Liposomes Loaded with Cisplatin and Magnetic Nanoparticles: Physicochemical Characterization, Pharmacokinetics, and In-Vitro Efficacy
Abstract
1. Introduction
2. Results
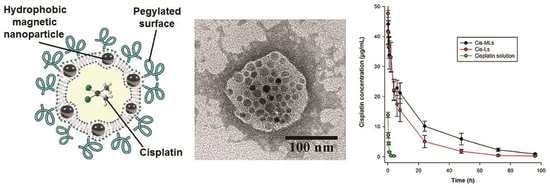
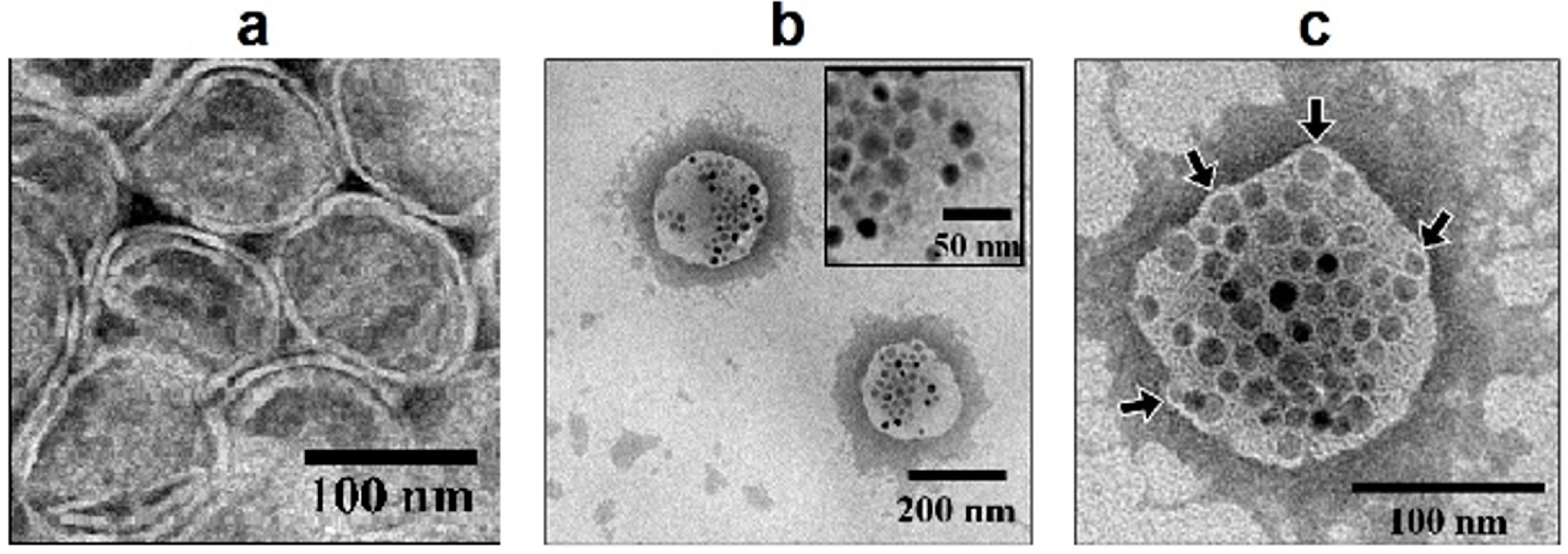
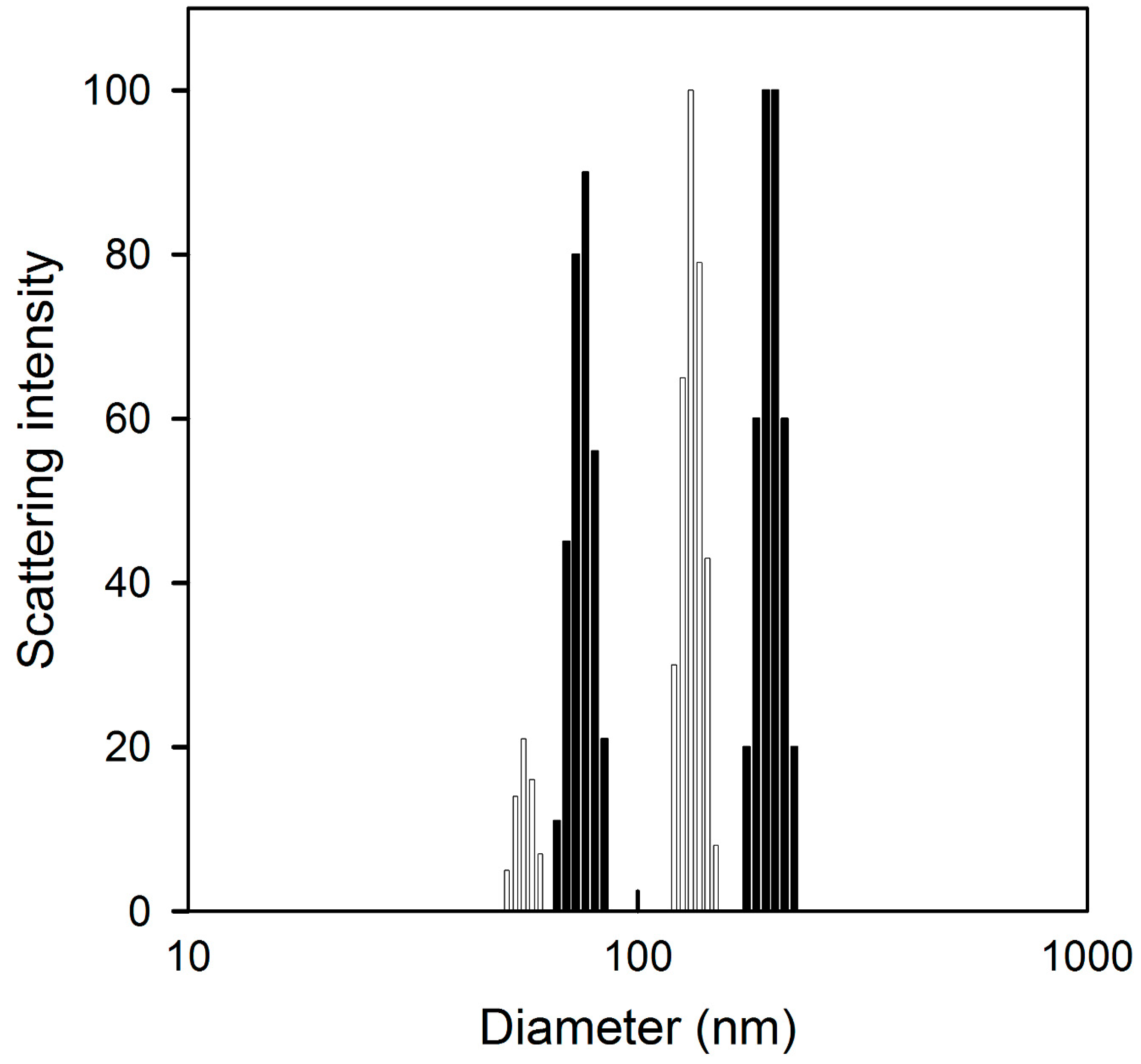
2.1. Physicochemical Characterization
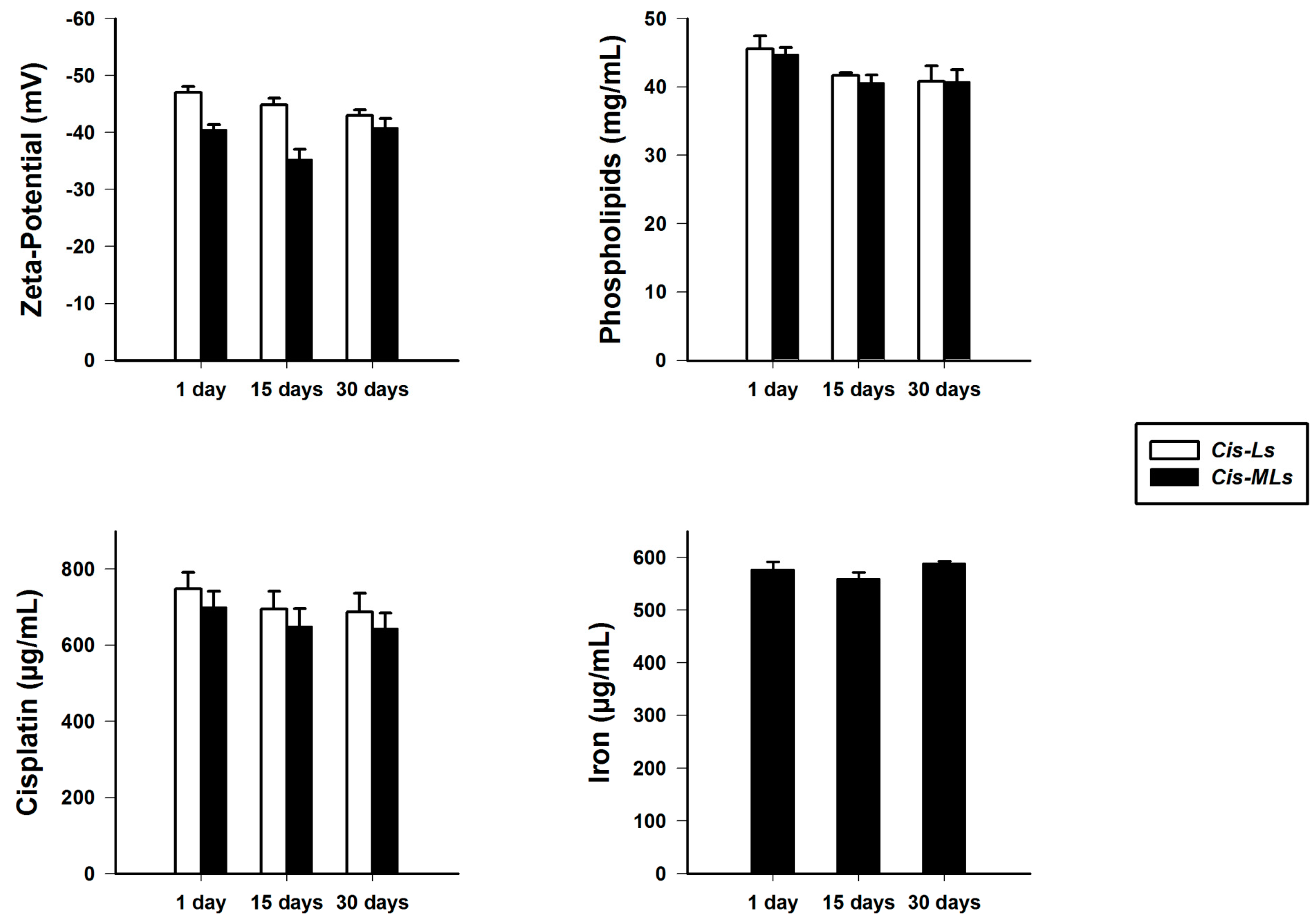
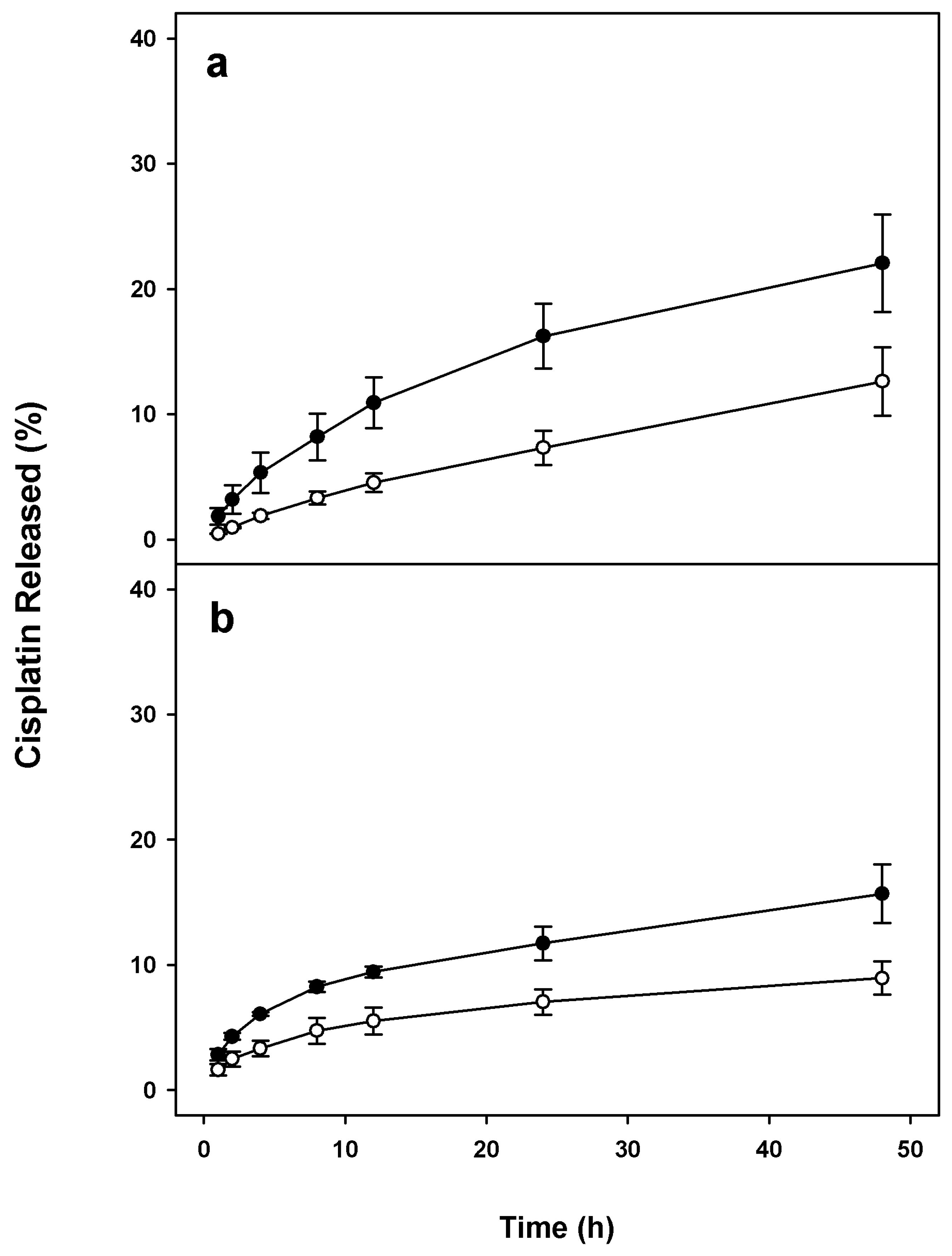
2.2. Stability of Cis-MLs
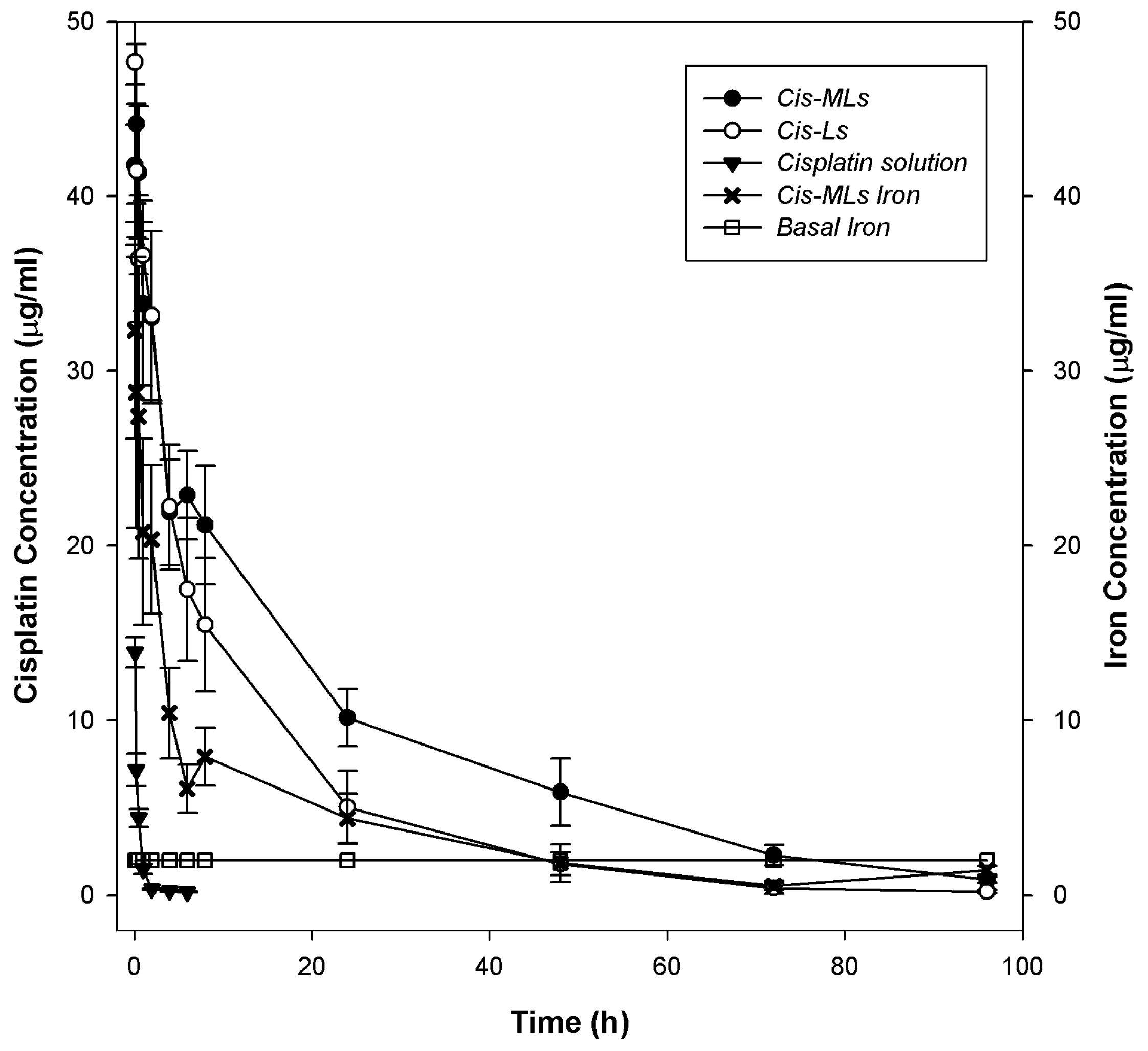
2.3. Pharmacokinetic and Biodistribution Studies
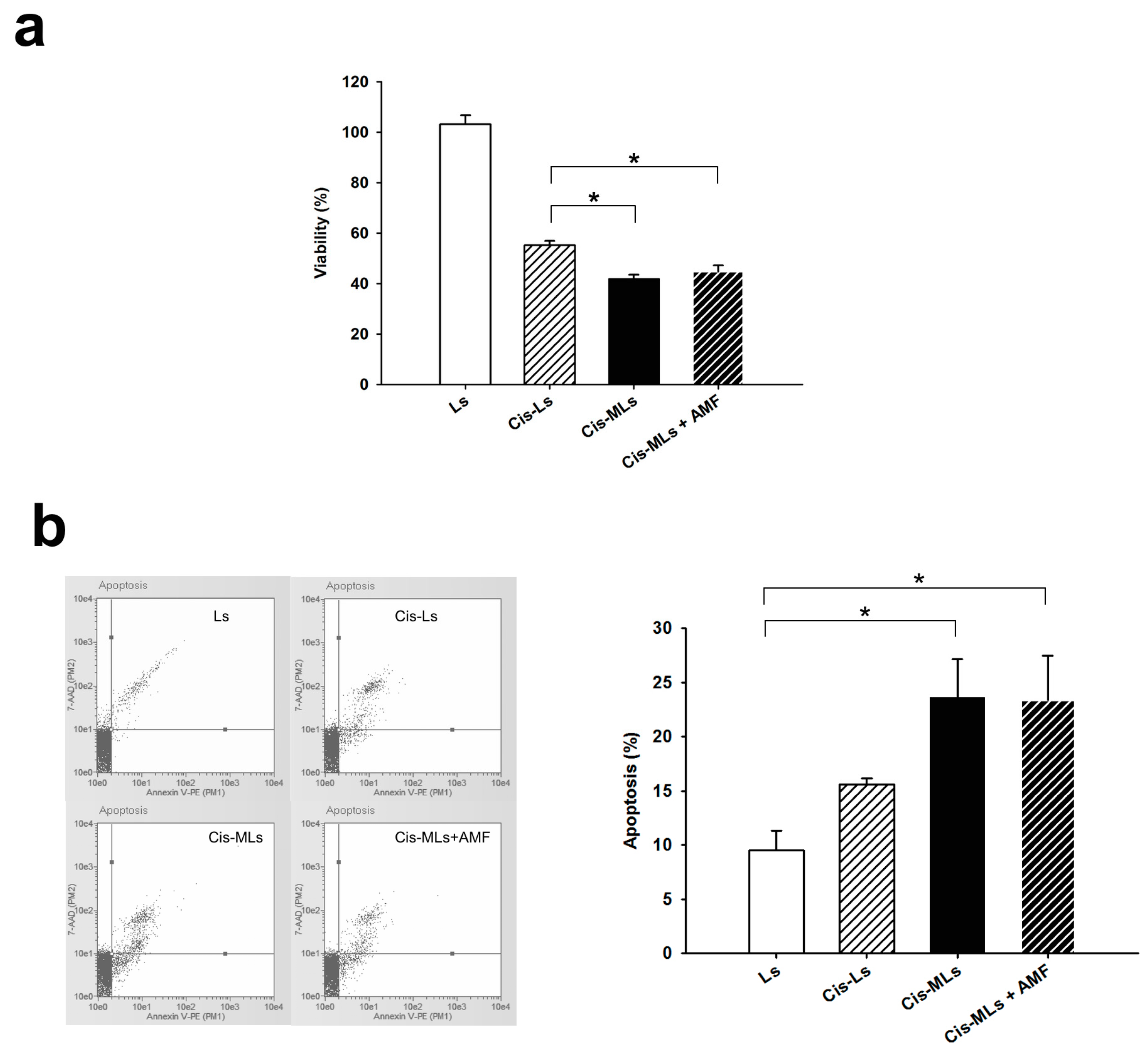
2.4. In Vitro Study
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of Cisplatin-Loaded Magnetic Liposomes (Cis-MLs)
4.3. Characterization of Cis-MLs
4.4. Stability of the Cis-MLs
4.5. Pharmacokinetics of Cis-MLs and Cis-Ls
4.6. In Vitro Study
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ardizzoni, A.; Boni, L.; Tiseo, M.; Fossella, F.V.; Schiller, J.H.; Paesmans, M.; Radosavljevic, D.; Paccagnella, A.; Zatloukal, P.; Mazzanti, P.; et al. Cisplatin-versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: An individual patient data meta-analysis. J. Natl. Cancer Inst. 2007, 99, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Markman, M. Antineoplastic agents in the management of ovarian cancer: Current status and emerging therapeutic strategies. Trends Pharmacol. Sci. 2008, 29, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Movva, S.; Rodriguez, L.; Arias-Pulido, H.; Verschraegen, C. Novel chemotherapy approaches for cervical cancer. Cancer 2009, 115, 3166–3180. [Google Scholar] [CrossRef] [PubMed]
- Chabner, B.A.; Longo, D.L. Cancer Chemotherapy and Biotherapy. In Principles and Practice, 5th ed.; Lipincott Williams & Wilkins (Wolters Kluwer): Philadelphia, PA, USA, 2011; pp. 96–105. ISBN 978-1-60547-431-1. [Google Scholar]
