K2S2O8-Promoted Aryl Thioamides Synthesis from Aryl Aldehydes Using Thiourea as the Sulfur Source
Abstract
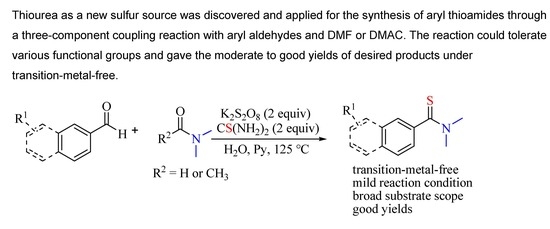
1. Introduction
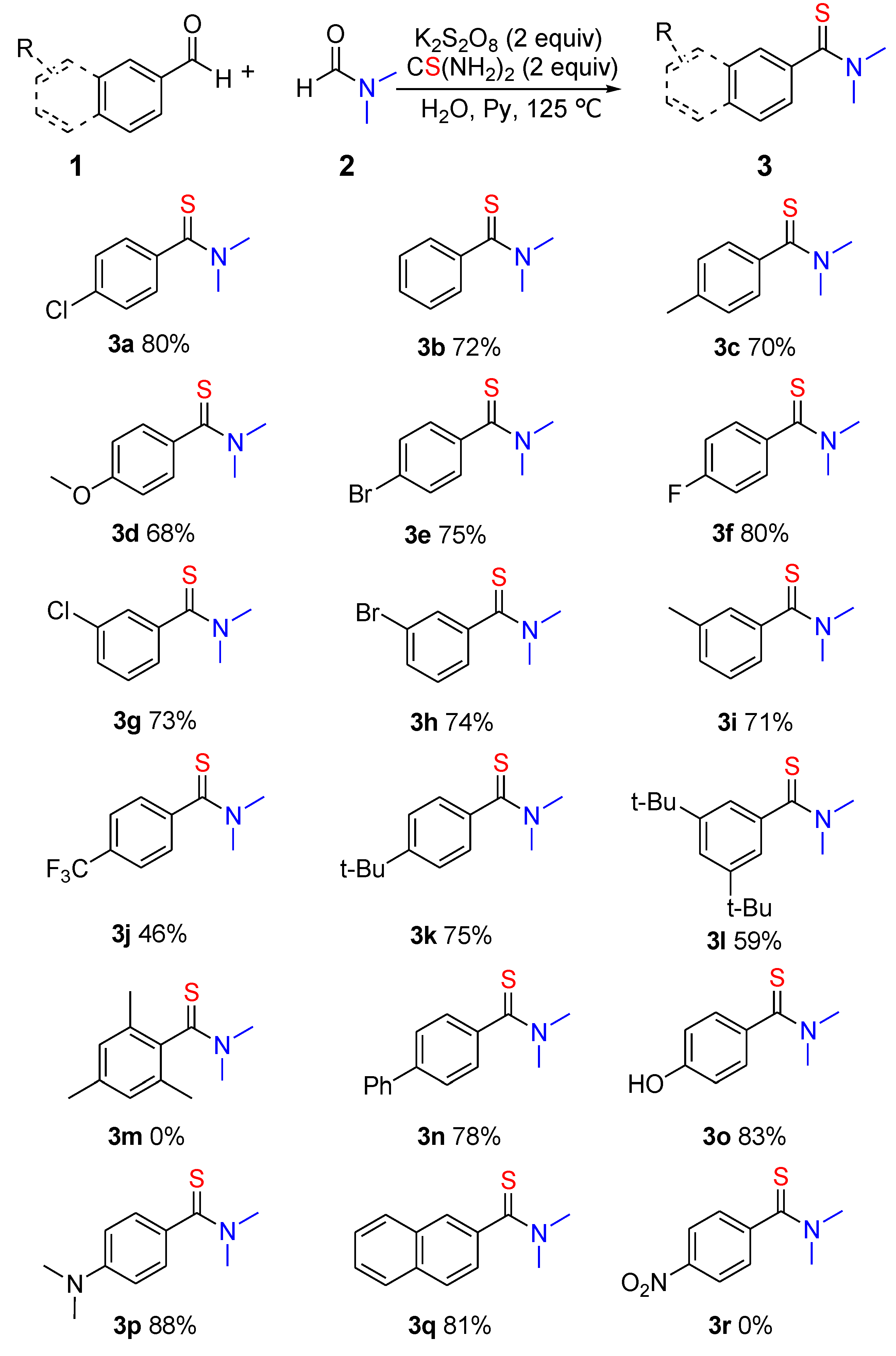
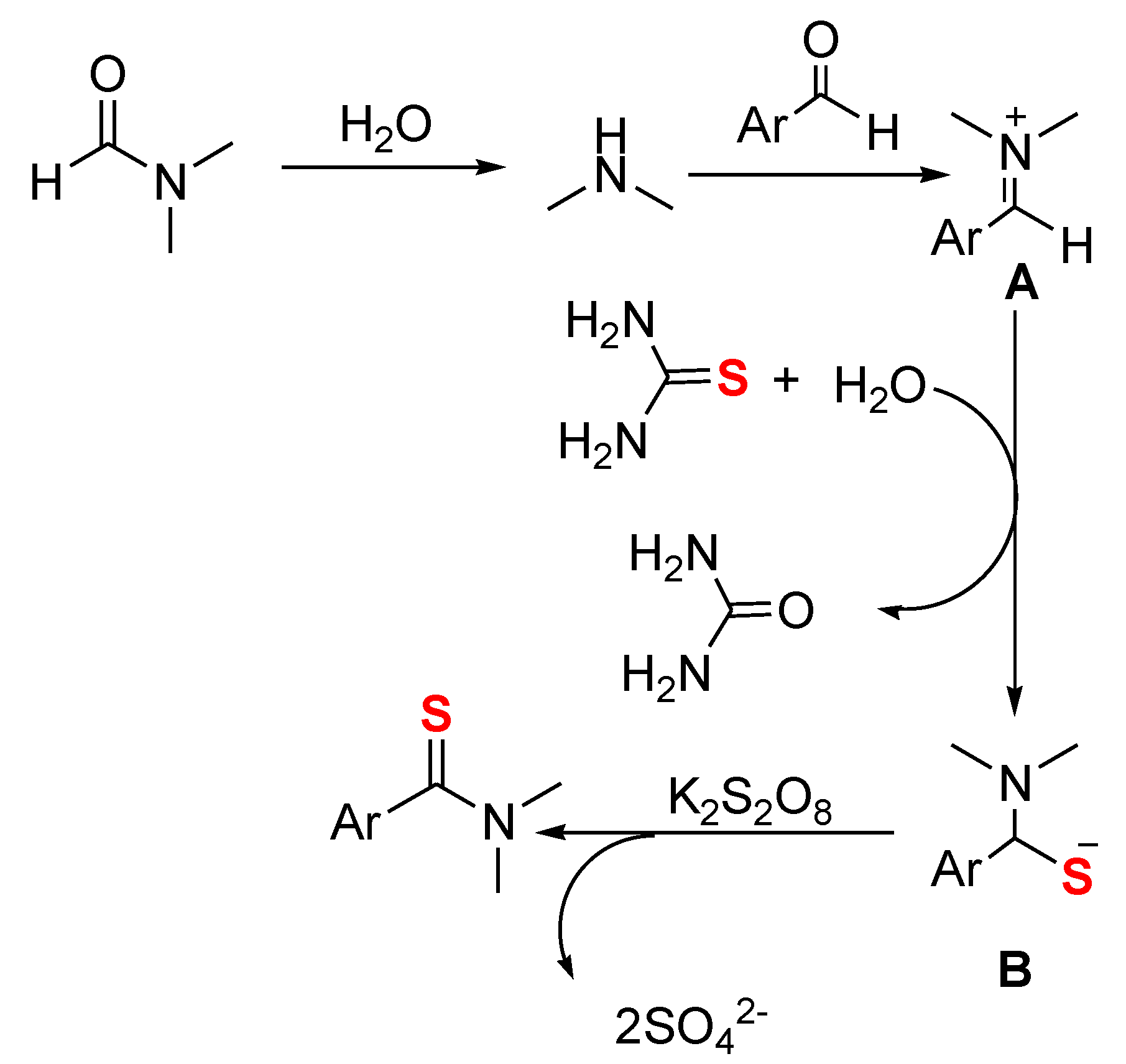
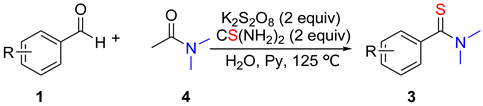
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Petrov, K.A.; Andreev, L.N. The Chemical Properties of Thioamides. Russ. Chem. Rev. 1971, 40, 505–524. [Google Scholar] [CrossRef]
- Cremlyn, R.J. An Introduction to Organo-Sulfur Chemistry; Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- Jiang, W.; Li, Y.; Wang, Z. Heteroarenes as high performance organic semiconductors. Chem. Soc. Rev. 2013, 42, 6113–6127. [Google Scholar] [CrossRef] [PubMed]
- Jagodziński, T.S. Thioamides as Useful Synthons in the Synthesis of Heterocycles. Chem. Rev. 2003, 103, 197–228. [Google Scholar] [CrossRef] [PubMed]
- Lincke, T.; Behnken, S.; Ishida, K.; Roth, M.; Hertweck, C. Closthioamide: An Unprecedented Polythioamide Antibiotic from the Strictly Anaerobic Bacterium Clostridium cellulolyticum. Angew. Chem. Int. Ed. 2010, 49, 2011–2013. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Zhang, P.F.; Sun, Q.; Bai, S.Q.; Andy Hor, T.S.; Liu, X.G. Recent advances in C–S bond formation via C–H bond functionalization and decarboxylation. Chem. Soc. Rev. 2015, 44, 291–314. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.E. Functionalized Acenes and Heteroacenes for Organic Electronics. Chem. Rev. 2006, 106, 5028–5048. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, M.; Shah, S.S.A.; Najam, T.; Ahmad, M.M.; Tabassum, R.; Rivera, G. Synthetic Thioamide, Benzimidazole, Quinolone and Derivatives with Carboxylic Acid and Ester Moieties: A Strategy in the Design of Antituberculosis Agents. Curr. Med. Chem. 2014, 21, 911–931. [Google Scholar] [CrossRef] [PubMed]
- Zoumpoulakis, P.; Camoutsis, C.; Pairas, G.; Sokovic, M.; Glamoclija, J.; Potamitis, C.; Pitsas, A. Synthesis of novel sulfonamide-1,2,4-triazoles, 1,3,4-thiadiazoles and 1,3,4-oxadiazoles, as potential antibacterial and antifungal agents. Biological evaluation and conformational analysis studies. Bioorg. Med. Chem. 2012, 20, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Fu, Y.Z. A Perspective on Energy Densities of Rechargeable Li-S Batteries and Alternative Sulfur-Based Cathode Materials. Energy Environ. Mater. 2018, 1, 20–27. [Google Scholar] [CrossRef]
