2.1. HILIC–ESI-FTMS Analysis of Phospholipids in Fresh Seafood Products
By analogy with previous investigations on mussel phospholipids [
17,
18,
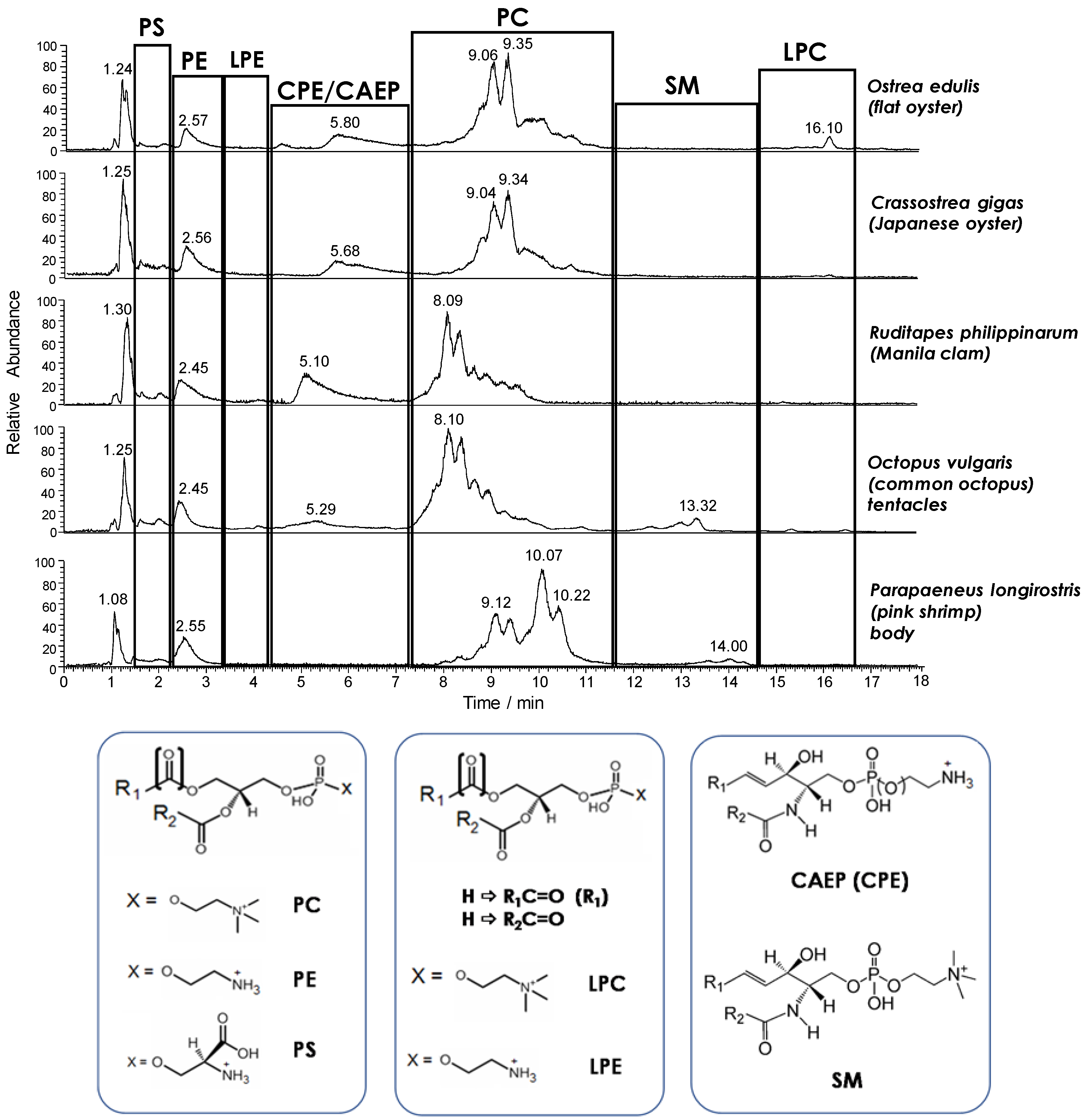
19], HILIC–ESI(+)-FTMS was adopted for the initial characterization of the main PL classes in the seafood products of interest, which were all examined as fresh products in the first step of the study. As expected, the total ion current (TIC) chromatogram resulting from HILIC separation provided a snapshot of the PL class distribution in each product (see
Figure 1). The retention time window of each PL class was easily inferred from searches on the LipidMaps database based on accurate
m/
z ratios retrieved from FTMS spectra averaged along the window, as described in the Materials and methods section. As emphasized in
Figure 1, besides glycerophospholipids such as PC, PE, and phosphatidylserines (PS), ceramide PLs were also detected. Notably, ceramide amino-ethyl-phosphonates (CAEP) and ceramide phosphoethanolamines (CPE) were only found in bivalve molluscs, like in the case of mussels [
17,
18,
19]. On the other hand, sphingomyelins (SM) were found in lipid extracts of shrimps, whereas all the three types of ceramide lipids were detected in the extracts of octopuses, thus marking a definite evolution of the ceramide lipid component in the marine organisms under study. A further important feature of lipid profiles compared in
Figure 1 was the generally low response observed for lysoPC (LPC) and lysoPE (LPE). This finding confirmed the very limited incidence of PL hydrolysis previously observed in fresh mussels [
18]. Last but not least, variations occurring between the profiles of bands related to the same PL class in different products could be inferred from HILIC–ESI(+)-FTMS traces like those of
Figure 1. The effect was very evident in the case of PC, representing the leading PL class in all seafood products, at least in terms of ESI-FTMS overall response. As apparent from
Figure 1, several distinguishable—although not completely resolved—peaks were always observed in the retention time interval related to PC, leading to characteristic chromatographic profiles. The PC profiles related to the two types of oysters under analysis were rather similar, as expected, with major peaks located between 8.5 and 9.5 min, whereas peaks eluting between 7.5 and 9 min prevailed in the case of clams and octopuses. It is worth noting that the TIC trace related to the lipid extract of octopus mantle, not shown in
Figure 1, was almost identical to the one related to the tentacle extract. The PC profile observed for shrimps was different from all the others, with two groups of peaks located around 9 and 10 min, respectively.
These variations, which clearly reflect the biological diversity of the examined products, could be evaluated in more detail from a molecular point of view by considering the ESI-FTMS spectra averaged in retention times intervals related to specific PL classes. In the present study, the attention was focused on the two main types of PL—PC and PE—and on their lyso forms, since, by analogy with mussels [
