Abstract
Benign prostatic hyperplasia (BPH) is the most common male clinical problem impacting the quality of life of older men. Clinical studies have indicated that the inhibition of α1A-/α1D adrenoceptors might offer effective therapy in lower urinary tract symptoms. Herein, a limited series of arylsulfonamide derivatives of (aryloxy)ethyl alicyclic amines was designed, synthesized, and biologically evaluated as potent α1-adrenoceptor antagonists with uroselective profile. Among them, compound 9 (3-chloro-2-fluoro-N-([1-(2-(2-(2,2,2-trifluoroethoxy)phenoxy]ethyl)piperidin-4-yl)methyl)benzenesulfonamide) behaved as an α1A-/α1D-adrenoceptor antagonist (Ki(α1) = 50 nM, EC50(α1A) = 0.8 nM, EC50(α1D) = 1.1 nM), displayed selectivity over α2-adrenoceptors (Ki(α2) = 858 nM), and a 5-fold functional preference over the α1B subtype. Compound 9 showed adequate metabolic stability in rat-liver microsome assay similar to the reference drug tamsulosin (Clint = 67 and 41 µL/min/mg, respectively). Compound 9 did not decrease systolic and diastolic blood pressure in normotensive anesthetized rats in the dose of 2 mg/kg, i.v. These data support development of uroselective agents in the group of arylsulfonamides of alicyclic amines with potential efficacy in the treatment of lower urinary tract symptoms associated to benign prostatic hyperplasia.
1. Introduction
α1-Adrenergic receptors (α1-ARs) belong to the G-protein-coupled receptor superfamily. They generally mediate their actions through Gq/11 proteins, which stimulate the activation of phospholipase C, via generation of the inositol triphosphate and diacylglycerol, liberation of calcium from the endoplasmic reticulum, and/or activation of genes. To date, three subtypes of α1-AR, i.e., α1A, α1B, and α1D have been identified in human tissues [1]. Although these subtypes display high structural homology, they differ in biological structure, tissue distributions, and pharmacological actions [2]. Several studies revealed that α1-AR subtypes are highly expressed in blood vessels—mainly α1B-ARs, in the urogenital area (prostate, urethra, bladder, ureter)—mainly α1A and α1D-ARs, and central nervous system [3]. α1-ARs play an important role in the pathogenesis of hypertension and benign prostatic hyperplasia (BPH) [4,5].
An increased α1-adrenergic prostate smooth muscle tone together with enhanced prostate volume are recognized causes of the disease [6]. BPH clinically manifests with lower urinary tract symptoms (LUTS) as storage (irritative) symptoms (nocturia, urgency, incontinence, altered bladder sensations, increased frequency) or obstructive (voiding) symptoms (hesitancy, slow stream, intermittency, splitting, straining, terminal dribble) [7]. Some of them commonly occur secondary to obstructive symptoms, and result from exaggerated, spontaneous detrusor contractions (known as bladder overactivity) [7,8]. BPH affects the majority of men with increasing frequency as they get older [9]. LUTS, if left untreated, result in significant impairment of quality of life and lead to long-term complications, such as recurrent urinary tract infections or renal insufficiency [10].
Despite several classes of BPH medications available, studies have shown that α1-adrenolitics are considered as the first-line drug treatment [11]. It has been suggested that enhanced, three-to-nine-fold greater expression of α1A- and α1D-ARs in an enlarged prostate and bladder neck, comparing to healthy tissue, remains in strong contribution with LUTS occurrence [12]. Consequently, an α1A- and α1D-AR blockade relieves obstructive and voiding symptoms by relaxation of the smooth muscle in the prostate and bladder detrusor, respectively [13].
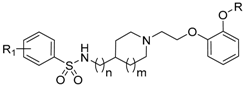
In contrast, a blockade of α1B-ARs, which are predominantly expressed in vascular smooth muscle [14], results in vasodilation of blood vessels leading to cardiovascular side effects, especially orthostatic hypotension [15]. The old α1-adrenolitics, bearing quinazoline scaffold, i.e., doxazosin or terazosin, display nonspecific interaction with all α1-AR subtypes [5]. On the other hand, naftopidil, tamsulosin, and silodosin (Figure 1), displaying relatively high α1A- and α1D-AR subtype selectivity, effectively relieve symptoms related to BHP/LUTS disease without undesirable side effects on blood pressure [16,17,18].
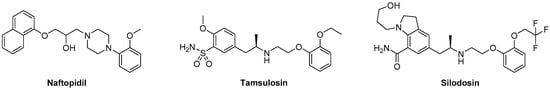
Figure 1.
Chemical structures of selective α1A- and α1D-AR antagonists.
Integrating a concept of arylpiperazine biomimetics recently adapted for development of selective and potent 5-HT7R antagonists [19], we explored the common chemical space with tamsulosin to propose modifications leading to increased α1A-AR properties. Associating arylsulfonamide and aryloxylalkyl fragments identified compound I, which behaved as an α1A-AR antagonist and displayed a moderate selectivity receptor profile over α1B-AR subtype [20]. In an attempt to further increase the uroselective profile, a limited series of compounds integrating silodosin-derived chemical scaffold was designed (Figure 2). Selection of the central amine core (4-aminomethyl-piperidine and 3-amino-pyrrolidine), as well as a kind of substituent at the arylsulfonamide moiety, was based on our previously reported data presenting their preference for α1A-AR over 5-HT1A, and 5-HT7R [20,21,22].
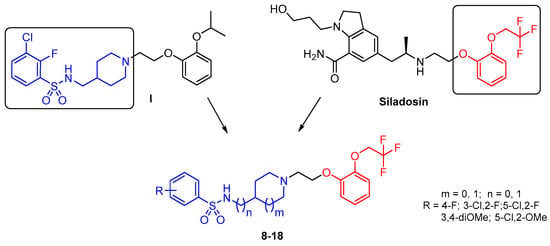
Figure 2.
Design strategy for arylsulfonamide derivatives of alicyclic amines as silodosin analogs.
All the synthesized derivatives were in vitro evaluated to assess their affinity for α1-AR and selectivity over α2-AR subtypes. Then, antagonist properties of selected derivatives against α1A-, α1B-, and α1D-AR subtypes were determined in cellular functional assays. The most representative compounds with uroselective functional profile were submitted under extended in vitro screening towards off-targets responsible for potential side effects, and were evaluated in metabolic stability in in vitro assay to assess their susceptibility to biotransformation. Finally, selected compounds were administered to normotensive anesthetized rats to evaluate their effects on blood pressure as a measure of their potential in vivo uroselectivity and to exclude hypotensive effects unfavorable to the treatment of lower urinary tract symptoms associated to benign prostatic hyperplasia.
2. Chemistry
The multistep protocol for synthesis of compounds 8–18 in outlined in Scheme 1 and Scheme 2. Initially, (2,2,2-trifluoroethoxy)phenol (3) was synthesized by alkylation of commercially available guaiacol 1, followed by demethylation of intermediate 2 in the presence of boron tribromide (Scheme 1).
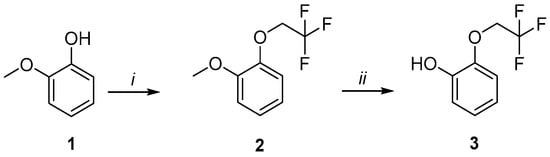
Scheme 1.
Synthesis of phenol 3. Reagents and conditions: (i) 2-iodo-1,1,1-trifluoroethane, K2CO3, KI, DMF, 90 °C, 24 h; (ii) BBr3, CH2Cl2 anh, 0 °C → r.t., 2 h.
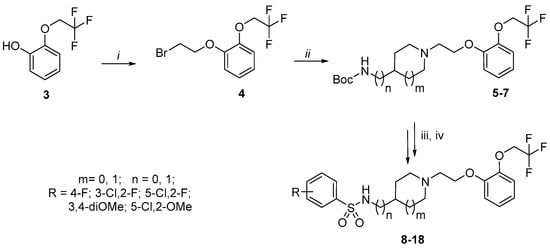
Scheme 2.
Synthesis of silodosin analogs 8–18. Reagents and conditions: (i) 1,2-dibromoethane, K2CO3, KI, (CH3)2CO, 60 °C, 48 h; (ii) alicyclic amine, K2CO3, KI, (CH3)2CO, 60 °C, 24 h; (iii) TFA/CH2Cl2 (80/20; v/v), r.t., 2 h; (iv) arylsulfonyl chloride, TEA, CH2Cl2, 0 °C, 2–6 h.