- Kiwada, H.; Sato, J.; Yamada, S.; Kato, Y. Feasibility of magnetic liposomes as a targeting device for drugs. Chem. Pharm. Bull. (Tokyo) 1986, 34, 4253–4258. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.V.; Martins, T.M.; Goes, A.M.; Fabris, J.D.; Cavalcante, L.C.; Outon, L.E.; Domingues, R.Z. Thermosensitive gemcitabine-magnetoliposomes for combined hyperthermia and chemotherapy. Nanotechnology 2016, 27, 085105. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiao, D.; Bose, A.; Deng, R.; Bothun, G.D. Low-dose chemotherapy of hepatocellular carcinoma through triggered-release from bilayer-decorated magnetoliposomes. Colloids Surf. B: Biointerfaces 2014, 116, 452–458. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qiu, D.; An, X. Controllable release from magnetoliposomes by magnetic stimulation and thermal stimulation. Colloids Surf. B Biointerfaces 2013, 104, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, M.; Krishnan, K.M. Synthesis of magnetoliposomes with monodisperse iron oxide nanocrystal cores for hyperthermia. J. Magn. Magn. Mater. 2005, 293, 265–270. [Google Scholar] [CrossRef]
- Huang, H.S.; Hainfeld, J.F. Intravenous magnetic nanoparticle cancer hyperthermia. Int. J. Nanomed. 2013, 8, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, P.; Gogoi, M.; Bahadur, D.; Banerjee, R. In vitro application of paclitaxel loaded magnetoliposomes for combined chemotherapy and hyperthermia. Colloids Surf. B Biointerfaces 2012, 96, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, P.; Giri, J.; Rieken, F.; Koch, C.; Mykhaylyk, O.; Döblinger, M.; Banerjee, R.; Bahadur, D.; Plank, C. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J. Control. Release 2010, 142, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Watanabe, Y.; Sato, M.; Maehara, T.; Aono, H.; Naohara, T.; Hirazawa, H.; Horiuchi, A.; Yukumi, S.; Sato, K.; et al. Feasibility of chemohyperthermia with docetaxel-embedded magnetoliposomes as minimally invasive local treatment for cancer. Int. J. Cancer 2010, 126, 1955–1965. [Google Scholar] [CrossRef] [PubMed]