18], the latter were expected to be increased after at least part of the performed low temperature treatments. As an example, the ESI(+)-FTMS spectra retrieved for PC and LPC classes after the analysis of lipid extracts obtained from two of the seafood products under investigation—Manila clams and Japanese oysters (analyzed as fresh products, soon after purchase)—are reported in
Figure 2. Cross-comparisons of ESI(+)-FTMS spectra obtained for the four PL classes of interest—PC, LPC, PE, and LPE—from all seafood products under investigation have been reported in
Figures S1–S4 of the
Supplementary Materials, respectively.
The overall composition related to the side chain(s) characteristic of each species detected in a PL class, as indicated by the conventional C:D notation (where C represents the total number of carbon atoms of side chains linked to glycerol and D the total number of C=C bonds), could be obtained from spectra like those shown in
Figure 2 and
Figures S1–S4. In detail, accurate
m/
z ratios related to the M+0 isotopologue of each species could be retrieved and used as input for a search on the LipidMaps database after setting a ±0.005 mass tolerance. This tolerance is higher than the actual accuracy (around ±0.001 units) available on
m/
z ratios provided by the Q-Exactive spectrometer when operated under high mass resolution/accuracy conditions, as in the present case. Nonetheless, the choice of a higher tolerance was aimed at compensating the worsening of accuracy occurring for low S/N signals. In any case, signals related to M+0 isotopologues and having a relative abundance higher than 5% were labeled with the corresponding overall chain composition in
Figure 2 and
Figures S1–S4. In accordance with the generally accepted nomenclature, PL characterized by the presence of an alkyl chain—a side chain linked to glycerol through an ether, instead of an ester, bond—were labeled with the C:D notation preceded by o- or p-. The two letters were related to plasmanyl-PL (bearing C=C bonds on the alkyl chain but not in the proximity of the ether bond) and plasmenyl-PL (in which a C=C bond is certainly adjacent to the ether O atom), respectively, as exemplified in
Figure 2 for o-PC and p-PC. It is worth noting that, as a convention, the C=C bond adjacent to the ether O atom is not accounted for in the C:D notation for p-PL; thus, PLs labeled as o-C:D and p-C:(D-1) are isobaric. MS/MS can provide diagnostic information to distinguish such isobaric PLs (see, for example, Reference [
17]), yet this level of structural elucidation was beyond the goals of the present study. Consequently, both the possible assignments were reported in spectra of
Figure 2 and
Figures S1–S4 when ions with
m/
z ratios compatible with the presence of isobaric plasmanyl/plasmenyl species were detected in ESI(+)-FTMS spectra.
