Hypoglycemic Efficacy of Docking Selected Natural Compounds against α-Glucosidase and α-Amylase
Abstract
1. Introduction
2. Results
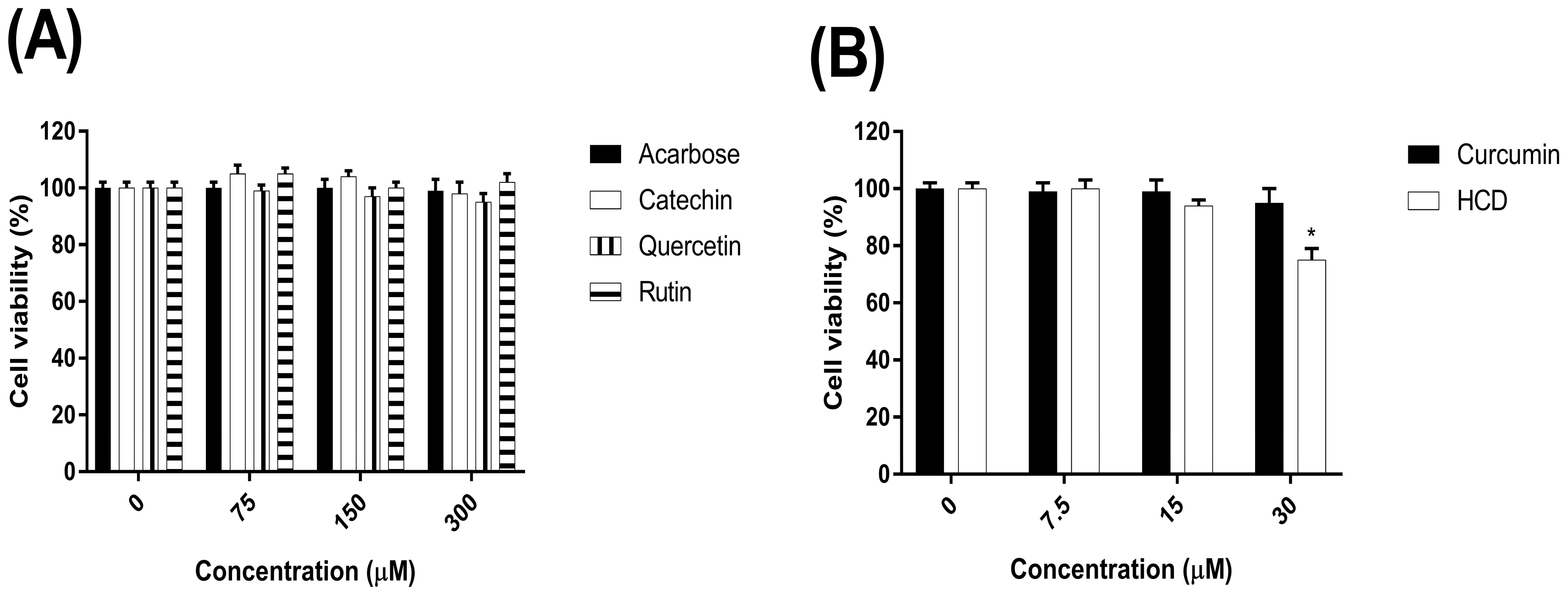
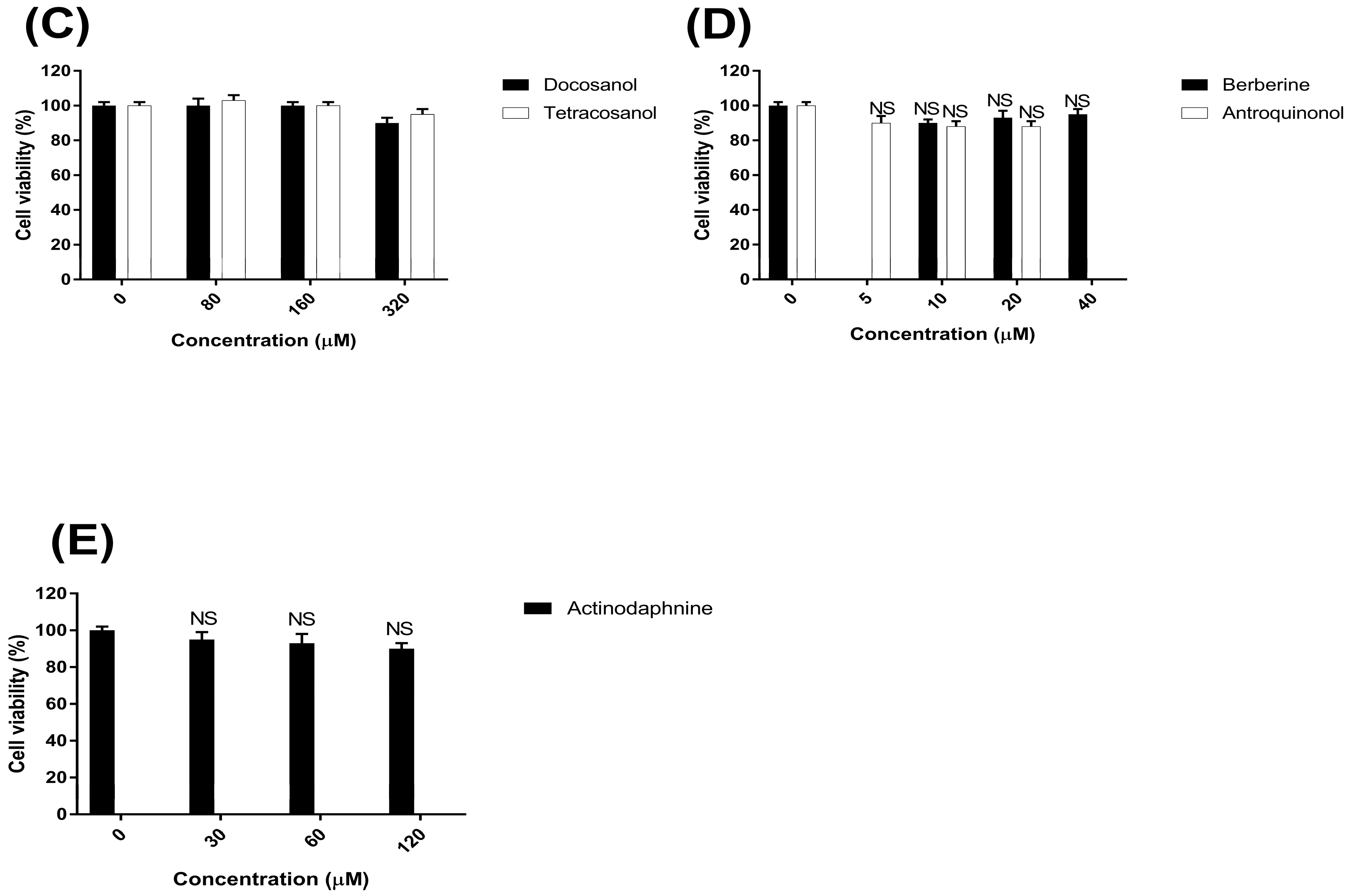
2.1. The Cytotoxicity of Natural Compounds on the Caco-2 Cells
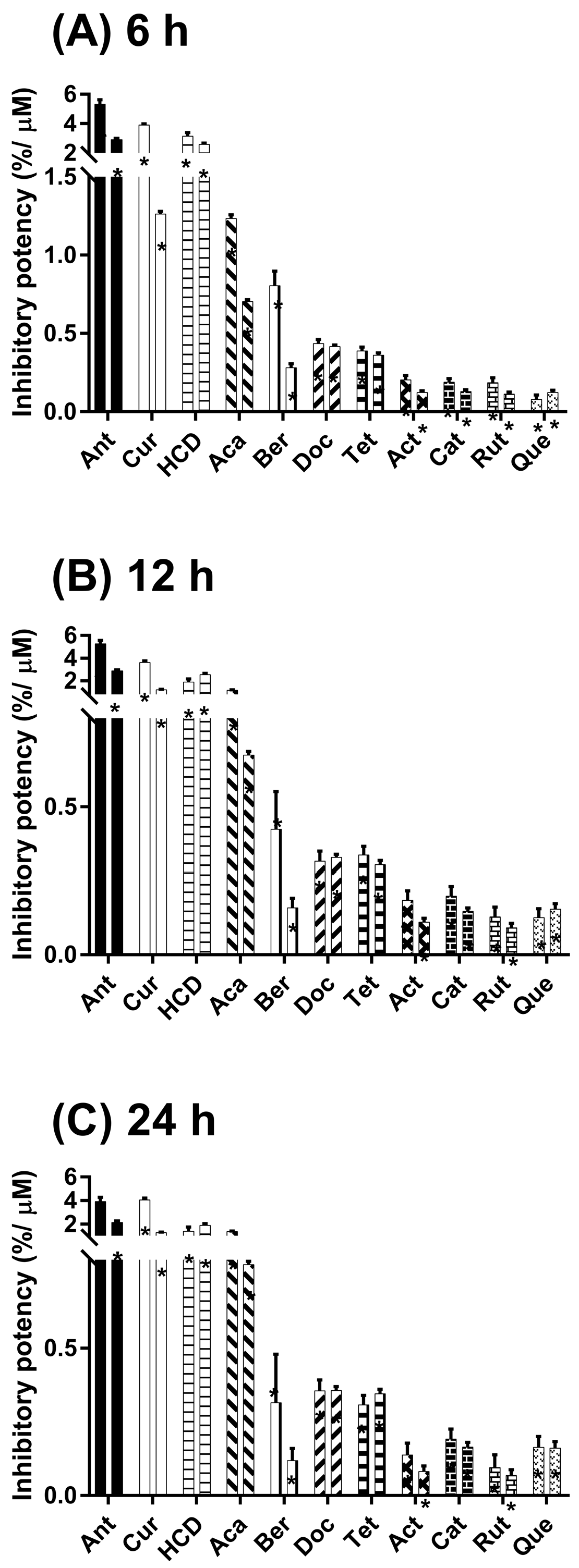
2.2. Inhibitory α-Glucosidase Activity of Selected Natural Compounds in Cells
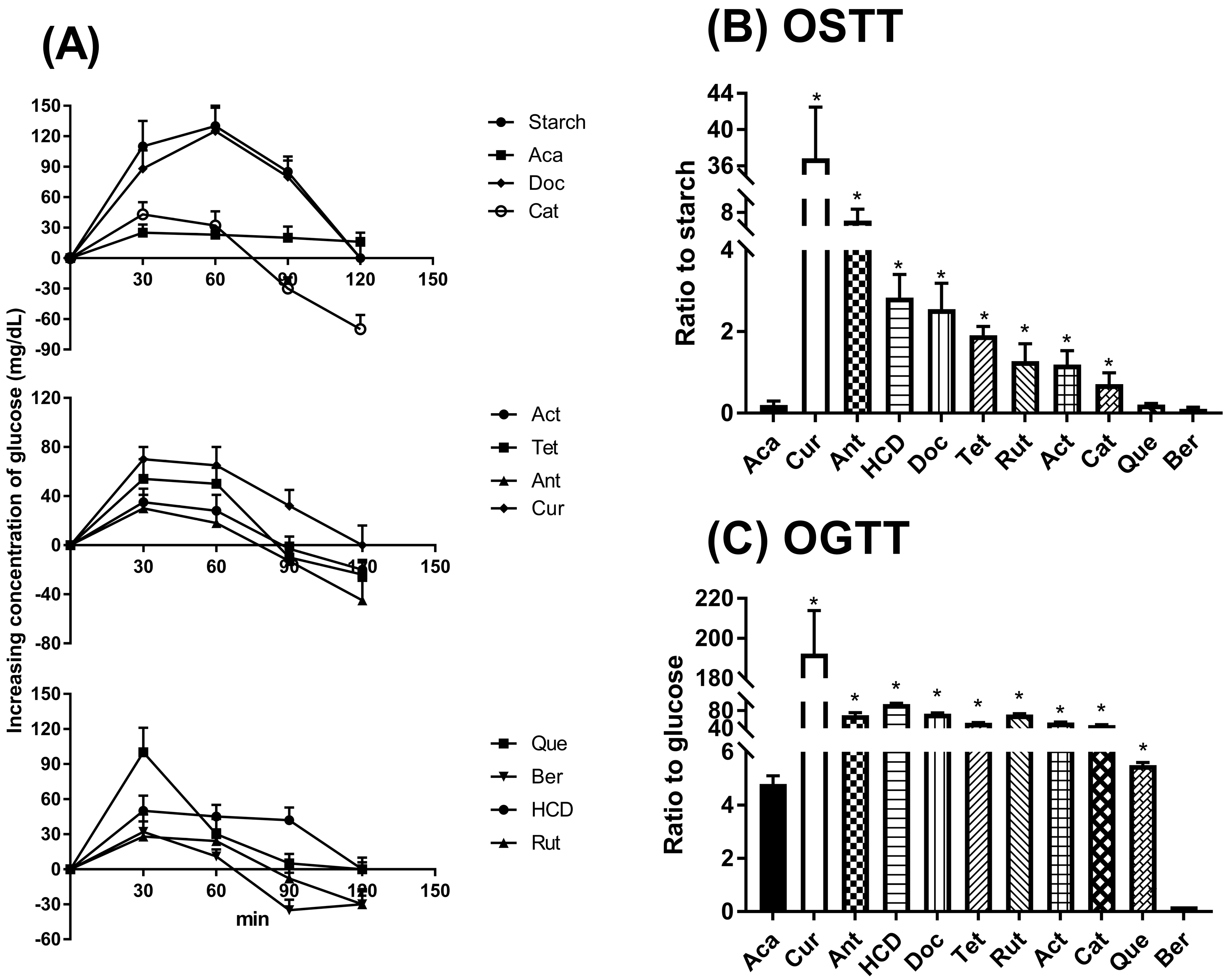
2.3. Hypoglycemic Effects in Oral Administration of Natural Compounds in Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture and Cell Viability Assay
4.3. In Vitro α-Glucosidase Activity Assay in Cells
4.4. Animals
4.5. Oral Glucose and Starch Tolerance Test (OGTT and OSTT)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Status Report on Noncommunicable Diseases 2014; WHO Press: Geneva, Switzerland, 2014; pp. 1–302. [Google Scholar]
- Van de Laar, F.A. Alpha-glucosidase inhibitors in the early treatment of type 2 diabetes. Vasc. Health Risk Manag. 2008, 4, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of hyperglycaemia in type 2 diabetes: A Patient-Centered Approach. Position Statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012, 55, 1577–1596. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, M. Cardiovascular benefits and safety profile of acarbose therapy in prediabetes and established type 2 diabetes. Cardiovasc. Diabetol. 2007, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.R.; Coleman, R.L.; Chan, J.C.N.; Chiasson, J.-L.; Feng, H.; Ge, J.; Gerstein, H.C.; Gray, R.; Huo, Y.; Lang, Z.; et al. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Diabetes Endocrinol. 2017, 5, 877–886. [Google Scholar] [CrossRef]
- Cefalu, W.T.; Stephens, J.M.; Ribnicky, D.M. Diabetes and Herbal (Botanical) Medicine. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 409–414. [Google Scholar]