- Bergs, J.W.; Franken, N.A.; Haveman, J.; Geijsen, E.D.; Crezee, J.; van Bree, C. Hyperthermia, cisplatin and radiation trimodality treatment: A promising cancer treatment? A review from preclinical studies to clinical application. Int. J. Hyperth. 2007, 23, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Babincová, M.; Kontrisova, K.; Durdík, S.; Bergemann, C.; Sourivong, P. Radiation enhanced efficiency of combined electromagnetic hyperthermia and chemotherapy of lung carcinoma using cisplatin functionalized magnetic nanoparticles. Pharmazie 2014, 69, 128–131. [Google Scholar] [PubMed]
- Attaluri, A.; Kandala, S.K.; Wabler, M.; Zhou, H.; Cornejo, C.; Armour, M.; Hedayati, M.; Zhang, Y.; DeWeese, T.L.; Herman, C.; et al. Magnetic nanoparticle hyperthermia enhances radiation therapy: A study in mouse models of human prostate cancer. Int. J. Hyperth. 2015, 31, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Huo, Z.; Wang, L.; Tong, X.; Xiao, Y.; Ni, K. Targeted delivery of methotrexate to skeletal muscular tissue by thermosensitive magnetoliposomes. Int. J. Pharm. 2009, 370, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Tong, J.; Liu, J.; Lin, W.; Zhang, C.; Chen, K.; Zhao, J.; Zhu, W. Combination of gemcitabine-containing magnetoliposome and oxaliplatin-containing magnetoliposome in breast cancer treatment: A possible mechanism with potential for clinical application. Oncotarget 2016, 7, 43762–43778. [Google Scholar] [CrossRef] [PubMed]
- Bixner, O.; Reimhult, E. Controlled magnetosomes: Embedding of magnetic nanoparticles into membranes of monodisperse lipid vesicles. J. Colloid Interface Sci. 2016, 466, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bose, A.; Bothun, G.D. Controlled release from bilayer-decorated magnetoliposomes via electromagnetic heating. ACS Nano 2010, 4, 3215–3221. [Google Scholar] [CrossRef] [PubMed]