Next, the alkylation of phenol 3 under biphasic conditions yielded the corresponding (aryloxy)ethyl bromide 4. Subsequently, this alkylating agent reacted with selected Boc-protected alicyclic amines (4-aminomethyl-piperidine, R-3-amino-pyrrolidine, and S-3-amino-pyrrolidine), giving intermediates 5–7. Removal of the protecting group, followed by the treatment with selected arylsulfonyl chloride, yielded final arylsulfonamide derivatives 8–18.
3. In Vitro Experiments
3.1. Radioligand Binding and Functional Evaluation
The pharmacological profile of the new compounds was assessed in radioligand-binding assays as the ability to displace [3H]-Prazosin or [3H]-Clonidine from α1- and α2-ARs, respectively, on rat cerebral cortex [23]. The inhibition constants (Ki) were calculated from the Cheng-Prusoff equation [24].
The intrinsic activity at α1A-ARs of the selected compounds was assessed by fluorescence detection (Invitrogen, Thermo Fisher Scientific, Carlsbad, CA, USA) of β-lactamase reporter genes using a FRET-enabled substrate. The intrinsic activity at α1B-ARs and α1D-ARs was determined by luminescence detection (PerkinElmer, Zaventem, Belgium) of calcium mobilization using the recombinant-expressed jellyfish photoprotein (Aequorin).
The most representative compounds, 9 and 10, with the highest functional selectivity were further tested to determine the affinity for 5-HT1A and 5-HT7Rs in screening radioligand-binding studies using [3H]-8-Hydroxy-2-(dipropylamino)tetralin ([3H]-8-OH-DPAT) and [3H]-Lysergic acid diethylamide ([3H]-LSD), respectively. Experiments were performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A and 5-HT7Rs according to the methods previously described [25].
Finally, the percentage of inhibition for selected compounds 9 and 10 for off-target histaminic H1R, muscarinic M1R, adrenergic β1-AR and potassium ion channel hERG were assessed at Eurofins (Celle-Lévescault, France) according to the procedure online at www.eurofins.com [26].
3.2. Metabolic Stability
In vitro biotransformation assays of selected compounds 9 and 10 were performed using rat-liver microsomes (RLM), potassium-phosphate buffer, NADPH-regenerating system (NADP, glucose-6-phosphate, glucose-6-phosphate dehydrogenase), and levallorphan as internal standard, according to the previously published procedure [27]. Compound I and the drug tamsulosin were used as reference standard. UPLC/MS analysis (Waters Corporation, Milford, MA, USA) was performed to determine the quantity of the starting material left in solution. The in vitro half-time (t1/2) for test compounds was determined from the slope of the linear regression of ln % parent compound remaining versus incubation time. The calculated t1/2 was incorporated into the following equation to obtain intrinsic clearance: (Clint) = (volume of incubation [µL]/protein in the incubation [mg]) × 0.693/t1/2.
4. In Vivo Pharmacology
Compounds 9 and 10, which displayed the highest α1B/α1A selectivity profile, were selected for in vivo evaluation to determine their influence on blood pressure of normotensive anaesthetized rats after acute administration at single dose of 2 mg/kg (i.v.). The experiments were performed to our previously reported method.
5. Results and Discussion
All synthesized compounds were in vitro evaluated in binding assays for their affinity for α1-AR and selectivity over α2-AR subtype. Compounds showed high-to-moderate affinity for α1-ARs (Ki = 19–171 nM), and low-to-moderate selectivity over α2-AR subtype (Table 1). Analysis of the influence of substituent in position-2 at the aryloxy fragment showed that an increase of its volume by replacing the isopropoxy group present in compound I and II with the 2,2,2-trifluoroethoxy one (present in a new series) only slightly increased the affinity for α1-ARs (I vs. 9, II vs. 16).

Table 1.
The biological data of compounds 8–18 for adrenergic α1- and α2-receptors.
In line with our previous results [20], the 4-aminomethylpiperidine core was more favorable for the binding at α1-AR than 3-aminopyrrolidine one (8 vs. 13 and 14, 12 vs. 17 and 18). Although among compounds with 3-aminopyrrolidine no stereochemical preference towards α1-AR was observed, the S enantiomers showed higher selectivity over α2-AR than their R counterparts (13 vs. 14, 15 vs. 16, 17 vs. 18).
Further modifications involved the introduction of different electron-withdrawing or electron-donating substituents at the phenyl ring of sulfonamide moiety. A fluorine atom in 4-position was sufficient for obtaining a potent α1-AR ligand 8 (Ki = 20 nM) among the 4-aminomethyl-piperidine subset, but did not significantly improve the affinity of pyrrolidine derivatives 13 and 14 for α1-AR (Ki = 188 and 134 nM, respectively). Interestingly, the presence of the 4-F substituent in both series led to derivatives with the highest selectivity over the α2-AR subtype (Sα2/α1 ≥ 46). An introduction of two halogen substituents did not affect the affinity for α1-AR while decreasing the selectivity over the α2-AR subtype (8 vs. 9 and 10, 13 vs. 15, and 14 vs. 16). Replacing one of the halogen substituents (e.g., 2-F) in compound 10, with an electron-donating substituent as the 2-methoxy, up to 4-fold reduced both affinity for α1-AR and selectivity over α2-AR (10 vs. 11). Finally, compounds 12, 17, and 18, with two methoxy groups in 3,4-position at the phenyl ring of sulfonamide moiety, showed higher affinity for α1-AR than the 4-F direct analogs (8 vs. 12, 13 vs. 17, and 14 vs. 18); however, this modification decreased the selectivity over α2-AR subtype. Selected compounds with the highest affinity for α1-ARs (Ki ≤ 50 nM) and selectivity ratio, which equals >15-fold over α2-AR subtype, behaved as potent antagonists at α1A-, α1B-, and α1D-ARs in in-vitro functional tests (Table 2). Compounds 8, 9, and 10 were classified as more potent antagonists than previously reported compound I at all tested α1A-, α1B-, and α1D-ARs. Compounds 9 and 10, bearing two halogen atoms in ortho and meta position (i.e., 3-Cl,2-F and 5-Cl,2-F) at the phenyl ring of sulfonamide moiety displayed the highest α1B/α1A selectivity ratio. An introduction of the strong electron-donating substituent in meta and para position (e.g., 3,4-diOMe), switched the functional-selectivity profile of compound 12, which behaved as a selective α1B-AR antagonist (IC50 = 0.022 nM).

Table 2.
The functional activity of selected derivatives and reference drugs for α1A-, α1B-, and α1D-ARs.
It is well known that a blockade of α1A- and α1D-ARs relaxes the enhanced prostate and bladder detrusor smooth muscle tone, whereas α1B-AR antagonism is involved in blood-pressure regulation. Normal detrusor, obtained from surgical patients, expresses predominantly α1D-ARs. Some pharmacological experiments revealed that highly selective α1A-AR antagonists are effective in relaxing prostate smooth muscle and therefore improving urine flow in men in this area. However, relaxation of smooth muscle of the prostate alone does not alter reported LUTS scores in men with BPH. Reduction of these symptoms is reported only when pharmacotherapy includes drugs with both α1A- and α1D-AR antagonistic activity. Such activity improves bladder-based symptoms in humans and is used in LUTS pharmacotherapy [13]. Compounds 8, 9, 10, and 12 in the intrinsic activity studies showed strong antagonistic properties against the α1D-AR subtype with EC50 in the range of 1.1 to 2.7 nM. However, among the tested compounds, only 9 and 10 showed a similar inhibitory effect on intrinsic signal transduction in cells with stable expression of human α1A- and α1D-ARs.
Some pieces of evidence suggest an involvement of serotonin 5-HT1A and 5-HT7Rs in regulation of rodent bladder and urethral-sphincter contractions in both in in vitro and in vivo models [28,29]. Thus, 5-HT1A and 5-HT7R ligands may be regarded as adjunctive agents in alleviating LUTS associated to BPH. Compounds 9 and 10 displayed high-to-moderate affinity for 5-HT1A and 5-HT7Rs (Table 3).

Table 3.
The binding data of selective compounds for 5-HT1A and 5-HT7Rs.
