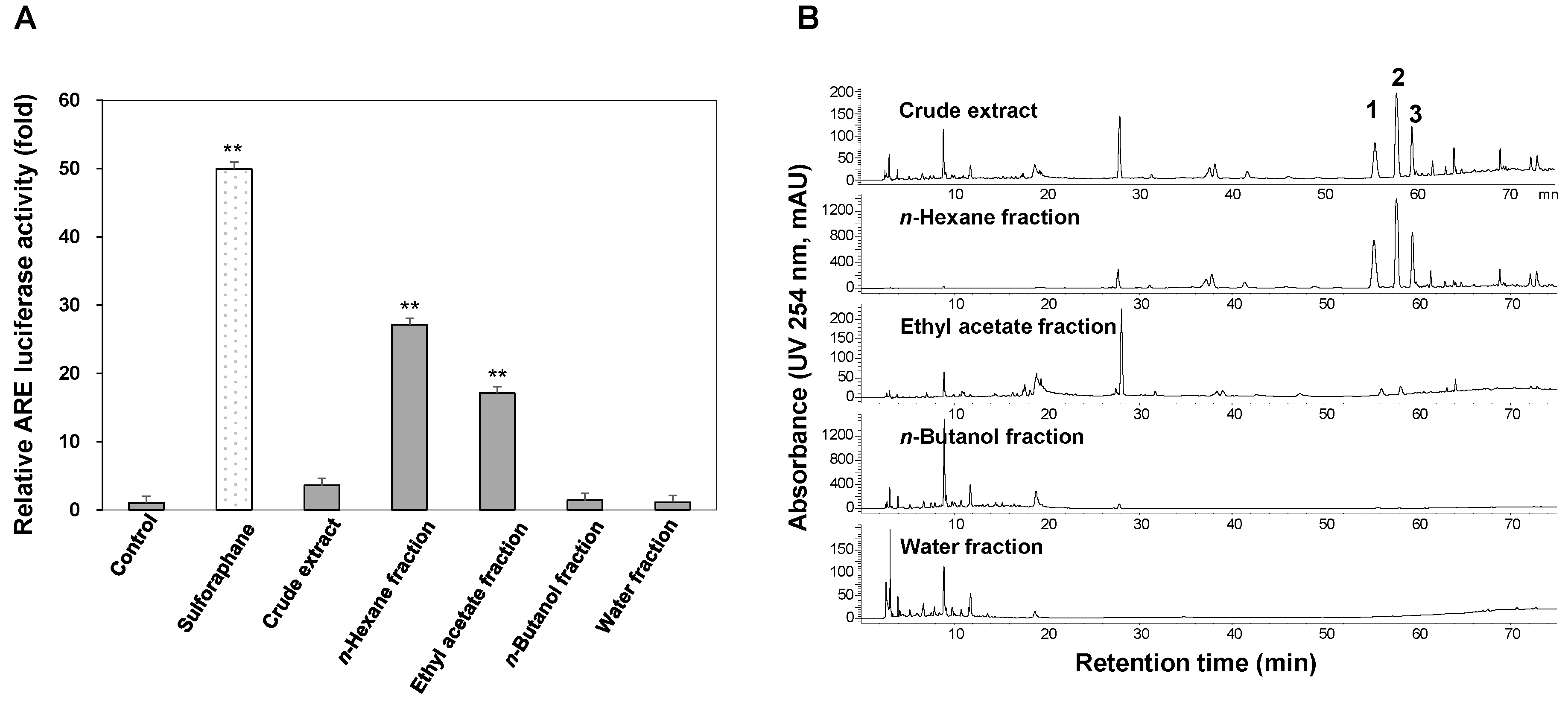
2.1. ARE Luciferase Assay and HPLC Analysis of the Crude Extract and Sub-Fractions
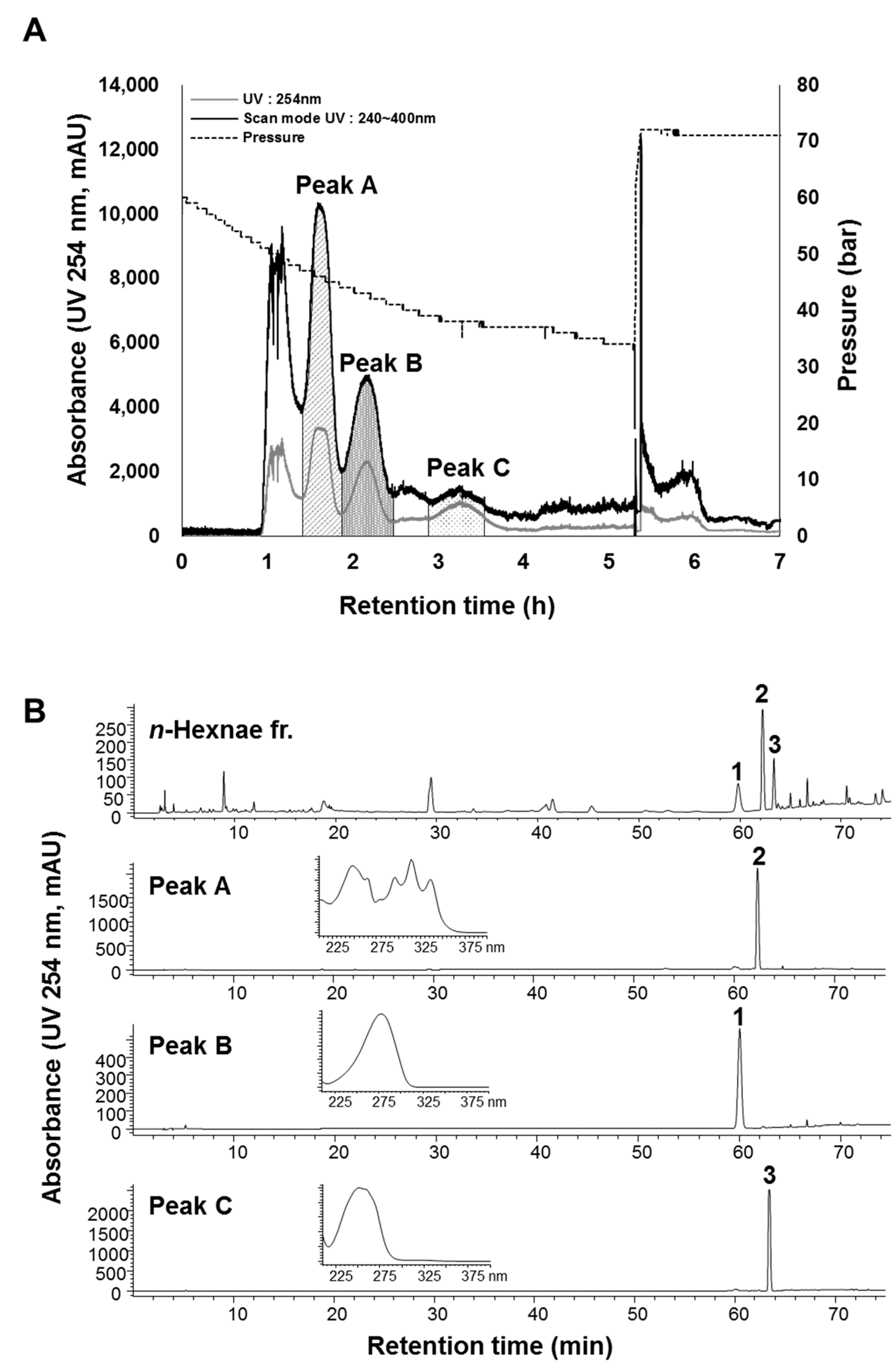
2.2. Optimization of the Two-Phase Solvent System and CPC Operation
2.3. ARE-Activating Effect of Purified Compounds 1–3
3. Materials and Methods
3.1. Apparatus and Materials
3.2. Plant Material and Preparation of Crude Extracts
3.3. Selection of the Two-Phase Solvent System
3.4. CPC Separation Procedure
3.5. HPLC Analysis and Identification of the CPC Peak Fractions
3.6. Structural Identification
3.7. Structural Identification of the Purified Compounds
3.8. Cell Culture and Cell Viability
3.9. ARE Luciferase Reporter Assay
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Conflicts of Interest
References
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Haixia, W.; Lijuan, M.; Yanduo, T. Isolation, purification and identification of antioxidants from Lepidium latifolium extracts. Med. Chem. Res. 2018, 27, 37–45. [Google Scholar] [CrossRef]
- Surh, Y.J.; Kundu, J.K.; Li, M.H.; Na, H.K.; Cha, Y.N. Role of Nrf2-mediated heme oxygenase-1 upregulation in adaptive survival response to nitrosative stress. Arch. Pharm. Res. 2009, 32, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Han, H.Y.; Yang, Y.S.; Kim, S.N.; Han, S.C.; Lee, J.H.; Jeong, J.Y.; Roh, H.; Seok, J.H.; Lee, J.S.; Kim, J.A.; et al. Two-week repeated dose toxicity of Atractylodis Rhizoma Alba in F344 rats. Nat. Prod. Sci. 2016, 22, 180–186. [Google Scholar] [CrossRef]
- Yun, B.R.; Weon, J.B.; Lee, B.H.; Lee, J.W.; Eom, M.R.; Ma, C.J. Quantitative analysis of atractylenolide I and III in Atractrylodes macrocephala. Kor. J. Pharmacogn. 2013, 44, 53–59. [Google Scholar]