- Amstad, E.; Kohlbrecher, J.; Müller, E.; Schweizer, T.; Textor, M.; Reimhult, E. Triggered release from liposomes through magnetic actuation of iron oxide nanoparticle containing membranes. Nano Lett. 2011, 11, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Floris, A.; Sinico, C.; Fadda, A.M.; Lai, F.; Marongiu, F.; Scano, A.; Pilloni, M.; Angius, F.; Vázquez-Vázquez, C.; Ennas, G. Characterization and cytotoxicity studies on liposome-hydrophobic magnetite hybrid colloids. J. Colloid Interface Sci. 2014, 425, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Dyego, M.; Carter, K.; Luo, D.; Shao, S.; Geng, J.; Li, C.; Chitgupi, U.; Turowski, S.G.; Li, N.; Atilla-Gokcumen, G.E.; et al. Multifunctional liposomes for image-guided intratumoral chemo-phototherapy. Adv. Healthc. Mater. 2017, 6, 1700253. [Google Scholar] [CrossRef]
- Pradhan, P.; Banerjee, R.; Bahadur, D.; Koch, C.; Mykhaylyk, O.; Plank, C. Targeted Magnetic Liposomes Loaded with Doxorubicin. Methods Mol. Biol. 2017, 1522, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Jehn, C.F.; Boulikas, T.; Kourvetaris, A.; Possinger, K.; Lüftner, D. Pharmacokinetics of liposomal cisplatin (lipoplatin) in combination with 5-FU in patients with advanced head and neck cancer: First results of a phase III study. Anticancer Res. 2007, 27, 471–475. [Google Scholar] [PubMed]
- Li, S.D.; Huang, L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Drummond, D.C.; Meyer, O.; Hong, K.; Kirpotin, D.B.; Papahadjopoulos, D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol. Rev. 1999, 51, 691–743. [Google Scholar] [PubMed]
- Luciani, N.; Wilhelm, C.; Gazeau, F. The role of cell-released microvesicles in the intercellular transfer of magnetic nanoparticles in the monocyte/macrophage system. Biomaterials 2010, 31, 7061–7069. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Chen, B.; Ding, J.; Xia, G.; Gao, C.; Cheng, J.; Jin, N.; Zhou, Y.; Li, X.; et al. Pharmacokinetic parameters and tissue distribution of magnetic Fe(3)O(4) nanoparticles in mice. Int. J. Nanomed. 