- Polshettiwar, V. Phosphorus Pentasulfide (P4S10). Synlett 2004, 12, 2245–2246. [Google Scholar] [CrossRef]
- Ozturk, T.; Ertas, E.; Mert, O. Use of Lawesson’s Reagent in Organic Syntheses. Chem. Rev. 2007, 107, 5210–5278. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Céspedes, S.; Ferry, A.; Candish, L.; Glorius, F. Heterogeneously Catalyzed Direct C–H Thiolation of Heteroarenes. Angew. Chem., Int. Ed. 2015, 54, 5772–5776. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Wei, L.; Ji, X.M.; Hu, M.L.; Tang, R.Y. Direct Introduction of Dithiocarbamates onto Imidazoheterocycles under Mild Conditions. Adv. Synth. Catal. 2016, 358, 268–275. [Google Scholar] [CrossRef]
- Rafique, J.; Saba, S.; Rosário, A.R.; Braga, A.L. Regioselective, Solvent- and Metal-Free Chalcogenation of Imidazo[1,2-a] pyridines by Employing I2/DMSO as the Catalytic Oxidation System. Chem. Eur. J. 2016, 22, 11854–11862. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.P.; Cao, B.P.; Yuan, J.J.; Liu, X.J.; Peng, Y.Y. Synthesis of thioethers via metal-free reductive coupling of tosylhydrazones with thiols. Org. Biomol. Chem. 2011, 9, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Ravi, C.; Mohan, D.C.; Adimurthy, S. N-Chlorosuccinimide-Promoted Regioselective Sulfenylation of Imidazoheterocycles at Room Temperature. Org. Lett. 2014, 16, 2978–2981. [Google Scholar] [CrossRef] [PubMed]
- Hiebel, M.A.; Berteina-Raboin, S. Iodine-catalyzed regioselective sulfenylation of imidazoheterocycles in PEG400. Green Chem. 2015, 17, 937–944. [Google Scholar] [CrossRef]
- Siddaraju, Y.; Prabhu, K.R. Iodine-Catalyzed Cross Dehydrogenative Coupling Reaction: A Regioselective Sulfenylation of Imidazoheterocycles Using Dimethyl Sulfoxide as an Oxidant. J. Org. Chem. 2016, 81, 7838–7846. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.L.; Tian, S.K. Iodine-Catalyzed Regioselective Sulfenylation of Indoles with Sulfonyl Hydrazides. Angew. Chem. Int. Ed. 2013, 52, 4929–4932. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, S.; Tang, L.; Hu, Y.B.; Zha, Z.G.; Wang, Z.Y. Catalyst-free thiolation of indoles with sulfonyl hydrazides for the synthesis of 3-sulfenylindoles in water. Green Chem. 2016, 18, 2609–2613. [Google Scholar] [CrossRef]