As apparent from
Figure 2, plasma(e)nyl PLs had a limited incidence, at least in terms of number of detected species, in the case of Manila clams (Ruditapes philippinarum) PC, which were dominated by diacylic species. This finding was confirmed in all the other seafood products, with only one further plasma(e)nyl-PC (o-34:1/p-34:0) detected in the lipid extract of octopus tentacle (see
Figure S1). Not surprisingly for marine organisms, most PC of the analyzed products (see
Figure 2 and
Figure S1) exhibited a remarkable degree of unsaturation in their side chains, with up to seven C=C bonds, whereas the abundance of saturated (i.e., 30:0, 31:0 and 38:0) or monounsaturated (i.e., 32:1, 33:1 and 34:1) species was generally low. With regard to the total number of carbon atoms, even values (the entire series from 30 to 42) prevailed, as expected; yet, odd values were not completely absent (see labels 31:0, 33:1, 39:5 and 39:6 in
Figure 2 and
Figure S1). Note that relative abundances observed in spectra related to a specific PL class—like those of
Figure 2 and
Figures S1–S4—can be reasonably considered as corresponding to relative concentrations. Indeed, a previous study performed on PL mixtures and based on HILIC–ESI-MS showed ESI yields for different PLs belonging to the same class to be similar due to the absence of competition for ionization with PLs of different classes and due to the almost-identical composition of the mobile phase in which they are eluted under HILIC conditions [
28]. Conceivably, the comparison between ESI(+)-FTMS spectra obtained for PC of different seafood products, reported in
Figure S1, also enabled an evaluation of similarities or differences between seafood products from a quantitative point of view. In particular, the PC spectral profiles obtained for the two types of oysters were very similar, with prevalence of diacylic species with compositions 36:5 and 38:6, followed by those with compositions 40:6 and 40:7. Clams exhibited a spectral profile for PC that was more similar to those obtained for octopus (both mantle and tentacles), with PC(38:6) still representing the leading species, but other PCs, such as those with compositions o-38:6/p-38:5 and 34:1, became more relevant, and PC(36:5) showed a lower incidence compared to oysters. Finally, the PC spectral profile of shrimps was peculiar, with PC having 34:1 and 32:1 compositions becoming major species. Based on the PC distribution similarities, the analyzed products could thus be divided into three general groups: (i) oysters (both species), (ii) clams and octopuses, and (iii) shrimps. Not surprisingly, this grouping was consistent with that enabled by a comparison of the chromatographic profiles obtained for PC (see
Figure 1).
In the case of each lysoPC, the composition of the single acyl or alkyl chain located on the molecular structure was easily inferred from a search on the LipidMaps database based on the accurate
m/
z ratio of the corresponding positive ion. The compositions of LPC detected in the lipid extracts of the analyzed products are reported in
Figure 2 and
Figure S2. Interestingly, the 16:0 chain was always prevailing, generally followed—in the case of oysters—by 18:0/18:1 and 20:1 chains and by the o-16:0 (in this case, no doubt exists on the chain identity due to the lack of C=C bonds) in the case of clams and octopuses (see
Figure S2). In fact, up to three plasmanyl LPC were found in octopuses lipid extracts, whereas the LPC profile of shrimps was rather different from all the others, with the contemporary presence of several plasmanyl PCs (o-14:0/o:15/o-16:0/o-18:0) and of the acylic LPC with 18:1 and 18:0 chain detected in oysters. The occurrence of LPCs with saturated or monounsaturated residual acyl or alkyl chains—despite the presence of many PCs with a high number of C=C bonds—suggested that PC hydrolysis occurring naturally in seafood products mainly involves the chain located on the sn
2 position of glycerol. Indeed, as emphasized by our recent study on mussels [
17], most PCs of marine organisms are expected to bear polyunsaturated chains on the sn
2 position.
The composition profiles of the PE class were quite different from those obtained for PCs; indeed, as emphasized in
Figure S3 and by analogy with the results obtained for mussels [
17], the PE class was dominated by plasmanyl/plasmenyl species and only a few diacylic species (with 36, 38, or 40 carbon atoms) were detected, although two of the latter (38:6 and 40:7) were the prevailing PEs in the case of shrimps. Interestingly, no saturated PE was detected—at least among signals exceeding the 5% abundance—and the number of C=C bonds could reach even 10 in the case of a PE detected in shrimp lipid extracts (40:10). Starting from this scenario, the prevalence of plasmanyl and plasmenyl species among LPE, inferred from
Figure S4, was not surprising; yet, an acyl LPE—the 22:6 one—was found to be relevant in the case of clams, shrimps, and octopuses (especially in the mantle). A comparison with the PE profiles (see
Figure S3) of these products suggested LPE(22:6) to be the hydrolysis byproduct of PEs with 38 or 40 carbon atoms, i.e., of PE in which the 22:6 chain was combined with a 16:0, 18:0, or 18:1 chain.