2010, 5, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, M.; Vigliotti, C.; Mosca, T.; Cammarota, M.R.; Capone, D. Emerging role of the spleen in the pharmacokinetics of monoclonal antibodies, nanoparticles and exosomes. Int. J. Mol. Sci. 2017, 18, 1249. [Google Scholar] [CrossRef]
- Jiskoot, W.; van Schie, R.M.; Carstens, M.G.; Schellekens, H. Immunological risk of injectable drug delivery systems. Pharm. Res. 2009, 26, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, D.; Di Stefano, A.; Carone, V.; Fagotti, A.; Pisconti, S.; Scambia, G. Pegylated liposomal doxorubicin-related palmar-plantar erythrodysesthesia (‘hand-foot’ syndrome). Ann. Oncol. 2007, 18, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, P.; Tarabishi, R.; Mishra, J.; Ma, Q.; Kourvetaris, A.; Vougiouka, M.; Boulikas, T. Low renal toxicity of lipoplatin compared to cisplatin in animals. Anticancer Res. 2004, 4, 2193–2200. [Google Scholar] [PubMed]
- Wang, S.; Mi, J.B.; Li, Y.Z.; Chang, W.B.; Ci, Y.X.; Zhao, M.Z.; Zhao, Y.K.; Zhu, L.Y.; Xu, G. Pharmacokinetics and tissue distribution of iv injection of polyphase liposome-encapsulated cisplatin (KM-1) in rats. Acta Pharmacol. Sin. 2003, 24, 589–592. [Google Scholar] [PubMed]
- Working, P.K.; Newman, M.S.; Sullivan, T.; Brunner, M.; Podell, M.; Sahenk, Z.; Turner, N. Comparative intravenous toxicity of cisplatin solution and cisplatin encapsulated in long-circulating, pegylated liposomes in cynomolgus monkeys. Toxicol. Sci. 1998, 46, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulos, G.P. Liposomal cisplatin: A new cisplatin formulation. Anticancer Drugs 2010, 21, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Boulikas, T. Molecular mechanisms of cisplatin and its liposomally encapsulated form, Lipoplatin™. Lipoplatin™ as a chemotherapy and antiangiogenesis drug. Cancer Ther. 2007, 5, 349–376. [Google Scholar]