- Frier, B.M. Hypoglycaemia in diabetes mellitus: Epidemiology and Clinical Implications. Nat. Rev. Endocrinol. 2014, 10, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.T.; Le, D.D.; Nguyen, P.H.; Ali, M.Y.; Choi, J.S.; Min, B.S.; Shin, H.M.; Rhee, H.I.; Woo, M.H. PTP1B, alpha-glucosidase, and DPP-IV inhibitory effects for chromene derivatives from the leaves of Smilax china L. Chem. Biol. Interact. 2016, 253, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Gao, L.X.; Meng, F.W.; Tang, C.L.; Zhang, R.J.; Li, J.Y.; Luo, C.; Li, J.; Zhao, W.M. PTP1B inhibitors from stems of Angelica keiskei (Ashitaba). Bioorg. Med. Chem. Lett. 2015, 25, 2028–2032. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Jannat, S.; Jung, H.A.; Jeong, H.O.; Chung, H.Y.; Choi, J.S. Coumarins from Angelica decursiva inhibit alpha-glucosidase activity and protein tyrosine phosphatase 1B. Chem. Biol. Interact. 2016, 252, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Bao, L.; Ma, K.; Zhang, J.; Chen, B.; Han, J.; Ren, J.; Luo, H.; Liu, H. A novel class of alpha-glucosidase and HMG-CoA reductase inhibitors from Ganoderma leucocontextum and the anti-diabetic properties of ganomycin I in KK-A(y) mice. Eur. J. Med. Chem. 2017, 127, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Thiagarajan, R.; Rastrelli, L.; Daglia, M.; Sobarzo-Sanchez, E.; Alinezhad, H.; Nabavi, S.M. Curcumin: A Natural Product for Diabetes and its Complications. Curr. Top. Med. Chem. 2015, 15, 2445–2455. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.R.; Muthenna, P.; Shankaraiah, G.; Akileshwari, C.; Babu, K.H.; Suresh, G.; Babu, K.S.; Chandra Kumar, R.S.; Prasad, K.R.; Yadav, P.A.; et al. Synthesis and biological evaluation of new piplartine analogues as potent aldose reductase inhibitors (ARIs). Eur. J. Med. Chem. 2012, 57, 344–361. [Google Scholar] [CrossRef] [PubMed]