The same compounds were further evaluated for their affinity for “off-target” receptor panels at Eurofins Cerep, and displayed weak affinity for histamine H1, muscarinic M1, adrenergic β1, and hERG channels (<50% @ 1 μM) [26]. These may suggest a low risk of compounds to evoke undesirable cardiovascular or CNS side effects. An initial assessment of the metabolic fate in liver was subsequently performed in an in-vitro RLM model. Compounds 9 and 10 showed relatively low clearances (Clint = 67 and 91.7 μL/min/mg, respectively, Table 4), with values similar to those of reference compound I and the drug tamsulosin (Clint = 87 and 41 μL/min/mg, respectively). The values of internal clearance calculated for the tested compounds are in line with the value of clearance of reference drugs (i.e., propranolol, verapamil) reported in the literature [30,31].

Table 4.
Metabolic stability of compounds I, 9, 10 and reference drug tamsulosin.
Identified compounds 9 and 10 with favorable α1B/α1D profile and acceptable metabolic stability were selected for in vivo tests to evaluate their influence on blood pressure. Hypotensive activity was determined after one time i.v. administration to normotensive anaesthetized rats at single doses of 2 mg/kg according to our previously reported method [32].
Compound 9 given at the dose of 2 mg/kg decreased SBP about 5.9–10.9 mmHg (5.1–9.4%), and DBP about 2–5.3 mmHg (2.4–6.5%) insignificantly.
Compound 10 in a dose of 2 mg/kg reduced both, SBP about 7–18 mmHg (5.8–15.1%), and DBP about 7–16.4 mmHg (7.8–18.3%). A statistically significant drop in systolic blood pressure was observed from 30 min after administration (Figure 3 and Figure 4).
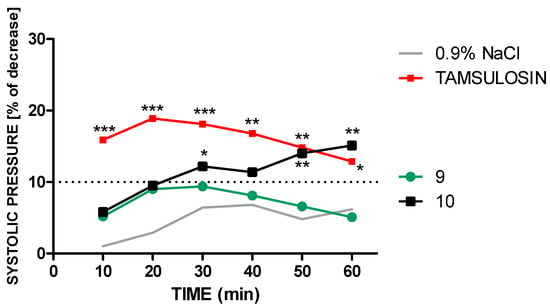
Figure 3.
An influence of tamsulosin and compounds 9 and 10, given in a dose of 2 mg/kg (i.v.), on systolic pressure in anaesthetized rats. Significant to control group (0.9% NaCl): * p < 0.05, ** p < 0.02, *** p < 0.01.
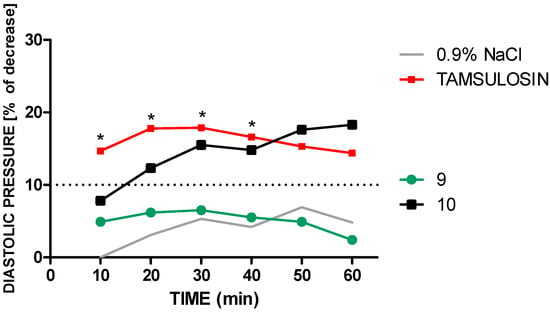
Figure 4.
An influence of tamsulosin and compounds 9 and 10, given in a dose of 2 mg/kg (i.v.), on diastolic pressure in anaesthetized rats. Significant to control group (0.9% NaCl): * p < 0.05.
In contrast, the highly α1A-AR-selective drug tamsulosin administered intravenously at a dose of 2 mg/kg decreased SBP 16.2–23.7 mmHg (12.9–18.9%) and the DBP about 13.3–16.6 mmHg (14.4–17.9%) significantly through whole period of observation (Figure 3 and Figure 4).
It thus seems that compound 9 revealed a potential uroselective profile, comparable to tamsulosin, without evoking hypotension as a side effect. These data warrant further investigation of compound 9 in ex vivo preclinical models of BPH disease.
6. Conclusions
By combining the 2-(2,2,2-trifluoroethoxy)phenoxy fragment of silodosin with an alicyclic amine core functionalized with arylsulfonamide moiety, derived from previously reported compound I, we designed and synthesized a new series of arylsulfonamides of (aryloxy)ethyl pyrrolidines and piperidines as α1-AR antagonists. Structure–activity relationship studies revealed that the 4-aminomethylpiperidine core was preferential for binding with the α1-AR over the 3-aminopyrrolidine analog. Additionally, a kind of substituent at the phenyl ring of sulfonamide significantly impacted the selectivity of evaluated compounds over α1B- and α2-AR subtypes. The study allowed the identification of compound 9 as a potent and metabolically stable α1A-AR antagonist with improved α1B/α1A selectivity ratio, comparing with previously reported series. Moreover, compound 9 showed α1D-AR antagonistic activity that may be beneficial in terms of LUTS therapy. In contrast to the reference drug tamsulosin, the tested compound did not decrease blood-pressure parameters after acute administration at the dose of 2 mg/kg (i.v.) in rats. Preliminary data for compound 9 are promising enough to warrant its further detailed mechanistic studies as a potential uroselective α1A- and α1D-AR antagonist in the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia.
7. Experimental
7.1. Chemistry
7.1.1. General Chemical Methods
Organic transformations were carried out at ambient temperature unless indicated otherwise. Organic solvents (Sigma-Aldrich, Merck Group, Darmstadt, Germany) used in this study were of reagent grade and were used without purification. All other commercially available reagents were of the highest purity (Sigma-Aldrich). All workup and purification procedures were carried out with reagent-grade solvents under ambient atmosphere.
Mass spectra were recorded on a UPLC-MS/MS system consisted of a Waters ACQUITY® UPLC® (Waters Corporation, Milford, MA, USA) coupled to a Waters TQD mass spectrometer (electrospray ionization mode ESI-tandem quadrupole). Chromatographic separations were carried out using the Acquity UPLC BEH (bridged ethyl hybrid) C18 column; 2.1 × 100 mm, and 1.7 µm particle size, equipped with Acquity UPLC BEH C18 VanGuard precolumn (Waters Corporation, Milford, MA, USA); 2.1 × 5 mm, and 1.7 µm particle size. The column was maintained at 40 °C, and eluted under gradient conditions from 95% to 0% of eluent A over 10 min, at a flow rate of 0.3 mL∙min−1. Eluent A: water/formic acid (0.1%, v/v); eluent B: acetonitrile/formic acid (0.1%, v/v). Chromatograms were made using Waters eλ PDA detector. Spectra were analyzed in the 200–700 nm range with 1.2 nm resolution and sampling rate 20 points/s. MS detection settings of Waters TQD mass spectrometer were as follows: source temperature 150 °C, desolvation temperature 350 °C, desolvation gas flow rate 600 L∙h−1, cone gas flow 100 L∙h−1, capillary potential 3.00 kV, cone potential 40 V. Nitrogen was used for both nebulizing and drying gas. The data were obtained in a scan mode ranging from 50 to 1000 m/z in time 0.5 s intervals. Data acquisition software was MassLynx V 4.1 (Waters Corporation, Milford, MA, USA). The UPLC/MS purity of all the final compounds was confirmed to be 95% or higher.
1H-NMR and 13C-NMR spectra were obtained in Varian BB 300 spectrometer (Varian, Palo Alto, CA, USA) in CDCl3 or d6-DMSO, and were recorded at 300 and 75 MHz, respectively. The J values are reported in Hertz (Hz), and the splitting patterns are designated as follows: s (singlet), br.s. (broad singlet), d (doublet), t (triplet), q (quartet), dd (doublet of doublets), dt (doublet of triplets), td (triplet of doublets), ddd (doublet of doublet of doublets), m (multiplet).
Elemental analyses for C, H, N and S were carried out using the elemental Vario EL III Elemental Analyser (Elementar Analysensysteme GmbH, Hanau, Germany). All values are given as percentages, and were within ±0.4% of the calculated values.
Melting points (mp) were determined with a Büchi apparatus (Flawil, Switzerland) and are uncorrected.
The general procedures used for the synthesis of intermediate and final compounds were in accordance with previously reported methodology [20].
Spectroscopic data (MS, 1H-NMR and 13C-NMR spectra) for representative final compounds are presented in Supplementary Materials.