- De Jonge, M.J.; Slingerland, M.; Loos, W.J.; Wiemer, E.A.; Burger, H.; Mathijssen, R.H.; Kroep, J.R.; den Hollander, M.A.; van der Biessen, D.; Lam, M.H.; et al. Early cessation of the clinical development of LiPlaCis, a liposomal cisplatin formulation. Eur. J. Cancer 2010, 46, 3016–3021. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Júnior, A.D.; Vieira, F.P.; Melo, V.J.; Lopes, M.T.; Silveira, J.N.; Ramaldes, G.A.; Garnier-Suillerot, A.; Pereira-Maia, E.C.; Oliveira, M.C. Preparation and cytotoxicity of cisplatin-containing liposomes. Braz. J. Med. Biol. Res. 2007, 40, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Mai, J.; Song, S.; Rui, M.; Liu, D.; Ding, Q.; Peng, J.; Xu, Y. A synthetic peptide mediated active targeting of cisplatin liposomes to Tie2 expressing cells. J. Control. Release 2009, 139, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Hirai, M.; Minematsu, H.; Hiramatsu, Y.; Kitagawa, H.; Otani, T.; Iwashita, S.; Kudoh, T.; Chen, L.; Li, Y.; Okada, M.; et al. Novel and simple loading procedure of cisplatin into liposomes and targeting tumor endothelial cells. Int. J. Pharm. 2010, 391, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Basel, M.T.; Balivada, S.; Wang, H.; Shrestha, T.B.; Seo, G.M.; Pyle, M.; Abayaweera, G.; Dani, R.; Koper, O.B.; Tamura, M.; et al. Cell-delivered magnetic nanoparticles caused hyperthermia-mediated increased survival in a murine pancreatic cancer model. Int. J. Nanomed. 2012, 7, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Y.; Han, Y.; Wang, J.; Yang, J. Preparation and characterization of cisplatin magnetic solid lipid nanoparticles (MSLNs): Effects of loading procedures of Fe3O4 nanoparticles. Pharm. Res. 2015, 32, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Tabuchi, Y.; Kondo, T. Hyperthermia: An effective strategy to induce apoptosis in cancer cells. Apoptosis 2015, 20, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Obaidat, I.M.; Issa, B.; Haik, Y. Magnetic Properties of Magnetic Nanoparticles for Efficient Hyperthermia. Nanomaterials (Basel) 2015, 5, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Deatsch, A.E.; Evans, B.A. Heating efficiency in magnetic nanoparticle hyperthermia. J. Magn. Magn. Mater. 