2.2. Effects of Thermal Treatments on Seafood Products: Evolution of LPE and LPC Species
By analogy with one of our previous studies concerning mussels [
18], the incidence of LPE and LPC—compared to their precursors, PE and PC—was exploited to evaluate if low temperature treatments, typically performed on the seafood products under study, could leave a mark on their PL profiles. For this aim, extracted ion current (XIC) chromatograms related to the four PL classes were first retrieved—both for fresh and for thermally treated products—by extracting from the HILIC–ESI(+)-FTMS TIC traces of the corresponding lipid extracts ion currents related to
m/
z intervals including all the species detected for each class, i.e., the
m/
z ranges of spectra shown in
Figure 2 and
Figures S1–S4. Afterwards, bands appearing in XIC traces in time intervals corresponding to those marked for PE, LPE, PC, and LPC in
Figure 1 were integrated, respectively, and the resulting areas were adopted as estimates of the overall ESI(+)-FTMS responses for each class. This assumption is reasonable as no interference due to PLs of other classes was observed in ESI-FTMS spectra like those shown in
Figure 2 and
Figures S1–S4. Finally, XIC band areas were used to calculate LPE/PE and LPC/PC response ratios. The mean values of these for different seafood products and different thermal treatments are reported in
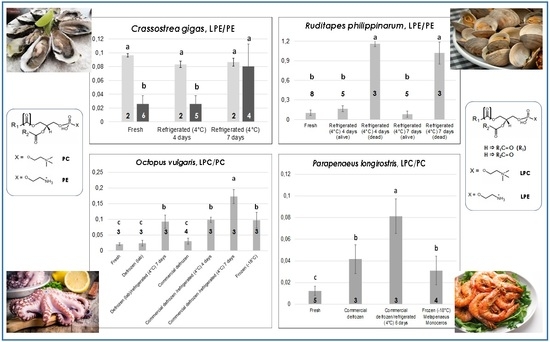
Figure 3,
Figure 4 and
Figure 5 and in
Figure S5 of the
Supplementary Materials, accompanied by error bars representing standard deviations and numbers of samples analyzed for each treatment. In each case, the Tukey–Kramer statistical test was applied to evaluate if significant differences (at a 95% confidence level) occurred between different types of treatments for the same product. Thus, the treatments could be grouped according to the literal annotation shown in the figures. Notably, ratios reported in
Figure 3,
Figure 4 and
Figure 5 and
Figure S5 could not be influenced by between-run signal fluctuations, which often occur when LC-ESI-MS is performed, because they arose from responses obtained during the same run. This feature could be easily appreciated by considering the usually low relative standard deviation observed for a specific ratio in a particular product/treatment despite the fact that replicated lipid extractions and analyses related to the same seafood product and thermal treatment were performed over a relatively long time range. It can thus be hypothesized that the more pronounced variabilities—often observed for LPL/PL ratios related to particularly severe thermal treatments—were not due to analytical fluctuations but due to the higher variability in the individual reaction to those treatments (vide infra).
Since thermal treatments were often diverse for different seafood products, data reported in
Figure 3,
Figure 4 and
Figure 5 and
Figure S5 will be described and discussed in three subsections. The first subsection will focus on the three types of bivalve mollusks under analysis as the same treatment (refrigeration at 4 °C) was performed on them. The second and third subsections will be dedicated to octopuses and shrimps, respectively.
2.2.1. Bivalve Molluscs: Oysters and Clams
In contrast to mussels, which can also be found on the market as glazed-frozen or defrozen products, oysters and clams are more valuable bivalves from a commercial point of view and are usually sold only as fresh (live) products. This feature sets severe transportation and storage constrains (see, for example, European Regulation 853/2004, Annexes VII and VIII), implying a constant refrigeration at a few °C (up to 4–6). For this reason, refrigeration at 4 °C (in a dry and clean container of a dedicated laboratory refrigerator) was applied to oysters and clams during our study. The process was initially prolonged for four days, a reasonable estimate of the maximum time that should be adopted for the storage of live bivalves at retail stores level. It was then purposely extended to seven days to simulate the effects of an inappropriate prolonged storage.
As shown in
Figure S5 of the
Supplementary Materials, prolonged refrigeration had a limited effect on oysters of
O. edulis sp., i.e., the European flat oyster, since both the LPE/PE and the LPC/PC ratios were slightly increased with refrigeration time, but the variabilities observed for mean values after four and seven days (especially for LPC/PC ratios) were high enough to make the increase not statistically significant. As anticipated before, the increase in variability can be interpreted as the different response of individual oysters to the same thermal treatment, a phenomenon that was also observed for the other analyzed bivalve mollusks (vide infra). Nevertheless, the limited extent of PE and PC hydrolysis occurring during refrigeration, inferred from data of
Figure S5, is consistent with previous studies dedicated to the low temperature tolerance of oysters of
O. edulis sp. [
29,
30]. These oysters were reported to survive for at least 11 weeks (even without external feeding like in our study) in seawater at 3 °C [
29].