7.1.2. Preparation of 1-Methoxy-2-(2,2,2-trifluoroethoxy)benzene (2)
2-Methoxy-phenol 1 (5.19 g, 0.04 mol) was dissolved in DMF (25 mL), after addition of K2CO3, (16.6 g, 0.12 mol) a mixture that was heated to 90 °C. Then 2-iodo-1,1,1-trifluoroethane (4.2 mL, 0.05 mol) was added dropwise in 30 min. The reaction mixture was then heated under reflux for 24 h. Inorganic residues were filtered off and organic mixture was concentrated under reduced pressure. The obtained crude product was purified using silica gel with AcOEt/Hexane (1/9, v/v) as an eluting system (isolated yield 65%). Yellow oil (5.6 g); UPLC/MS purity 99%, tR = 6.52. C9H9F3O2, MW 206.16, Monoisotopic Mass 206.06, [M + H]+ 207.1. 1H-NMR (300 MHz, CDCl3) δ 3.91 (s, 3H, O–CH3), 4.36 (q, J = 8.4 Hz, 2H, O–CH2–CF3), 6.81–7.11 (m, 4H, Ar–H). 13C-NMR (75 MHz, CDCl3) δ 56.4, 68.3, 119.0, 121.5, 122.3, 123.8, 126.1, 127.2, 138.9.
7.1.3. Preparation of 2-(2,2,2-Trifluoroethoxy)phenol (3)
A 1 M solution of boron tribromide (30 mL, 0.03 mol) in CH2Cl2 was added to a solution of intermediate 2 (4.3 g, 0.02 mol) in anhydrous CH2Cl2 (50 mL) at −20 °C. The reaction mixture was warmed to room temperature and, after, quenched by addition of excess saturated aqueous sodium bicarbonate solution (40 mL). The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude material was sufficiently pure to be used directly in the next step (yield 98%). Yellow oil (3.8 g); UPLC/MS purity 99%, tR = 5.37. C8H7F3O2, MW 192.14, Monoisotopic Mass 192.04, [M + H]− 191.0. 1H-NMR (300 MHz, CDCl3) δ 4.36 (q, J = 8.4 Hz, 2H, O–CH2–CF3), 6.81–7.11 (m, 4H, Ar–H), 9.72 (br.s., 1H, O–H). 13C-NMR (75 MHz, CDCl3) δ 56.2, 68.1, 119.2, 121.5, 122.4, 123.7, 126.4, 127.3, 136.4
7.1.4. Preparation of 1-(2-Bromoethoxy)-2-(2,2,2-trifluoroethoxy)benzene (4)
Phenol 3 (4.8 g, 0.025 mol) was dissolved in acetone (30 mL). Then K2CO3 (10.4 g, 0.075 mol) and catalytic amount of KI (0.08 g, 0.0005 mol) were added, followed by dropwise addition of 1,2-dibromoethane (12.9 mL, 0.15 mol). The reaction was refluxed for 48 h. Inorganic residues were filtered off and organic mixture was concentrated under reduced pressure. The obtained crude product was purified using silica gel with AcOEt/Hexane (0.5/9, v/v) as an eluting system (isolated yield 75%). Yellow oil (5.61 g); UPLC/MS purity 97%, tR = 7.41. C10H10BrF3O2, MW 299.09, Monoisotopic Mass 297.98, [M + H]+ 300.2. 1H-NMR (300 MHz, CDCl3) δ 3.45 (t, J = 6.5 Hz, 2H, N–CH2–CH2), 4.29 (t, J = 6.5 Hz, 2H, O–CH2–CH2), 4.36 (q, J = 8.4 Hz, 2H, O–CH2–CF3), 6.82 (dd, J = 5.2, 1.0 Hz, 1H, Ar–H), 6.99 (td, J = 7.6, 1.1 Hz, 1H, Ar–H), 7.14 (td, J = 8.0, 1.8 Hz, 1H, Ar–H), 7.24–7.28 (m, 1H, Ar–H). 13C-NMR (75 MHz, CDCl3) δ 56.2, 64.1, 65.8, 68.1, 119.3, 121.5, 122.4, 123.7, 126.4, 127.3, 136.4.
7.1.5. General Procedure for the Alkylation of Boc-Protected Amines (5–7)
Commercial Boc-protected amines (1 eq) were dissolved in acetone (15 mL). Then, K2CO3 (3 eq) and a catalytic amount of KI (0.02 eq) were added, followed by dropwise addition of (aryloxy)ethyl bromide 4 (1.2 eq) in 30 min. The reaction was heated under reflux for 48 h. Inorganic residues were filtered off and organic mixture was concentrated under reduced pressure. The obtained crude products were purified according to the methods described below (isolated yields 68–75%).
tert-Butyl ((1-(2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl)piperidin-4-yl)methyl)carbamate (5)
Compound 5 was prepared using 4-Boc-aminomethyl-piperidine (1.1 g, 5.8 mmol), K2CO3 (2.4 g, 17.4 mmol), KI (0.02 g, 0.12 mmol) and (aryloxy)ethyl bromide 4 (2.1 g, 6.96 mmol). Yellow oil 2.06 g, (isolated yield 68%), following chromatographic purification over silica gel with CH2Cl2/MeOH (9/0.7, v/v); UPLC/MS purity 97%, tR = 4.57. C21H31F3N2O4, MW 432.48, Monoisotopic Mass 432.22, [M + H]+ 433.5. 1H-NMR (300 MHz, CDCl3) δ 1.08–1.20 (m, 2H), 1.34–1.42 (m, 1H, piperidine), 1.45 (s, 9H, (CH3)3–C), 1.56–1.60 (m, 2H, piperidine), 1.92–1.99 (m, 2H, piperidine), 2.79–2.88 (m, 4H, piperidine), 3.45 (t, J = 6.5 Hz, 2H, N–CH2–CH2), 4.29 (t, J = 6.5 Hz, 2H, O–CH2–CH2), 4.36 (q, J = 8.4 Hz, 2H, O–CH2–CF3), 4.52 (br.s, 1H, SO2–NH–CH2), 6.82 (dd, J = 5.2, 1.0 Hz, 1H, Ar–H), 6.99 (td, J = 7.6, 1.1 Hz, 1H, Ar–H), 7.14 (td, J = 8.0, 1.8 Hz, 1H, Ar–H), 7.24–7.28 (m, 1H, Ar–H). 13C-NMR (75 MHz, CDCl3) δ 27.0, 28.3, 34.0, 47.6, 52.6, 55.4, 64.1, 65.8, 66.3, 79.2, 120.7, 122.1,122.7, 123.4, 126.3, 128.9, 135.6, 155.3.
tert-Butyl (R)-(1-{2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl}pyrrolidin-3-yl)carbamate (6)
Compound 6 was prepared using (R)-3-Boc-amino-pyrrolidine (0.75 g, 4.03 mmol), K2CO3 (1.67 g, 12.09 mmol), KI (0.01 g, 0.08 mmol), and (aryloxy)ethyl bromide 4 (1.5 g, 4.84 mmol). Yellow oil 1.17 g, (isolated yield 72%), following chromatographic purification over silica gel with CH2Cl2/MeOH (9/0.7 v/v); UPLC/MS purity 97%, tR = 4.72. C19H27F3N2O4, MW 404.43, Monoisotopic Mass 404.19, [M + H]+ 405.1. 1H-NMR (300 MHz, CDCl3) δ 1.43 (2, 9H, (CH3)3–C), 1.51–1.64 (m, 1H, pyrrolidine), 2.04–2.18 (m, 1H, piperidine), 2.33 (td, J = 8.91, 7.16 Hz, 1H, piperidine), 2.71–2.92 (m, 4H, piperidine), 3.45 (t, J = 6.5 Hz, 2H, N–CH2–CH2), 3.79 (br.s, 1H, SO2–NH–CH), 4.29 (t, J = 6.5 Hz, 2H, O–CH2–CH2), 4.36 (q, J = 8.4 Hz, 2H, O–CH2–CF3), 6.82 (dd, J = 5.2, 1.0 Hz, 1H, Ar–H), 6.99 (td, J = 7.6, 1.1 Hz, 1H, Ar–H), 7.14 (td, J = 8.0, 1.8 Hz, 1H, Ar–H), 7.24–7.28 (m, 1H, Ar–H). 13C-NMR (75 MHz, CDCl3) δ 28.3, 29.0, 32.5, 52.7, 54.0, 60.8, 64.1, 65.8, 66.3, 79.2, 120.7, 122.1,122.7, 123.4, 126.3, 128.9, 135.6, 156.1.