2014, 354, 163–172. [Google Scholar] [CrossRef]
- Piñeiro-Redondo, Y.; Bañobre-López, M.; Pardiñas-Blanco, I.; Goya, G.; López-Quintela, M.A.; Rivas, J. The influence of colloidal parameters on the specific power absorption of PAA-coated magnetite nanoparticles. Nanoscale Res. Lett. 2011, 6, 383. [Google Scholar] [CrossRef] [PubMed]
- Szoka, F.; Papahadjopoulos, D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc. Natl. Acad. Sci. USA 1978, 75, 4194–4198. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.C. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 1980, 104, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Jeitner, T.M. Optimized ferrozine-based assay for dissolved iron. Anal. Biochem. 2014, 454, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Toro-Córdova, A.; Ledezma-Gallegos, F.; Mondragon-Fuentes, L.; Jurado, R.; Medina, L.A.; Pérez-Rojas, J.M.; Garcia-Lopez, P. Determination of Liposomal Cisplatin by High-Performance Liquid Chromatography and Its Application in Pharmacokinetic. J. Chromatogr. Sci. 2016, 54, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


| Parameter | Cis-Ls | Cis-MLs |
|---|---|---|
| Particle size (nm) | 115.36 ± 4.99 | 104.43 ± 11.53 |
| ζ-potential (mV) | −47.09 ± 0.95 | −40.49 ± 0.87 |
| Lipids (mg/mL) | 45.59 ± 1.85 | 44.79 ± 1.00 |
| Cholesterol (mg/mL) | 6.41 ± 0.14 | 7.29 ± 0.19 |
| Cisplatin (µg/mL) | 748.05 ± 43.72 | 700.17 ± 41.85 |
| Iron (µg/mL) | - | 577.29 ± 14.49 |
| Parameter | Cisplatin Solution | Cisplatin Liposomes | Cis-MLs |
|---|---|---|---|
| AUC0→t (µg·h/mL·kg) | 7.49 ± 0.73 * | 519.27 ± 71.07 | 819.56 ± 140.58 |
| t1/2 (h) | 3.89 ± 2.10 * | 22.13 ± 7.14 | 22.44 ± 1.98 |