- Chang, Y.H.; Kim, C.T.; Jung, M.H.; Lim, Y.H.; Lee, S.H.; Kang, S.J. Inhibition of melanogenesis by selina-4,7-dien-8-one isolated from Atracylodis Rhizome Alba. Biol. Pharm. Bull. 2007, 30, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Jan, Y.S.; Tsai, P.W.; Norimoto, H.; Michihara, S.; Murayama, C.; Wang, C.C. Anti-inflammatory and antinociceptive constituents of Atractylodes japonica Koidzumi. J. Agric. Food Chem. 2016, 64, 2254–2262. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, J.; Han, W.; Mai, W.; Wang, L.; Li, Q.; Lin, M.; Bai, M.; Zhang, L.; Chen, D. Antioxidant ability and mechanism of rhizoma Atractylodes macrocephala. Molecules 2012, 17, 13457–13472. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; He, L.; Huang, M.; Dong, Y. Anti-inflammatory components isolated from Atractylodes macrocephala Koidzmi. Nat. Prod. Res. 2008, 22, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y.; Komatsu, K.I.; Saito, K.I.; Bando, H.; Sakurai, T. A new polyacetylene compound from Atractylodes rhizome. Chem. Pharm. Bull. 1989, 37, 193–194. [Google Scholar] [CrossRef]
- Yao, C.M.; Yang, X.W. Bioactivity-guided isolation of polyacetylenes with inhibitory activity against NO production in LPS-activated RAW264.7 macrophages from the rhizomes of Atractylodes macrocephala. J. Ethnopharmacol. 2014, 151, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.J.; Liang, J.W.; Lin, H.R. Sesquiterpenoids from Atractylodes macrocephala act as farnesoid X receptor and progesterone receptor modulators. Bioorg. Med. Chem. Lett. 2012, 22, 2326–2329. [Google Scholar] [CrossRef] [PubMed]