tert-Butyl (S)-(1-(2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl)pyrrolidin-3-yl)carbamate (7)
Compound 7 was prepared using (S)-3-Boc-amino-pyrrolidine (0.75 g, 4.03 mmol), K2CO3 (1.67 g, 12.09 mmol), KI (0.01 g, 0.08 mmol), and (aryloxy)ethyl bromide 4 (1.5 g, 4.84 mmol). Yellow oil 1.22 g, (isolated yield 75%), following chromatographic purification over silica gel with CH2Cl2/MeOH (9/0.7 v/v); UPLC/MS purity 98%, tR = 4.84. C19H27F3N2O4, MW 404.43, Monoisotopic Mass 404.19, [M + H]+ 405.3. 1H-NMR (300 MHz, CDCl3) δ 1.43 (2, 9H, (CH3)3–C), 1.51–1.64 (m, 1H, pyrrolidine), 2.04–2.18 (m, 1H, pyrrolidine), 2.33 (td, J = 8.91, 7.16 Hz, 1H, pyrrolidine), 2.71–2.92 (m, 4H, pyrrolidine), 3.45 (t, J = 6.5 Hz, 2H, N–CH2–CH2), 3.79 (br.s, 1H, SO2–NH–CH), 4.29 (t, J = 6.5 Hz, 2H, O–CH2–CH2), 4.36 (q, J = 8.4 Hz, 2H, O–CH2–CF3), 6.82 (dd, J = 5.2, 1.0 Hz, 1H, Ar–H), 6.99 (td, J = 7.6, 1.1 Hz, 1H, Ar–H), 7.14 (td, J = 8.0, 1.8 Hz, 1H, Ar–H), 7.24–7.28 (m, 1H, Ar–H). 13C-NMR (75 MHz, CDCl3) δ 28.2, 29.0, 32.4, 52.7, 54.0, 60.7, 64.1, 65.8, 66.1, 79.2, 120.7, 122.0,122.7, 123.5, 126.3, 128.9, 135.3, 156.5.
7.1.6. General Procedure for Preparation of Final Compounds (8–18)
Intermediates 5–7 were converted into their TFA salts by treatment with a mixture of TFA/CH2Cl2 (4 mL/1 mL). The excess reagent and solvent were removed under reduced pressure and maintained overnight under vacuum. A mixture of the appropriate (aryloxy)ethyl alicyclic amine (1 eq) in CH2Cl2 (3 mL) and TEA (3 eq) was then cooled in an ice bath, and the proper arylsulfonyl chloride (1.2 eq) was added at 0 °C (the entire amount was added at the same time). The reaction mixture was stirred for 2–6 h under cooling. The solvent was evaporated, and the sulfonamides were a purified silica-gel column with CH2Cl2/MeOH (9/0.7, v/v) as an eluting system (isolated yields 55–87%). Compounds 9 and 10, selected for in vivo pharmacological evaluation, were further converted into the hydrochloride salts by treatment of their solution in anhydrous ethanol with 1.25 M HCl in MeOH.
4-Fluoro-N-[(1-{2-[(2,2,2-trifluoroethoxy)phenoxy]ethyl}piperidin-4-yl)methyl]benzenesulfonamide (8)
Compound 8 was prepared using intermediate 5 (150 mg, 0.45 mmol), TEA (0.19 mL, 1.35 mmol), and 4-fluorobenzenesulfonyl chloride (110 mg, 0.54 mmol). Yellow oil 200 mg (isolated yield 87%); UPLC/MS purity 100%, tR = 4.92. C22H26F4N2O4S, MW 490.51, Monoisotopic Mass 490.15, [M + H]+ 491.4. 1H-NMR (300 MHz, CDCl3) δ 1.25–1.37 (m, 2H, piperidine), 1.51–1.54 (m, 1H, piperidine), 1.70 (d, J = 13.5 Hz, 2H, piperidine), 2.22 (t, J = 11.5 Hz, 2H, piperidine), 2.83 (t, J = 6.5 Hz, 2H, piperidine), 2.91 (t, J = 5.4 Hz, 2H, N–CH2–CH2), 3.12 (d, J = 11.3 Hz, 2H, NH–CH2–CH), 4.17 (t, J = 5.5 Hz, 2H, O–CH2–CH2), 4.38 (q, J = 8.4 Hz, 2H, O–CH2–CF3), 6.88–6.95 (m, 3H, Ar–H), 6.96–7.06 (m, 1H, Ar–H), 7.14–7.22 (m, 2H, Ar–H), 7.83–7.91 (m, 2H, Ar–H). Anal. calcd for C22H26F4N2O4S HCl: C: 50.14, H: 5.16, N: 5.32, S: 6.08; Found C: 49.96, H: 4.96, N: 5.17, S: 5.78. Mp for C22H26F4N2O4S HCl:155.5–158.2 °C.
3-Chloro-2-fluoro-N-[(1-{2-[(2,2,2-trifluoroethoxy)phenoxy]ethyl}piperidin-4-yl)methyl]benzenesulfonamide (9)
Compound 9 was prepared using intermediate 5 (150 mg, 0.45 mmol), TEA (0.19 mL, 1.35 mmol), and 3-chloro-2-fluorobenzenesulfonyl chloride (0.08 mL, 0.54 mmol). Yellow oil, 190 mg (isolated yield 82%); UPLC/MS purity 100%, tR = 5.35. C22H25ClF4N2O4S, MW 524.96, Monoisotopic Mass 524.12, [M + H]+ 525.3. 1H-NMR (300 MHz, CDCl3) δ 1.15–1.30 (m, 2H, piperidine), 1.48–1.55 (m, 1H, piperidine), 1.70 (d, J = 13.1 Hz, 2H, piperidine), 2.11 (td, J = 11.7, 2.0 Hz, 2H, piperidine), 2.82 (t, J = 5.7 Hz, 2H, piperidine), 2.88 (t, J = 6.2 Hz, 2H, N–CH2–CH2), 3.02 (d, J = 11.7 Hz, 2H, NH–CH2–CH), 4.12 (t, J = 5.7 Hz, 2H, O–CH2–CH2), 4.39 (q, J = 8.5 Hz, 2H, O–CH2–CF3), 6.89–6.93 (m, 2H, Ar–H), 6.96–7.06 (m, 2H, Ar–H), 7.19–7.25 (m, 1H, Ar–H), 7.62 (ddd, J = 8.2, 6.7, 1.7 Hz, 1H, Ar–H), 7.79 (ddd, J = 7.9, 6.3, 1.7 Hz, 1H, Ar–H). 13C-NMR (75 MHz, DMSO-d6) δ 27.0, 34.0, 47.6, 52.6, 55.4, 64.1, 65.8, 66.3, 115.0 (d, J = 80.6 Hz), 121.7, 122.1 (d, J = 10.4 Hz), 122.7, 123.4, 126.2 (d, J = 4.6 Hz), 126.3, 128.9, 130.5 (d, J = 13.8 Hz), 135.6, 147.1 (d, J = 58.7 Hz), 154.0 (d, J = 255.7 Hz). Anal. calcd for C22H25ClF4N2O4S HCl: C: 47.07, H: 4.67, N: 4.99, S: 5.71; Found C: 47.09, H: 4.48, N: 4.74, S: 5.32. Mp for C22H25ClF4N2O4S HCl: 163.1–164.9 °C.