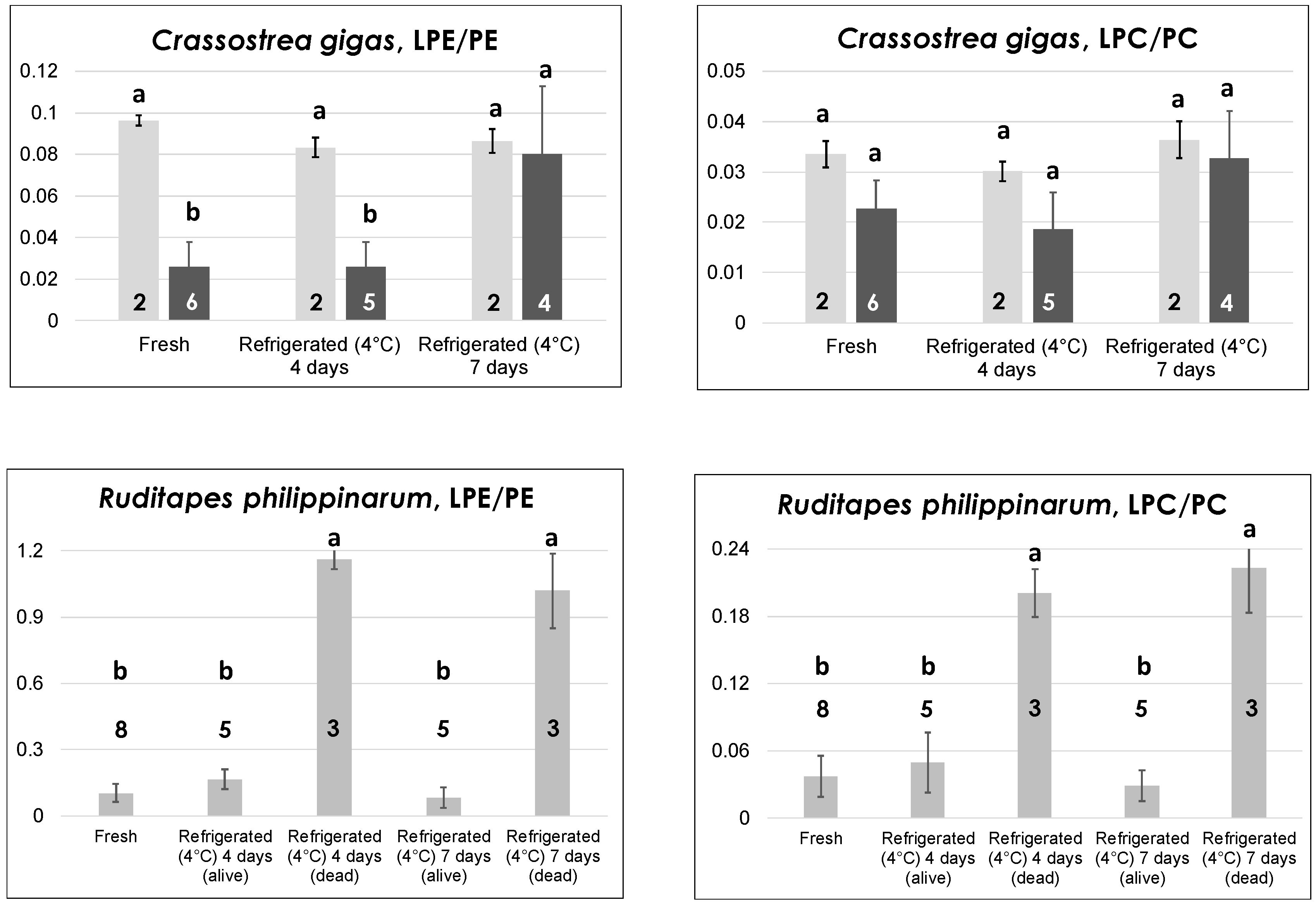
A different scenario was observed for the other oyster species studied in the present work—
Crassostrea gigas, i.e., the Japanese or Pacific oyster. Indeed, as shown by the darker bars in
Figure 3, while the LPC/PC ratio exhibited a behavior like that observed for
O. edulis, the LPE/PE ratio underwent a significant increase when passing from four to seven days of refrigeration at 4 °C. This result is in accordance with the generally lower capacity of
C. gigas to survive at low temperatures, with high mortalities (>95%) reported after 3–7 weeks at 3 °C [
29]. Interestingly, the sensitivity to thermal stress was greater for the LPE/PE ratio, in accordance with results previously obtained for mussels [
18]. The data represented by light grey bars in the two graphs dedicated to
C. gigas in
Figure 3 deserve specific discussion. They are related to oysters of this species belonging to two lots purchased in the month of July, characterized by LPE/PE ratios significantly higher than those observed for fresh oysters purchased in other months. As apparent, both the LPE/PE and the LPC/PC ratio seemed to be unresponsive to prolonged refrigeration for these oysters, probably because an anomalously pronounced PE and PC hydrolysis had already occurred during storage in the fishery shop before purchase. Note that both
C. gigas (originating from France) and
O. edulis (originating from Greece or Italy) oysters analyzed in the present work were stored either in a seawater-filled (not refrigerated container) or in a dry container put in a refrigerating bench at the moment of purchase. Not surprisingly, anomalous LPE/PE values were observed for fresh
C. gigas oysters stored in seawater purchased in July, the month that is usually characterized by the highest yearly temperatures. By analogy with data previously observed for mussels exposed to relatively high temperatures [
18], the effect could be due to the combination of two factors: (i) the relatively high temperature reached by the seawater in which those oysters were stored (instead of being kept dry and into a refrigerated bench as prescribed by the current European legislation); and (ii) the increase in seawater temperature already at the level of the aquaculture plant. The result, which showed how sensitive to environmental conditions LPL/PL ratios of bivalve mollusks can be, was likely emphasized by the low capacity of
C. gigas oysters to adapt to increasing temperatures [
31].
The evolution of both LPL/PL ratios in the case of clams of