- Walasek, M.; Grzegorczyk, A.; Malm, A.; Skalicka-Woźniak, K. Bioactivity-guided isolation of antimicrobial coumarins from Heracleum mantegazzianum Sommier & Levier (Apiaceae) fruits by high-performance counter-current chromatography. Food Chem. 2015, 186, 133–138. [Google Scholar] [PubMed]
- Foucault, A.P.; Chevolot, L. Counter-current chromatography: Instrumentation, solvent selection and some recent applications to natural product purification. J. Chromatogr. A 1988, 808, 3–22. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, X.; Liu, P.; Li, M.; Dong, H.; Qiao, X. Semi-preparative separation of 10 caffeoylquinic acid derivatives using high speed counter-current chromatography combined with semi-preparative HPLC from the roots of burdock (Arctium lappa L.). Molecules 2018, 23, 429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Luo, J.G.; Han, C.; Xu, J.F.; Kong, L.Y. Bioassay-guided preparative separation of angiotensin-converting enzyme inhibitory C-flavone glycosides from Desmodium styracifolium by recycling complexation high-speed counter-current chromatography. J. Pharm. Biomed. Anal. 2015, 102, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Peng, Y.; Wang, X.; Li, Z.; Sun, Y. Preparative separation and purification of four glycosides from Gentianae radix by high-speed counter-current chromatography and comparison of their anti-NO production effects. Molecules 2017, 22, 2002. [Google Scholar] [CrossRef] [PubMed]
- Budryn, G.; Nebesny, E.; Pałecz, B.; Rachwał-Rosiak, D.; Hodurek, P.; Miśkiewicz, K.; Oracz, J.; Żyżelewicz, D. Inclusion complexes of β-cyclodextrin with chlorogenic acids (CHAs) from crude and purified aqueous extracts of green Robusta coffee beans (Coffea canephora L.). Food Res. Int. 2014, 61, 202–213. [Google Scholar] [CrossRef]