5-Chloro-2-fluoro-N-[(1-{2-[(2,2,2-trifluoroethoxy)phenoxy]ethyl}piperidin-4-yl)methyl]benzenesulfonamide (10)
Compound 10 was prepared using intermediate 5 (150 mg, 0.45 mmol), TEA (0.19 mL, 1.35 mmol), and 5-chloro-2-fluorobenzenesulfonyl chloride (120 mg, 0.54 mmol). Yellow oil, 190 mg (isolated yield 81%); UPLC/MS purity 100%, tR = 5.41. C22H25ClF4N2O4S, MW 524.96, Monoisotopic Mass 524.12, [M + H]+ 525.3. 1H-NMR (300 MHz, CDCl3) δ 1.17–1.32 (m, 2H, piperidine), 1.43–1.57 (m, 1H, piperidine), 1.70 (d, J = 13.3 Hz, 2H, piperidine), 2.08–2.18 (m, 2H, piperidine), 2.83 (t, J = 5.7 Hz, 2H, piperidine), 2.87 (t, J = 6.2 Hz, 2H, N–CH2–CH2), 3.04 (d, J = 11.8 Hz, 2H, NH–CH2–CH), 4.13 (t, J = 5.6 Hz, 2H, O–CH2–CH2), 4.41 (q, J = 8.5 Hz, 2H, O–CH2–CF3), 4.90 (br.s., 1H, SO2–NH–CH2), 6.89–6.93 (m, 2H, Ar–H), 6.96–7.06 (m, 2H, Ar–H), 7.12–7.19 (m, 1H, Ar–H), 7.52 (ddd, J = 8.8, 4.3, 2.7 Hz, 1H, Ar–H), 7.87 (dd, J = 6.1, 2.7 Hz, 1H, Ar–H). 13C-NMR (75 MHz, DMSO-d6) δ 27.0, 34.0, 47.5, 52.6, 55.4, 64.1, 65.6, 66.2, 115.0 (d, J = 74.9 Hz), 119.9 (d, J = 21.9 Hz), 122.1, 123.4, 129.1 (d, J = 3.5 Hz), 130.4 (d, J = 16.1 Hz), 135.3 (d, J = 9.2 Hz), 147.4 (d, J = 57.6 Hz), 157.3 (d, J = 249.9 Hz). Anal. calcd for C22H25ClF4N2O4S HCl: C: 47.07, H: 4.67, N: 4.99, S: 5.71; Found C: 47.17, H: 4.32, N: 4.74, S: 5.39. Mp for C22H25ClF4N2O4S HCl: 165.0–166.6 °C.
5-Chloro-2-methoxy-N-[(1-{2-[(2,2,2-trifluoroethoxy)phenoxy]ethyl}piperidin-4-yl)methyl]benzenesulfonamide (11)
Compound 11 was prepared using intermediate 5 (150 mg, 0.45 mmol), TEA (0.19 mL, 1.35 mmol), and 5-chloro-2-methoxybenzenesulfonyl chloride (130 mg, 0.54 mmol). Yellow oil, 140 mg (isolated yield 58%); UPLC/MS purity 98%, tR = 5.35. C23H28ClF3N2O5S, MW 536.14, Monoisotopic Mass 536.99, [M + H]+ 537.2. 1H-NMR (300 MHz, CDCl3) δ 1.14–1.29 (m, 2H, piperidine), 1.50–1.58 (m, 1H, piperidine), 1.70 (d, J = 12.5 Hz, 2H, piperidine), 2.17 (t, J = 11.0 Hz, 2H, piperidine), 2.73 (t, J = 6.6 Hz, 2H, piperidine), 2.86 (t, J = 5.5 Hz, 2H, N–CH2–CH2), 3.04–3.11 (m, 2H, NH–CH2–CH), 3.95 (s, 3H, O–CH3), 4.15 (t, J = 5.5 Hz, 2H, O–CH2–CH2), 4.38 (q, J = 8.4 Hz, 2H, O–CH2–CF3), 5.04 (br.s., 1H, SO2–NH–CH2), 6.94 (dd, J = 16.5, 8.1 Hz, 4H, Ar–H), 6.99–7.05 (m, 1H, Ar–H), 7.48 (dd, J = 8.9, 2.7 Hz, 1H, Ar–H), 7.87 (d, J = 2.7 Hz, 1H, Ar–H). Anal. calcd for C23H28ClF3N2O5S: C: 51.44, H: 5.26, N: 5.22, S: 5.97; Found C: 51.13, H: 5.06, N: 5.07, S: 5.65.
3,4-Dimethoxy-N-[(1-{2-[(2,2,2-trifluoroethoxy)phenoxy]ethyl}piperidin-4-yl)methyl]benzenesulfonamide (12)
Compound 12 was prepared using intermediate 5 (150 mg, 0.45 mmol), TEA (0.19 mL, 1.35 mmol), and 3,4-dimethoxybenzenesulfonyl chloride (130 mg, 0.54 mmol). Yellow oil, 180 mg (isolated yield 77%); UPLC/MS purity 98%, tR = 4.71. C24H31F3N2O6S, MW 532.57, Monoisotopic Mass 532.19, [M + H]+ 533.4. 1H-NMR (300 MHz, CDCl3) δ 1.14–1.29 (m, 2H, piperidine), 1.46–1.54 (m, 1H, piperidine), 1.67 (d, J = 12.8 Hz, 2H, piperidine), 2.11 (t, J = 11.0 Hz, 2H, piperidine), 2.77–2.80 (m, 2H,), 2.82–2.86 (m, 2H, N–CH2–CH2), 3.02 (d, J = 11.5 Hz, 2H, NH–CH2–CH), 3.91 (s, 3H, O–CH3), 3.93 (s, 3H, O–CH3), 4.12 (t, J = 5.6 Hz, 2H, O–CH2–CH2), 4.39 (q, J = 8.5 Hz, 2H, O–CH2–CF3), 4.68 (br.s., 1H, SO2–NH–CH2), 6.86–6.97 (m, 4H, Ar–H), 6.98–7.05 (m, 1H, Ar–H), 7.33 (d, J = 2.1 Hz, 1H, Ar–H), 7.46 (dd, J = 8.5, 2.2 Hz, 1H, Ar–H). Anal. calcd for C24H31F3N2O6S: C: 54.13, H: 5.87, N: 5.26, S: 6.02; Found C: 54.33, H: 6.01, N: 5.45, S: 6.34.
(R)-4-Fluoro-N-(1-{2-[(2,2,2-trifluoroethoxy)phenoxy]ethyl}pyrrolidin-3-yl)benzenesulfonamide (13)
Compound 13 was prepared using intermediate 6 (150 mg, 0.5 mmol), TEA (0.21 mL, 1.5 mmol), and 4-fluorobenzenesulfonyl chloride (120 mg, 0.6 mmol). Yellow oil, 150 mg (isolated yield 65%); UPLC/MS purity 95%, tR = 5.07. C20H22F4N2O4S, MW 462.46, Monoisotopic Mass 462.12, [M + H]+ 463.3. 1H-NMR (300 MHz, CDCl3) δ 1.52–1.64 (m, 2H, pyrrolidine), 2.09–2.11 (m 1H, pyrrolidine), 2.31–2.41 (m, 1H, pyrrolidine), 2.58–2.64 (m, 1H, pyrrolidine), 2.82–2.86 (m, 2H, pyrrolidine), 2.93 (td, J = 9.0, 4.3 Hz, 2H, NH–CH2–CH2), 4.06 (t, J = 1.0 Hz, 2H, O–CH2–CH2), 4.36 (q, J = 8.4 Hz, 2H, O–CH2–CF3), 5.11 (br.s., 1H, SO2–NH–CH), 6.87–6.96 (m, 3H, Ar–H), 6.97–7.07 (m, 1H, Ar–H), 7.09–7.17 (m, 2H, Ar–H), 7.81–7.89 (m, 2H, Ar–H). 13C-NMR (75 MHz, CDCl3) δ 29.1, 32.5, 52.7, 54.0, 60.8, 67.7, 115.3 (d, J = 131.3 Hz), 116.8 (d, J = 59.9 Hz), 121.6, 121.7, 124.1, 129.7 (d, J = 9.2 Hz), 148.3 (d, J = 155.5 Hz), 165.0 (d, J = 252.2 Hz). Anal. calcd for C20H22F4N2O4S: C: 51.94, H: 4.80, N: 6.06, S: 6.93; Found C: 51.75, H: 4.64, N: 6.35, S: 6.97.