R. philippinarum sp. (Manila clams) evidenced the presence of a more marked interindividual variability in the reaction to low temperature treatments compared to oysters. As shown in the bottom panels of
Figure 3, low LPE/PE and LPC/PC ratios were always observed—in eight different lots—for fresh clams. However, refrigeration at 4 °C led to a surprising outcome. In three of the five lots that were subjected to refrigeration, a partial mortality already occurred after four days, as evidenced by the irreversible opening of valves for dead clams. The latter were thus separated from still-alive ones and lipid extraction was carried out separately. Then, HILIC–ESI(+)-FTMS analysis was performed on the lipid extracts arising from the two subgroups. As a result, both LPL/PL ratios were remarkably increased in dead clams, in strict analogy with results observed on mussels refrigerated at 4 °C [
18]. Not surprisingly, a higher proportion of clams belonging to the three lots already displaying mortality after four days of refrigeration (but still alive after that time) were found dead when refrigerated for seven consecutive days at 4 °C. Nonetheless, their LPL/PL ratios were comparable to those of clams that were already dead after four days, whereas the few clams that were still alive after seven days of refrigeration exhibited ratios comparable to those of fresh ones (see
Figure 3). It is worth noting that all clam samples examined in this work were stored in a seawater-filled container at room temperature, not in a refrigerating bench, at the moment of purchase. Once again, the three lots interested by a partial premature death were purchased in summer (July or early September), whereas the remaining two lots involved in prolonged refrigeration, which did not evidence any mortality even after seven days of refrigeration, were acquired in spring. Consequently, although all clams appeared comparable in terms of LPL/PL ratios when purchased, the more pronounced thermal stress expected for those purchased in hotter months due to the lack of a correct storage appeared to influence their subsequent behavior upon refrigeration. Apart from the discovery of such subtle effects, the monitoring of LPL/PL ratios in the case of clams could be very useful to preserve consumer safety and unveil inappropriate commercial conducts if the target product was represented by deshelled clams, sometimes available in fishery shops (like mussels). Indeed, clams dead before being deshelled due to a too-long or inappropriate storage would be easily recognized from those still alive before their valves were removed.