- Jalil, A.; Ashfaq, U.A.; Shahzadi, S.; Rasul, I.; Rehman, S.U.; Shah, M.; Javed, M.R. Screening and design of anti-diabetic compounds sourced from the leaves of neem (Azadirachta indica). Bioinformation 2013, 9, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, A.R.; Manaharan, T.; Merican, A.F.; Mohamad, S.B. Experimental and computational approaches to reveal the potential of Ficus deltoidea leaves extract as alpha-amylase inhibitor. Nat. Prod. Res. 2018, 32, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Dixit, V.A.; Rathi, P.C.; Bhagat, S.; Gohlke, H.; Petersen, R.K.; Kristiansen, K.; Chakraborti, A.K.; Bharatam, P.V. Design and synthesis of novel Y-shaped barbituric acid derivatives as PPARgamma activators. Eur. J. Med. Chem. 2016, 108, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.J.; Raj, N.N.; Sugappriya, M.; Priyadarshini, S.M. Modeling of ATP-sensitive inward rectifier potassium channel 11 and inhibition mechanism of the natural ligand, ellagic acid, using molecular docking. Adv. Exp. Med. Biol. 2010, 680, 489–495. [Google Scholar] [PubMed]
- Heng, S.; Harris, K.M.; Kantrowitz, E.R. Designing inhibitors against fructose 1,6-bisphosphatase: Exploring Natural Products for Novel Inhibitor Scaffolds. Eur. J. Med. Chem. 2010, 45, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Sales, P.M.; Souza, P.M.; Simeoni, L.A.; Silveira, D. alpha-Amylase inhibitors: A Review of Raw Material and Isolated Compounds from Plant Source. J. Pharm. Pharm. Sci. 2012, 15, 141–183. [Google Scholar] [CrossRef] [PubMed]