(S)-4-Fluoro-N-(1-{2-[(2,2,2-trifluoroethoxy)phenoxy]ethyl}pyrrolidin-3-yl)benzenesulfonamide (14)
Compound 14 was prepared using intermediate 7 (150 mg, 0.5 mmol), TEA (0.21 mL, 1.5 mmol), and 4-fluorobenzenesulfonyl chloride (120 mg, 0.6 mmol). Yellow oil, 140 mg (isolated yield 61%); UPLC/MS purity 95%, tR = 4.80. C20H22F4N2O4S, MW 462.46, Monoisotopic Mass 462.12, [M + H]+ 463.2. 1H-NMR (300 MHz, CDCl3) δ 1.52–1.64 (m, 2H, pyrrolidine), 2.09–2.11 (m 1H, pyrrolidine), 2.31–2.41 (m, 1H, pyrrolidine), 2.58–2.64 (m, 1H, pyrrolidine), 2.82–2.86 (m, 2H, pyrrolidine), 2.93 (td, J = 9.0, 4.3 Hz, 2H, NH–CH2–CH2), 4.06 (t, J = 1.0 Hz, 2H, O–CH2–CH2), 4.36 (q, J = 8.4 Hz, 2H, O–CH2–CF3), 5.11 (br.s., 1H, SO2–NH–CH), 6.87–6.96 (m, 3H, Ar–H), 6.97–7.07 (m, 1H, Ar–H), 7.09–7.17 (m, 2H, Ar–H), 7.81–7.89 (m, 2H, Ar–H). Anal. calcd for C20H22F4N2O4S: C: 51.94, H: 4.80, N: 6.06, S: 6.93; Found C: 51.73, H: 4.62, N: 6.33, S: 6.95.
(R)-5-Chloro-2-fluoro-N-(1-{2-[(2,2,2-trifluoroethoxy)phenoxy]ethyl}pyrrolidin-3-yl)benzenesulfonamide (15)
Compound 15 was prepared using intermediate 6 (150 mg, 0.5 mmol), TEA (0.21 mL, 1.5 mmol), and 5-chloro-2-fluorobenzenesulfonyl chloride (140 mg, 0.6 mmol). Yellow oil, 170 mg (isolated yield 68%); UPLC/MS purity 95%, tR = 5.19. C20H21ClF4N2O4S, MW 496.90, Monoisotopic Mass 496.08, [M + H]+ 497.3. 1H-NMR (300 MHz, CDCl3) δ 1.60–1.72 (m, 2H, pyrrolidine), 2.11–2.16 (m, 1H, pyrrolidine), 2.32–2.42 (m, 1H, pyrrolidine), 2.50–2.57 (m, 1H, pyrrolidine), 2.67–2.73 (m, 1H, pyrrolidine), 2.88 (dt, J = 8.0, 5.4 Hz, 2H, NH–CH2–CH2), 2.94–3.02 (m, 1H, pyrrolidine), 4.08 (t, J = 5.4 Hz, 2H, O–CH2–CH2), 4.34 (q, J = 8.3 Hz, 2H, O–CH2–CF3), 6.88–6.98 (m, 3H, Ar–H), 7.00–7.07 (m, 2H, Ar–H), 7.43 (ddd, J = 8.8, 4.3, 2.7 Hz, 1H, Ar–H), 7.87 (dd, J = 6.1, 2.7 Hz, 1H, Ar–H). Anal. calcd for C20H21ClF4N2O4S: C: 48.34, H: 4.26, N: 5.64, S: 6.45; Found C: 48.47, H: 4.55, N: 5.99, S: 6.75.
(S)-5-Chloro-2-fluoro-N-(1-{2-[(2,2,2-trifluoroethoxy)phenoxy]ethyl}pyrrolidin-3-yl)benzenesulfonamide (16)
Compound 16 was prepared using intermediate 7 (150 mg, 0.5 mmol), TEA (0.21 mL, 1.5 mmol), and 5-chloro-2-fluorobenzenesulfonyl chloride (140 mg, 0.6 mmol). Yellow oil, 130 mg (isolated yield 55%); UPLC/MS purity 98%, tR = 5.20. C20H21ClF4N2O4S, MW 496.90, Monoisotopic Mass 496.08, [M + H]+ 497.2. 1H-NMR (300 MHz, CDCl3) δ 1.60–1.72 (m, 2H, pyrrolidine), 2.07–2.20 (m, 1H, pyrrolidine), 2.31–2.41 (m, 1H, pyrrolidine), 2.51–2.57 (m, 1H, pyrrolidine), 2.65–2.70 (m, 1H, pyrrolidine), 2.79–2.90 (m, 2H, NH–CH2–CH2), 2.92–3.00 (m, 1H, pyrrolidine), 3.95 (br. s., 1H, SO2–NH–CH), 4.07 (t, J = 5.2 Hz, 2H, O–CH2–CH2), 4.34 (q, J = 8.2 Hz, 2H, O–CH2–CF3), 6.87–6.97 (m, 3H, Ar–H), 7.03 (t, J = 8.7 Hz, 2H, Ar–H), 7.40–7.46 (m, 1H, Ar–H), 7.86 (dd, J = 5.7, 2.2 Hz, 1H, Ar–H). Anal. calcd for C20H21ClF4N2O4S: C: 48.34, H: 4.26, N: 5.64, S: 6.45; Found C: 48.49, H: 4.57, N: 6.02, S: 6.78.
(R)-3,4-Dimethoxy-N-(1-{2-[(2,2,2-trifluoroethoxy)phenoxy]ethyl}pyrrolidin-3-yl)benzenesulfonamide (17)
Compound 17 was prepared using intermediate 6 (150 mg, 0.5 mmol), TEA (0.21 mL, 1.5 mmol), and 3,4-dimethoxybenzenesulfonyl chloride (140 mg, 0.6 mmol). Yellow oil, 180 mg (isolated yield 74%); UPLC/MS purity 95%, tR = 4.54. C22H27F3N2O6S, MW 504.52, Monoisotopic Mass 504.15, [M + H]+ 505.3. 1H-NMR (300 MHz, CDCl3) δ 1.52–1.64 (m, 2H, pyrrolidine), 2.04–2.17 (m, 1H, pyrrolidine), 2.33–2.42 (m, 1H, pyrrolidine), 2.52–2.59 (m, 1H, pyrrolidine), 2.60–2.65 (m, 1H, pyrrolidine), 2.86 (td, J = 5.5, 1.5 Hz, 2H, NH–CH2–CH2), 2.90–2.97 (m, 1H, pyrrolidine), 3.90 (s, 3H, O–CH3), 3.92 (s, 3H, O–CH3), 4.07 (t, J = 5.5 Hz, 2H, O–CH2–CH2), 4.36 (q, J = 8.4 Hz, 2H, O–CH2–CF3), 4.67 (br.s., 1H, SO2–NH–CH), 6.87–6.93 (m, 3H, Ar–H), 6.95 (dd, J = 3.3, 1.8 Hz, 1H, Ar–H), 7.00–7.06 (m, 1H, Ar–H), 7.31 (d, J = 2.2 Hz, 1H, Ar–H), 7.46 (dd, J = 8.5, 2.2 Hz, 1H, Ar–H). Anal. calcd for C22H27F3N2O6S: C: 52.37, H: 5.39, N: 5.55, S: 6.35; Found C: 52.19, H: 5.15, N: 5.24, S: 6.05
(S)-3,4-Dimethoxy-N-(1-{2-[(2,2,2-trifluoroethoxy)phenoxy]ethyl}pyrrolidin-3-yl)benzenesulfonamide (18)
Compound 18 was prepared using intermediate 7 (150 mg, 0.5 mmol), TEA (0.21 mL, 1.5 mmol), and 3,4-dimethoxybenzenesulfonyl chloride (140 mg, 0.6 mmol). Yellow oil, 170 mg (isolated yield 68%); UPLC/MS purity 97%, tR = 4.57. C22H27F3N2O6S, MW 504.52, Monoisotopic Mass 504.15, [M + H]+ 505.3. 1H-NMR (300 MHz, CDCl3) δ 1.50–1.62 (m, 2H, pyrrolidine), 2.01–2.14 (m, 1H, pyrrolidine), 2.37 (td, J = 8.9, 6.9 Hz, 1H, pyrrolidine), 2.57 (d, J = 5.1 Hz, 2H, pyrrolidine), 2.80–2.85 (m, 2H, NH–CH2–CH2), 2.85–2.93 (m, 1H, pyrrolidine), 3.88 (s, 3H, O–CH3), 3.90 (s, 3H, O–CH3), 4.04 (t, J = 5.6 Hz, 2H, O–CH2–CH2), 4.35 (q, J = 8.4 Hz, 2H, O–CH2–CF3), 5.13 (br.s., 1H, SO2–NH–CH), 6.86–6.90 (m, 3H, Ar–H), 6.93 (dd, J = 5.6, 1.7 Hz, 1H, Ar–H), 6.96–7.05 (m, 1H, Ar–H), 7.32 (d, J = 2.2 Hz, 1H, Ar–H), 7.46 (dd, J = 8.5, 2.2 Hz, 1H, Ar–H). 13C-NMR (75 MHz, CDCl3) δ 32.4, 52.6, 52.7, 54.1, 56.1, 56.2, 60.8, 67.5, 67.7, 67.9, 109.5, 110.5, 114.4, 117.4, 120.9, 121.5, 124.0, 132.4, 147.3, 149.0, 152.4. Anal. calcd for C22H27F3N2O6S: C: 52.37, H: 5.39, N: 5.55, S: 6.35; Found C: 52.21, H: 5.18, N: 5.29, S: 6.09.