- Zhao, C.; Chaohong, H.E. Preparative isolation and purification of atractylon and atractylenolide III from the Chinese medicinal plant Atractylodes macrocephala by high-speed counter-current chromatography. J. Sep. Sci. 2006, 29, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Conway, W.D. Counter-current chromatography: Simple process and confusing terminology. J. Chromatogr. A 2011, 1218, 6015–6023. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lim, H.; Kim, H.P.; Kwon, Y.S. Downregulation of matrix metalloproteinase-13 by the root extract of Cyathula officinalis Kuan and its constituents in IL-1β-treated chondrocytes. Planta Med. 2011, 77, 1528–1530. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.I.; Kim, S.Y.; Kim, S.J.; Hwang, B.S.; Kwon, T.H.; Yu, K.Y.; Han, S.H.; Suzuki, K.; Kim, K.J. Antibacterial activity of phytochemicals isolated from Atractylodes japonica against methicillin-resistant Staphylococcus aureus. Molecules 2010, 15, 7395–7402. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.S.; Park, C.L.; Syed, A.S.; Kim, Y.M.; Cho, I.J.; Kim, C.Y. Preparative separation of sesamin and sesamolin from defatted sesame meal via centrifugal partition chromatography with consecutive sample injection. J. Chromatogr. B 2016, 1011, 108–113. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–3 are from the authors. |
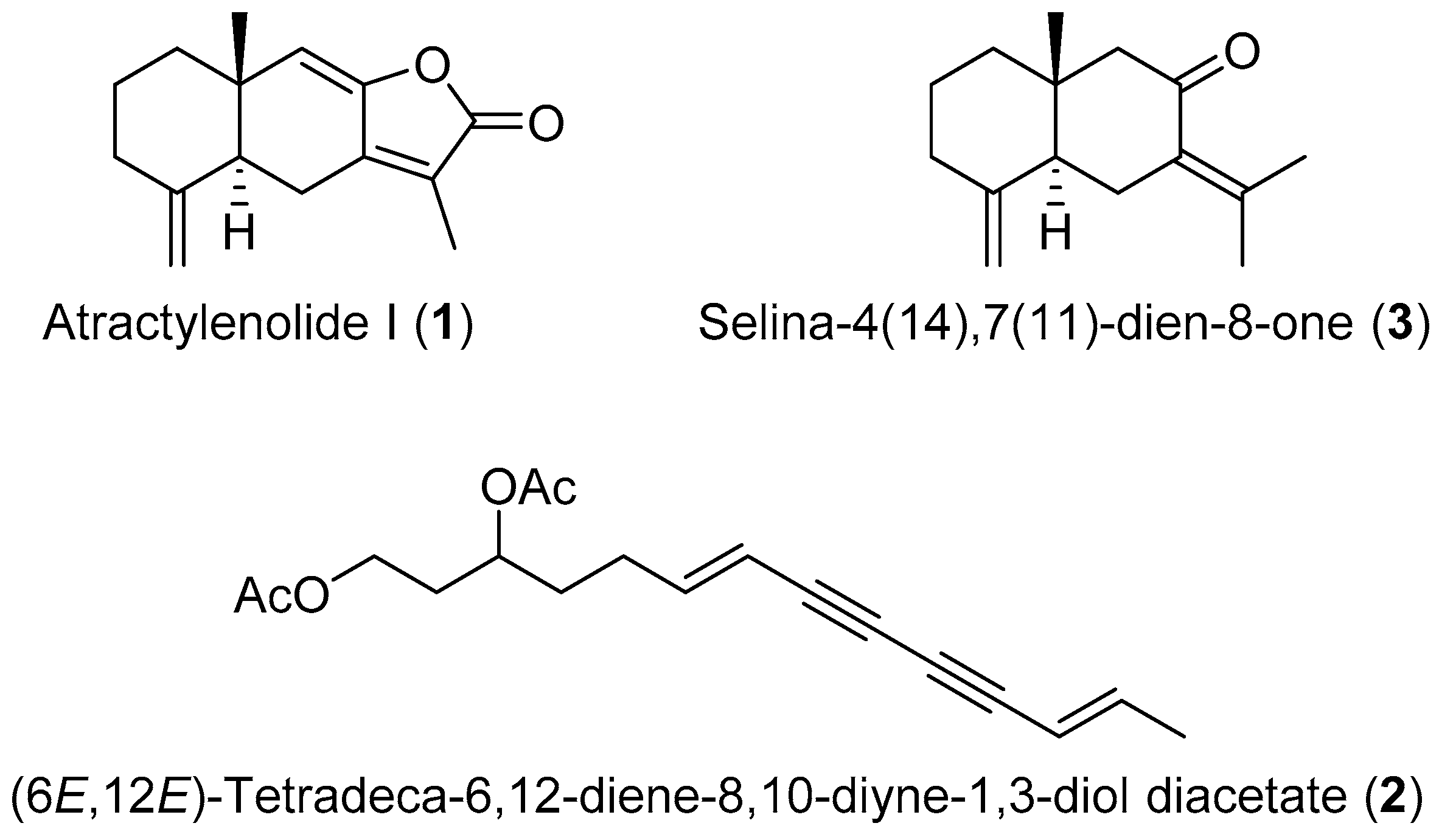

| Solvent Systems (HEMW, v/v) | K-Value | ||
|---|---|---|---|
| Atractylenolide I (1) | (6E,12E)-Tetradeca-6,12-dien-8,10-diyne-1,3-diyl diacetate (2) | Selina-4(14),7(11)-dien-8-one (3) | |
| 9:1:9:1 | 0.82 | 0.40 | 1.93 |
| 8:2:8:2 | 1.54 | 0.86 | 3.62 |
| 7:3:7:3 | 3.37 | 2.35 | 7.80 |
| CPC Peaks | Peak A | Peak B | Peak C |