- Singh, R.; Allam, K.B.; Singh, N.; Kumari, K.; Singh, S.K. A Direct Metal-Free Decarboxylative Sulfono Functionalization (DSF) of Cinnamic Acids to α, β-Unsaturated Phenyl Sulfones. Org. Lett. 2015, 17, 2656–2659. [Google Scholar] [CrossRef] [PubMed]
- Senadi, G.C.; Guo, B.C.; Hu, W.P.; Wang, J.J. Iodine-promoted cyclization of N-propynyl amides and N-allyl amides via sulfonylation and sulfenylation. Chem. Commun. 2016, 52, 11410–11413. [Google Scholar] [CrossRef] [PubMed]
- Handa, S.; Fennewald, J.C.; Lipshutz, B.H. Aerobic Oxidation in Nanomicelles of Aryl Alkynes, in Water at Room Temperature. Angew. Chem. Int. Ed. 2014, 53, 3432–3435. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.H.; Shi, B.F. Copper(II)-Catalyzed Direct Sulfonylation of C(sp2)–H Bonds with Sodium Sulfinates. Org. Lett. 2015, 17, 2784–2787. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wu, W.; Zhao, W.; Li, Y.; Xie, P.; Huang, Y.; Liu, Y.; Zhou, A. Generation of thioethers via direct C–H functionalization with sodium benzenesulfinate as a sulfur source. Org. Biomol. Chem. 2016, 14, 1428–1431. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Chen, S.; Tian, J.; Huang, H.; Liu, Y.; Deng, G.J. Chemoselective cross-coupling reaction of sodium sulfinates with phenols under aqueous conditions. Green Chem. 2016, 18, 1538–1546. [Google Scholar] [CrossRef]
- Guo, Y.J.; Lu, S.; Tian, L.L.; Huang, E.L.; Hao, X.Q.; Zhu, X.J.; Shao, T.; Song, M.P. Iodine-Mediated Difunctionalization of Imidazopyridines with Sodium Sulfinates: Synthesis of Sulfones and Sulfides. J. Org. Chem. 2018, 83, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, Y.; Chen, J.F.; Yao, E.; Cheng, J. Multicomponent Coupling Reactions of Two N-Tosyl Hydrazones and Elemental Sulfur: Selective Denitrogenation Pathway toward Unsymmetric 2,5-Disubstituted 1,3,4-Thiadiazoles. Org. Lett. 2016, 18, 5268–5271. [Google Scholar] [CrossRef] [PubMed]