2.2.2. Octopuses
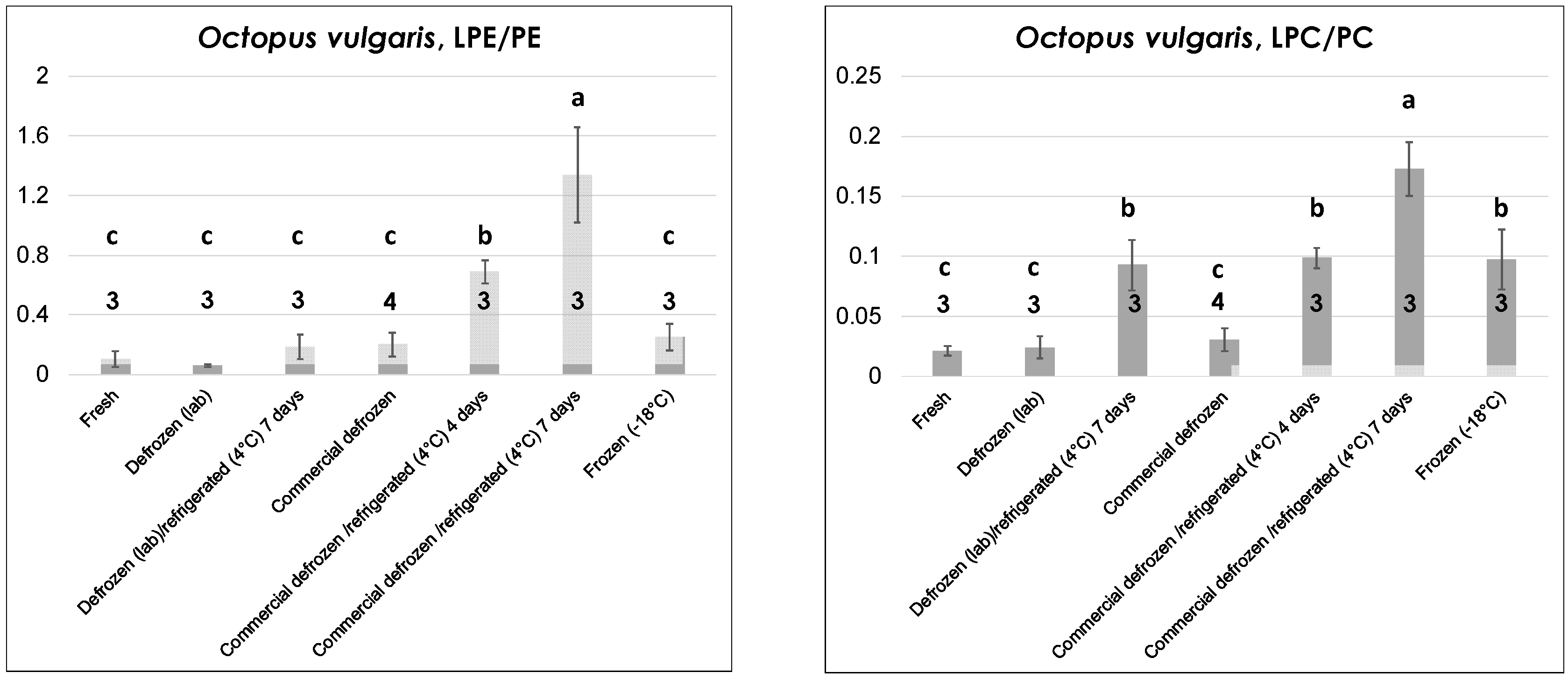
As emphasized by graphs of LPE/PE and LPC/PC ratios in
Figure 4, a higher number of thermal treatments had to be considered in the case of octopuses (all of
O. vulgaris sp.), to account for the greater variety of thermal histories expected for this seafood product compared to bivalve mollusks. Tentacles were considered for lipid extraction and analysis, although, as discussed before and as shown in
Figures S1–S4, the profiles of the four PL classes of interest were similar for the octopus’s mantle, which can also be used as edible part, once internal organs are removed. Two octopuses purchased in local fishery shops (where they were properly stored in a refrigerated bench) and one captured along the coasts of Apulia, 20 km north of Bari, and kept at 4 °C for ca. 24 h before being transferred to the laboratory were considered as fresh products. After the sampling of one tentacle for lipid extraction, each of the three octopuses was purposely frozen at −18 °C. Then, after thawing at 4 °C, a new tentacle was sampled and the lipid extraction/LC-MS analysis sequence was performed in each case. The resulting samples were considered as arising from “lab-made” defrozen octopuses. The octopus samples set was completed by defrozen and frozen (vacuum-sealed)
O. vulgaris octopuses obtained commercially and by lab-made or commercially defrozen octopuses purposely stored for up to seven days in the lab refrigerator at 4 °C.
Data reported in
Figure 4 indicate peculiar differences in the evolution of the LPE/PE and the LPC/PC ratios. Indeed, both were low and comparable in the case of fresh octopuses and defrozen ones (both lab-made and commercial) that were subjected to lipid extraction and analysis soon after preparation and without any further refrigeration. On the other hand, only the LPC/PC ratio was significantly increased in commercial frozen octopuses (compared to fresh or defrozen ones). This feature is very interesting as it suggests the possibility of distinguishing fresh octopuses from those claimed as fresh but obtained after thawing of frozen products, which represents a typical fraud. Lab-made defrozen octopuses stored for seven days at 4 °C (i.e., for an inappropriate time range) exhibited a significant increase of the LPC/PC ratio, whereas both LPL/PL ratios appeared sensitive to progressive refrigeration of commercially defrozen octopuses, with a steady increase occurring after four and seven days of refrigeration (see
Figure 4). Based on these considerations, octopuses represented the only product, among those under consideration in this study, for which the LPC/PC ratio provided more refined information than the LPE/PE in terms of distinction between different thermal treatments.