| Cmax (µg/mL) | 21.3 ± 3.61 * | 56.2 ± 6.37 | 45.2 ± 4.47 |
| Cl (mL/h) | 849.9 ± 102.5 * | 12.54 ± 1.9 | 8.32 ± 1.25 |
| Vd (mL/kg) | 313.11 ± 39.6 * | 112.7 ± 13.24 | 139.52 ± 13.94 |
| Parameter | Cis-MLs |
|---|---|
| AUC0→t (µg·h/mL·kg) | 352.84 ± 81.65 |
| t1/2 (h) | 29.48 ± 9.15 |
| Cmax (µg/mL) | 94.64 ± 59.32 |
| Cl (mL/h) | 10.12 ± 3.31 |
| Vd (mL/kg) | 62.91 ± 18.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toro-Cordova, A.; Flores-Cruz, M.; Santoyo-Salazar, J.; Carrillo-Nava, E.; Jurado, R.; Figueroa-Rodriguez, P.A.; Lopez-Sanchez, P.; Medina, L.A.; Garcia-Lopez, P. Liposomes Loaded with Cisplatin and Magnetic Nanoparticles: Physicochemical Characterization, Pharmacokinetics, and In-Vitro Efficacy. Molecules 2018, 23, 2272. https://doi.org/10.3390/molecules23092272
Toro-Cordova A, Flores-Cruz M, Santoyo-Salazar J, Carrillo-Nava E, Jurado R, Figueroa-Rodriguez PA, Lopez-Sanchez P, Medina LA, Garcia-Lopez P. Liposomes Loaded with Cisplatin and Magnetic Nanoparticles: Physicochemical Characterization, Pharmacokinetics, and In-Vitro Efficacy. Molecules. 2018; 23(9):2272. https://doi.org/10.3390/molecules23092272
Chicago/Turabian StyleToro-Cordova, Alfonso, Mario Flores-Cruz, Jaime Santoyo-Salazar, Ernesto Carrillo-Nava, Rafael Jurado, Pavel A. Figueroa-Rodriguez, Pedro Lopez-Sanchez, Luis A. Medina, and Patricia Garcia-Lopez. 2018. "Liposomes Loaded with Cisplatin and Magnetic Nanoparticles: Physicochemical Characterization, Pharmacokinetics, and In-Vitro Efficacy" Molecules 23, no. 9: 2272. https://doi.org/10.3390/molecules23092272
APA StyleToro-Cordova, A., Flores-Cruz, M., Santoyo-Salazar, J., Carrillo-Nava, E., Jurado, R., Figueroa-Rodriguez, P. A., Lopez-Sanchez, P., Medina, L. A., & Garcia-Lopez, P. (2018). Liposomes Loaded with Cisplatin and Magnetic Nanoparticles: Physicochemical Characterization, Pharmacokinetics, and In-Vitro Efficacy. Molecules, 23(9), 2272. https://doi.org/10.3390/molecules23092272