- Ravi, C.; Reddy, N.N.K.; Pappula, V.; Samanta, S.; Adimurthy, S. Copper-Catalyzed Three-Component System for Arylsulfenylation of Imidazopyridines with Elemental Sulfur. J. Org. Chem. 2016, 81, 9964–9972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.R.; Liao, Y.Y.; Deng, J.C.; Feng, K.Y.; Zhang, M.; Ning, Y.Y.; Lin, Z.W.; Tang, R.Y. Oxidative dual C–H thiolation of imidazopyridines with ethers or alkanes using elemental sulphur. Chem. Commun. 2017, 53, 7784–7787. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.M.; Yang, Y.Z.; Xiao, G.H.; Song, J.X.; Liang, Y.; Deng, G.B. Double C–S bond formation via C–H bond functionalization: Synthesis of benzothiazoles and naphtho[2,1-d]thiazoles from N-substituted arylamines and elemental sulfur. Chem. Commun. 2017, 53, 11917–11920. [Google Scholar] [CrossRef] [PubMed]
- Bergman, J. Comparison of Two Reagents for Thionations. Synthesis 2018, 50, 2323–2328. [Google Scholar] [CrossRef]
- Hurd, R.N.; Delamater, G. The Preparation and Chemical Properties of Thionamides. Chem. Rev. 1961, 61, 45–86. [Google Scholar] [CrossRef]
- Wei, J.P.; Li, Y.M.; Jiang, X.F. Aqueous Compatible Protocol to Both Alkyl and Aryl Thioamide Synthesis. Org. Lett. 2016, 18, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yu, J.T.; Zhou, Y.N.; Jiang, Y.; Cheng, J. Aqueous MCRs of quaternary ammoniums, N-substituted formamides and sodium disulfide towards aryl thioamides. Org. Chem. Front. 2017, 4, 413–416. [Google Scholar] [CrossRef]
- Xu, K.; Li, Z.Y.; Cheng, F.Y.; Zuo, Z.Z.; Wang, T.; Wang, M.C.; Liu, L.T. Transition-Metal-Free Cleavage of C–C Triple Bonds in Aromatic Alkynes with S8 and Amides Leading to Aryl Thioamides. Org. Lett. 2018, 20, 2228–2231. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Vanjari, R.; Guntreddi, T.; Singh, K.N. Sulfur promoted decarboxylative thioamidation of carboxylic acids using formamides as amine proxy. Tetrahedron 2016, 72, 2012–2017. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Tran, M.Q.; Ermolenko, L.; Al-Mourabit, A. Three-Component Reaction between Alkynes, Elemental Sulfur, and Aliphatic Amines: A General, Straightforward, and Atom Economical Approach to Thioamides. Org. Lett. 2014, 16, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.M.; Jayalakshmi, M.; Reddy, R.S. Time-selective Hydrothermal Synthesis of SnS Nanorods and Nanoparticles by Thiourea Hydrolysis. Chem. Lett. 2004, 33, 1044–1045. [Google Scholar] [CrossRef]