2.2.3. Shrimps
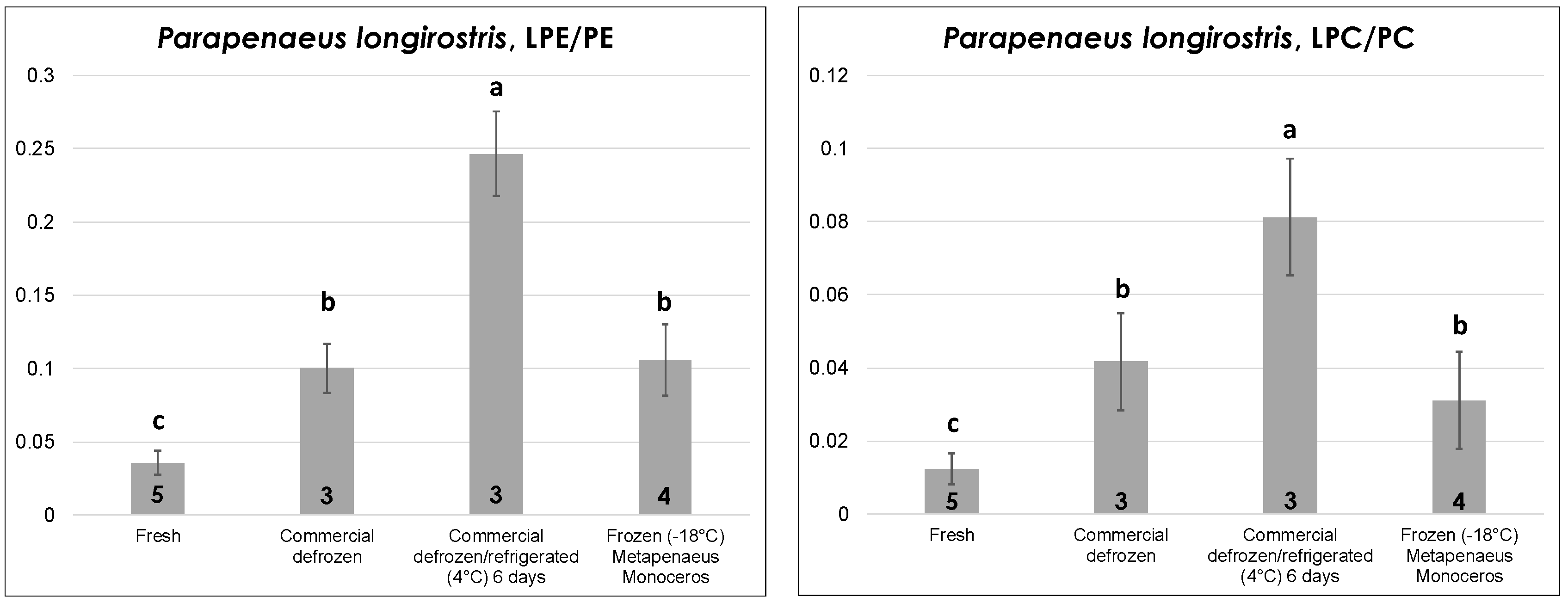
The edible part (i.e., the deshelled body) of fresh and commercial defrozen shrimps of
P. longirostris sp. (pink shrimp)—one of the leading shrimp species from a commercial point of view in the Mediterranean area—was subjected to lipid extraction and analysis during this study after purchase from local fisheries. Unfortunately, frozen shrimps of that species were not available locally; thus—for the sake of comparison—peeled, vacuum-sealed frozen shrimps of
Metapenaeus monoceros sp. (speckled/brown shrimp), which are native of Indian or Pacific Oceans and are intensively imported in Europe as frozen product, were examined. As shown in
Figure 5, the LPE/PE and LPC/PC ratios had a very similar trend with thermal history, with fresh shrimps displaying the lowest values, as expected. In contrast to octopuses, defrozen shrimps displayed significantly higher LPL/PL ratios than fresh ones, thus indicating a subtler correlation of this parameter to the thermal history in the case of shrimps. As observed before for fresh bivalves and for defrozen octopuses, prolonged refrigeration (up to six days) at 4 °C led to a significant increase in the LPL/PL ratio. Finally, frozen shrimps displayed LPL/PL ratios statistically comparable to those obtained for defrozen ones, which is reasonable given both products experienced a freezing/thawing sequence before lipid extraction and analysis.