- Stumpfe, D.; Geppert, H.; Bajorath, J. In SilicoScreening. In Lead Generation Approaches in Drug Discovery; Wiley & Sons Press: Hoboken, NJ, USA, 2010; pp. 73–103. [Google Scholar]
- Jhong, C.H.; Riyaphan, J.; Lin, S.H.; Chia, Y.C.; Weng, C.F. Screening alpha-glucosidase and alpha-amylase inhibitors from natural compounds by molecular docking in silico. Biofactors 2015, 41, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Zuo, D.Y.; Qie, X.D.; Qi, H.; Zhao, M.Q.; Wu, Y.L. Berberine acutely inhibits the digestion of maltose in the intestine. J. Ethnopharmacol. 2012, 142, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Zhang, M.; Yu, Y.; Lan, X.; Yao, F.; Yan, X.; Chen, L.; Hatch, G.M. Berberine Attenuates Development of the Hepatic Gluconeogenesis and Lipid Metabolism Disorder in Type 2 Diabetic Mice and in Palmitate-Incubated HepG2 Cells through Suppression of the HNF-4alpha miR122 Pathway. PLoS ONE 2016, 11, e0152097. [Google Scholar] [CrossRef] [PubMed]
- Rayasam, G.V.; Tulasi, V.K.; Sundaram, S.; Singh, W.; Kant, R.; Davis, J.A.; Saini, K.S.; Ray, A. Identification of berberine as a novel agonist of fatty acid receptor GPR40. Phytother. Res. 2010, 24, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Chiasson, J.L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M.; Group, S.-N.T.R. Acarbose for prevention of type 2 diabetes mellitus: The STOP-NIDDM Randomised Trial. Lancet 2002, 359, 2072–2077. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Amjad, M.W.; Hussain, F.; Ahmed, T.A.; Khan, S. Exploring recent developments to improve antioxidant, anti-inflammatory and antimicrobial efficacy of curcumin: A review of new trends and future perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Lertpiriyapong, K.; Steelman, L.S.; Abrams, S.L.; Cocco, L.; Ratti, S.; Martelli, A.M.; Candido, S.; Libra, M.; Montalto, G.; et al. Regulation of GSK-3 activity by curcumin, berberine and resveratrol: Potential effects on multiple diseases. Adv. Biol. Regul. 