- Jayalakshmi, M.; Rao, M.M. Synthesis of zinc sulphide nanoparticles by thiourea hydrolysis and their characterization for electrochemical capacitor applications. J. Power Sources 2006, 157, 624–629. [Google Scholar] [CrossRef]
- Zhang, K.; Han, Q.; Wang, X.; Zhu, J. One-Step Synthesis of Bi2S3/BiOX and Bi2S3/(BiO)2CO3 Heterojunction Photocatalysts by Using Aqueous Thiourea Solution as Both Solvent and Sulfur Source. ChemistrySelect 2016, 1, 6136–6145. [Google Scholar] [CrossRef]
- Manivel, P.; Prabakaran, K.; Krishnakumar, V.; Khan, F.N.; Maiyalagan, T. Thiourea-Mediated Regioselective Synthesis of Symmetrical and Unsymmetrical Diversified Thioethers. Ind. Eng. Chem. Res. 2014, 53, 7866–7870. [Google Scholar] [CrossRef]
- Niu, H.; Xia, C.; Qu, G.; Wu, S.; Jiang, Y.; Jin, X.; Guo, H. Microwave-Promoted “One-Pot” Synthesis of 4-Nitrobenzylthioinosine Analogues Using Thiourea as a Sulfur Precursor. Chem. Asian J. 2012, 7, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Firouzabadi, H.; Iranpoor, N.; Gholinejad, M. One-Pot Thioetherification of Aryl Halides Using Thiourea and Alkyl Bromides Catalyzed by Copper(I) Iodide Free from Foul-Smelling Thiols in Wet Polyethylene Glycol (PEG 200). Adv. Synth. Catal. 2010, 352, 119–124. [Google Scholar] [CrossRef]
- Mondal, J.; Modak, A.; Dutta, A.; Basu, S.; Jha, S.N.; Bhattacharyya, D.; Bhaumik, A. One-pot thioetherification of aryl halides with thiourea and benzylbromide in water catalyzed by Cu-grafted furfural imine-functionalized mesoporous SBA-15. Chem. Commun. 2012, 48, 8000–8002. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yu, L.; Su, C.; Yang, Y.; Li, H.; Xu, Q. Efficient Generation of C-S Bonds via a By-Product-Promoted Selective Coupling of Alcohols, Organic Halides, and Thiourea. Adv. Synth. Catal. 2017, 359, 1649–1655. [Google Scholar] [CrossRef]
- Abbasi, M.; Khalifeh, R. One-pot odourless synthesis of thioesters via in situ generation of thiobenzoic acids using benzoic anhydrides and thiourea. Beilstein J. Org. Chem. 2015, 11, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.P.; Chou, Y.; Hou, D. Thioesterifications Free of Activating Agent and Thiol: A Three-Component Reaction of Carboxylic Acids, Thioureas, and Michael Acceptors. Adv. Synth. Catal. 2015, 357, 2644–2650. [Google Scholar] [CrossRef]