|---|---|---|---|
| Compounds 1–3 | (6E,12E)-tetradeca-6,12-dien-8,10-diyne-1,3-diyl diacetate (2) | atractylenolide I (1) | selina-4(14),7(11)-dien-8-one (3) |
| K-values of compounds 1–3 | 0.86 | 1.54 | 3.62 |
| Retention time (calculated) | 102.16 min | 140.24 min | 251.12 min |
| Retention time (experimental data) | 90–10 min | 120–146 min | 178–227 min |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.I.; Kim, J.H.; Syed, A.S.; Kim, Y.-M.; Choe, K.K.; Kim, C.Y. Application of Centrifugal Partition Chromatography for Bioactivity-Guided Purification of Antioxidant-Response-Element-Inducing Constituents from Atractylodis Rhizoma Alba. Molecules 2018, 23, 2274. https://doi.org/10.3390/molecules23092274
Kim MI, Kim JH, Syed AS, Kim Y-M, Choe KK, Kim CY. Application of Centrifugal Partition Chromatography for Bioactivity-Guided Purification of Antioxidant-Response-Element-Inducing Constituents from Atractylodis Rhizoma Alba. Molecules. 2018; 23(9):2274. https://doi.org/10.3390/molecules23092274
Chicago/Turabian StyleKim, Myeong Il, Ji Hoon Kim, Ahmed Shah Syed, Young-Mi Kim, Kevin Kyungsik Choe, and Chul Young Kim. 2018. "Application of Centrifugal Partition Chromatography for Bioactivity-Guided Purification of Antioxidant-Response-Element-Inducing Constituents from Atractylodis Rhizoma Alba" Molecules 23, no. 9: 2274. https://doi.org/10.3390/molecules23092274
APA StyleKim, M. I., Kim, J. H., Syed, A. S., Kim, Y.-M., Choe, K. K., & Kim, C. Y. (2018). Application of Centrifugal Partition Chromatography for Bioactivity-Guided Purification of Antioxidant-Response-Element-Inducing Constituents from Atractylodis Rhizoma Alba. Molecules, 23(9), 2274. https://doi.org/10.3390/molecules23092274