2017, 65, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.K.; Lin, S.R.; Chang, C.H.; Tsai, M.J.; Lee, D.N.; Weng, C.F. Natural phenolic compounds potentiate hypoglycemia via inhibition of Dipeptidyl peptidase IV. Sci. Rep. 2018, in press. [Google Scholar]
- Du, Z.Y.; Liu, R.R.; Shao, W.Y.; Mao, X.P.; Ma, L.; Gu, L.Q.; Huang, Z.S.; Chan, A.S. Alpha-glucosidase inhibition of natural curcuminoids and curcumin analogs. Eur. J. Med. Chem. 2006, 41, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Sulake, R.S.; Huang, P.K.; Shih, H.Y.; Sie, H.W.; Lai, Y.K.; Chen, C.; Weng, C.F. Synthetic (+)-antroquinonol exhibits dual actions against insulin resistance by triggering AMP kinase and inhibiting dipeptidyl peptidase IV activities. Br. J. Pharmacol. 2015, 172, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Beidokhti, M.N.; Jager, A.K. Review of antidiabetic fruits, vegetables, beverages, oils and spices commonly consumed in the diet. J. Ethnopharmacol. 2017, 201, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.X.; Ge, A.H.; Donnapee, S.; Li, J.; Bai, Y.; Liu, J.; He, J.; Yang, X.; Song, L.J.; Zhang, B.L.; et al. The multi-targets integrated fingerprinting for screening anti-diabetic compounds from a Chinese medicine Jinqi Jiangtang Tablet. J. Ethnopharmacol. 2015, 164, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Zhou, F.C.; Gao, F.; Bian, J.S.; Shan, F. Comparative Evaluation of Quercetin, Isoquercetin and Rutin as Inhibitors of α-Glucosidase. J. Agric. Food Chem. 2009, 57, 11463–11468. [Google Scholar] [CrossRef] [PubMed]