- Bezgubenko, L.V.; Pipko, S.E.; Sinitsa, A.D. Dichlorothiophosphoric acid and dichlorothiophosphate anion as thionating agents in the synthesis of thioamides. Russ. J. Gen. Chem. 2008, 78, 1341–1344. [Google Scholar] [CrossRef]
- Meltzer, R.I.; Lewis, A.D.; King, J.A. Antitubercular Substances. IV. Thioamides. J. Am. Chem. Soc. 1955, 77, 4062–4066. [Google Scholar] [CrossRef]
- Perregaard, J.; Lawesson, S.O. Studies on Organophosphoous Compounds. XI.* Oxidation of Aromatic Compounds with Sulfur in Hexamethylphosphoric Triamide (HMPA). A New Method for Preparation of N,N-Dimethylthiocarboxamides. Acta Chem. Scand. B 1975, 29, 604–608. [Google Scholar]
Sample Availability: Not available. |


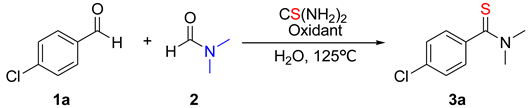
| Entry | Oxidant (Equiv) | Concentration of H2O (M) | Yield (%) b |
|---|---|---|---|
| 1 | BPO (2) | 14 | 58 |
| 2 | BQ (2) | 14 | 0 |
| 3 | DTBP (2) | 14 | <5 |
| 4 | TBHP (2) | 14 | 20 |
| 5 | K2S2O8 (2) | 14 | 69 |
| 6 | (NH4)2S2O8 (2) | 14 | 55 |
| 7 | K2S2O8 (2) | 42 | 0 c |
| 8 | K2S2O8 (2) | 8 | 65 d |
| 9 | K2S2O8 (2) | 0 | 0 e |
| 10 | K2S2O8 (3) | 14 | 68 |
| 11 | K2S2O8 (1.8) | 14 | 61 |
| 12 | K2S2O8 (2) | 14 | 54 f |
| 13 | K2S2O8 (2) | 14 | 80 g |
| Entry | R | Yield (%) of 3 b |
|---|---|---|
| 1 | 4-Cl | 63 (3a) |
| 2 | H | 52 (3b) |
| 3 | 4-CH3 | 55 (3c) |
| 4 | 3-Br | 48 (3h) |
| 5 | 3-CH3 | 51 (3i) |
| 6 | 4-Ph | 60 (3n) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, Y.; Qu, X.; Chen, Y.; Li, J.; Liu, L. K2S2O8-Promoted Aryl Thioamides Synthesis from Aryl Aldehydes Using Thiourea as the Sulfur Source. Molecules 2018, 23, 2225. https://doi.org/10.3390/molecules23092225
Bian Y, Qu X, Chen Y, Li J, Liu L. K2S2O8-Promoted Aryl Thioamides Synthesis from Aryl Aldehydes Using Thiourea as the Sulfur Source. Molecules. 2018; 23(9):2225. https://doi.org/10.3390/molecules23092225
Chicago/Turabian StyleBian, Yongjun, Xingyu Qu, Yongqiang Chen, Jun Li, and Leng Liu. 2018. "K2S2O8-Promoted Aryl Thioamides Synthesis from Aryl Aldehydes Using Thiourea as the Sulfur Source" Molecules 23, no. 9: 2225. https://doi.org/10.3390/molecules23092225
APA StyleBian, Y., Qu, X., Chen, Y., Li, J., & Liu, L. (2018). K2S2O8-Promoted Aryl Thioamides Synthesis from Aryl Aldehydes Using Thiourea as the Sulfur Source. Molecules, 23(9), 2225. https://doi.org/10.3390/molecules23092225