- Kinnings, S.L.; Liu, N.; Buchmeier, N.; Tonge, P.J.; Xie, L.; Bourne, P.E. Drug discovery using chemical systems biology: Repositioning the Safe Medicine Comtan to Treat Multi-Drug and Extensively Drug Resistant Tuberculosis. PLoS Comput. Biol. 2009, 5, e1000423. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Shih, H.Y.; Chia, Y.C.; Lee, C.H.; Ashida, H.; Lai, Y.K.; Weng, C.F. Rutin potentiates insulin receptor kinase to enhance insulin-dependent glucose transporter 4 translocation. Mol. Nutr. Food Res. 2014, 58, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei-Malazy, O.; Larijani, B.; Abdollahi, M. A novel management of diabetes by means of strong antioxidants’ combination. J. Med. Hypotheses Ideas 2013, 7, 25–30. [Google Scholar] [CrossRef]
- Cheng, M.F.; Lin, C.S.; Chen, Y.H.; Sung, P.J.; Lin, S.R.; Tong, Y.W.; Weng, C.F. Inhibitory Growth of Oral Squamous Cell Carcinoma Cancer via Bacterial Prodigiosin. Mar. Drugs 2017, 15, 224. [Google Scholar] [CrossRef] [PubMed]
- Hansawasdi, C.; Kawabata, J. Alpha-glucosidase inhibitory effect of mulberry (Morus alba) leaves on Caco-2. Fitoterapia 2006, 77, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, C.; Guo, L.; Zhang, Y.; Wang, J.; Ma, B.; Wang, Y.; Wang, Y.; Ren, J.; Yang, X.; et al. Ultrafiltration liquid chromatography combined with high-speed countercurrent chromatography for screening and isolating potential alpha-glucosidase and xanthine oxidase inhibitors from Cortex Phellodendri. J. Sep. Sci. 2014, 37, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.K.; Lin, S.X.; Tsai, M.J.; Leong, M.K.; Lin, S.R.; Kankala, R.K.; Lee, C.H.; Weng, C.F. Encapsulation of 16-Hydroxycleroda-3,13-Dine-16,15-Olide in Mesoporous Silica Nanoparticles as a Natural Dipeptidyl Peptidase-4 Inhibitor Potentiated Hypoglycemia in Diabetic Mice. Nanomaterials 2017, 7, 112. [Google Scholar] [CrossRef] [PubMed]
| Potency a | Compounds | Fold of Lowering Glucose Concentration | |
|---|---|---|---|
| Dosage (mg/(kg·B.wt)) | Fold b | ||
| Acarbose | 17.0 | 1.0 ± 0.6 | |
| I | Curcumin | 0.3 | 206.0 ± 12.3 c |
| II | Antroquinonol | 1.0 | 37.6 ± 2.8 c |
| HCD | 3.0 | 17.6 ± 3.4 c | |
| Docosanol | 4.0 | 15.5 ± 3.8 c | |
| Tetracosanol | 4.0 | 10.9 ± 0.9 c | |
| III | Rutin | 4.0 | 7.2 ± 2.9 c |
| Actinodaphnine | 5.0 | 6.4 ± 2.5 c | |
| Catechin | 6.0 | 4.4 ± 1.4 c | |
| IV | Quercetin | 60.0 | 1.2 ± 0.2 d |
| Berberine | 100.0 | 0.7 ± 0.2 d | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riyaphan, J.; Jhong, C.-H.; Lin, S.-R.; Chang, C.-H.; Tsai, M.-J.; Lee, D.-N.; Sung, P.-J.; Leong, M.K.; Weng, C.-F. Hypoglycemic Efficacy of Docking Selected Natural Compounds against α-Glucosidase and α-Amylase. Molecules 2018, 23, 2260. https://doi.org/10.3390/molecules23092260
Riyaphan J, Jhong C-H, Lin S-R, Chang C-H, Tsai M-J, Lee D-N, Sung P-J, Leong MK, Weng C-F. Hypoglycemic Efficacy of Docking Selected Natural Compounds against α-Glucosidase and α-Amylase. Molecules. 2018; 23(9):2260. https://doi.org/10.3390/molecules23092260
Chicago/Turabian StyleRiyaphan, Jirawat, Chien-Hung Jhong, Shian-Ren Lin, Chia-Hsiang Chang, May-Jwan Tsai, Der-Nan Lee, Ping-Jyun Sung, Max K. Leong, and Ching-Feng Weng. 2018. "Hypoglycemic Efficacy of Docking Selected Natural Compounds against α-Glucosidase and α-Amylase" Molecules 23, no. 9: 2260. https://doi.org/10.3390/molecules23092260
APA StyleRiyaphan, J., Jhong, C.-H., Lin, S.-R., Chang, C.-H., Tsai, M.-J., Lee, D.-N., Sung, P.-J., Leong, M. K., & Weng, C.-F. (2018). Hypoglycemic Efficacy of Docking Selected Natural Compounds against α-Glucosidase and α-Amylase. Molecules, 23(9), 2260. https://doi.org/10.3390/molecules23092260