7.2. In Vitro Pharmacology
7.2.1. Determination of the Affinity of the Tested Compounds at the α1- and α2-ARs
The affinity of the obtained compounds was evaluated by radioligand-binding assays (the ability to displace [3H]-Prazosin and [3H]-Clonidine from α1- and α2-ARs, respectively) on rat cerebral cortex. The brains are homogenized in 20 volumes of an ice-cold 50 mM Tris-HCl buffer (pH 7.6) and is centrifuged (MPW Med. Instruments, Warsaw, Poland) at 20,000 g for 20 min (0–4 °C). The cell pellet is resuspended in the Tris-HCl buffer and centrifuged again. Radioligand-binding assays are performed in plates (MultiScreen/Millipore, Burlington, MA, USA). The final incubation mixture (final volume 300 µL) consisted of 240 µL of the membrane suspension, 30 µL of [3H]-Prazosin (0.2 nM) or [3H]-Clonidine (2 nM) solution, and 30 µL of the buffer containing seven to eight concentrations (10−11 to 10−4 M) of the tested compounds. For measuring the unspecific binding, phentolamine, 10 µM (in the case of [3H]-Prazosin) and clonidine, and 10 µM (in the case of [3H]-Clonidine), were applied. The incubation was terminated by rapid filtration over glass fiber filters (Whatman GF/C, Sigma-Aldrich) using a vacuum manifold (Millipore). The filters were then washed twice with the assay buffer and placed in scintillation vials with a liquid-scintillation cocktail. Radioactivity was measured in a WALLAC 1409 DSA liquid-scintillation counter (BioSurplus, San Diego, CA, USA). All the assays were made in duplicate.
7.2.2. Determination of the Affinity of the Tested Compounds at the 5-HT1A and 5-HT7Rs
Binding experiments were conducted in 96-well microplates in a total volume of 250 μL of appropriate buffers. The composition of the assay buffers was as follows: 50 mM Tris-HCl, 0.1 mM EDTA, 10 mM MgCl2. The reaction mix included 50 μL solution of test compound, 50 μL of radioligand, and 150 μL of diluted membranes. All assays were incubated for 1 h (5-HT1ARs) or 2 h (5-HT7Rs) at 37 °C. Radioactivity was counted in MicroBeta2 scintillation counter (PerkinElmer, Waltham, MA, USA). Nonspecific binding is defined with 10 µM of 5-HT and 10 µM of methiothepine in 5-HT1AR and 5-HT7R binding experiments, respectively. Each compound was tested in screening assay at two final concentrations of 10 µM and 1 µM.
7.2.3. Determination of the Intrinsic Activity of the α1A-ARs
Intrinsic activity assay was performed according to the manufacturer of the assay kit (Invitrogen, Thermo Fisher Scientific corporation, Carlsbad, CA, USA). The cells were harvested and suspended in Assay Medium to a density of 312,500 cells/mL. Of the cell suspension, 32 µL per well was added to the Test Compound wells, the Unstimulated Control wells, and Stimulated Control wells and incubated per 16–24 h. To perform an agonist assay, 8 concentrations of 8 µL of the tested compound (10−4–10−11 M), e.g., in 5-fold higher concentration in comparison to the final tested concentration in the well, were added to the cells. To perform an antagonist assay, 8 concentrations of 4 µL of the tested compound (10−4–10−11M), e.g., in 10-fold higher concentration in comparison to the final tested concentration in the well, were added to the cells. Then, after 30 min, 4 µL of standard agonist in EC80 (10-fold higher concentration in comparison to the EC80 in the well), in Assay Medium, was added to the cells. Then, both the agonist and the antagonist plate were incubated in a humidified 37 °C/5% CO2 incubator for 5 h. After the incubation 8 µL of LiveBLAzer™-FRET B/G Substrate Mixture (CCF4-AM, Thermo Fisher Scientific corporation) was loaded cells in the absence of direct strong lighting, covered, and incubated at room temperature for 2 h.
7.2.4. Determination of the Intrinsic Activity of the α1B-ARs and α1D-ARs
Intrinsic activity assay to α1B- and α1D-ARs was performed according to the manufacturer of the ready-to-use cells with stable expression of the α1B-adrenoreceptors and α1D-adrenoreceptors, respectively (PerkinElmer, Zaventem, Belgium). For measurement, cells (frozen, ready to use) were thawed and resuspended in 10 mL of assay buffer containing 5 µM coelenterazine h. This cells suspension was put in a 10 mL Falcon tube, fixed onto a rotating heel, and incubated for overnight at rt in the dark (8 rpm; 45° angle). Cells were diluted with Assay Buffer to 5000 cells/20 µL. Agonistic ligands 2 × (50 µL/well), diluted in Assay Buffer, were prepared in ½ white polystyrene area plates, and the cell suspension was dispensed in 50 µL volume on the ligands using the injector. The light emitted was recorded for 20 s. Cells with antagonist were incubated for 15 min at room temperature. Thereafter, 50 µL of agonist (3 × EC80 final concentration) was injected into the mix of cells and antagonist and the light emitted was recorded for 20 s.
7.2.5. Determination of In Vitro Metabolic Stability
Metabolic stability of tested compound was analyzed using incubation systems, composed of: tested compounds (10 μM), RLMs (microsomes from rat male liver, pooled; 0.2 mg/mL; Sigma Aldrich), NADPH-regenerating system (NADP+, glucose-6-phosphate and glucose-6-phosphate dehydrogenase in 100mM potassium buffer, pH 7.4; all from Sigma Aldrich), and potassium buffer, pH 7.4. Stock solution of tested compounds was prepared in methanol (the final methanol concentration in incubation mixture does not exceed 0.2%). Firstly, all samples contained incubation mixture (without NADPH-regenerating system) were preincubated in a thermoblock at 37 °C for 10 min. Then reaction was initiated by the addition of NADPH-regenerating system. In control samples NADPH-regenerating system was replaced with potassium buffer. Probes were incubated in thermoblock for 15 and 30 min at 37 °C. After addition of internal standard (levallorphan, 10 μM), the biotransformation process was stopped by addition of perchloric acid (69–72%, by volume). Next, samples were centrifuged (Centrifuge 5427 R, Eppendorf, Hamburg, Germany) and supernatants were analyzed using UPLC/MS (Waters Corporation). All experiments were run in duplicates. Half-life time was evaluated using a nonlinear regression model using Graph Pad Prism software (Graph Pad Software, La Jolla, San Diego, CA, USA) and intrinsic clearance was calculated from the equation Clint = (volume of incubation [μL]/protein in the incubation [mg]) ×∙0.693/t1/2 [33].
7.3. In Vivo Pharmacology
7.3.1. Animals
The experiments were carried out on male Wistar rats (body weight 200–250 g). The animals were housed in pairs in plastic cages in constant-temperature facilities exposed to a 12:12 h light/dark cycle; water and food were available ad libitum. Experimental groups consisted of 6 animals each. All experiments were conducted in accordance with the NIH Guide for Care and Use of Laboratory Animals and were approved by the Animal Use and Care Committee of the Jagiellonian University (2012, Kraków, Poland).
7.3.2. Determination of the Effect of the Tested Compounds on Blood Pressure after a Single Administration in Rats
The normotensive rats were anesthetized with thiopental (70 mg/kg) by i.p. injection. The left carotid artery was cannulated with polyethylene tubing filled with heparin solution in saline to facilitate pressure measurements using PowerLab Apparatus (ADInstruments, Sydney, Australia). Blood pressure was measured: before administration of the compounds—time 0 min (control pressure), and 60 min thereafter. For each compound, studies were performed in the dose of 2 mg/kg b.w. Compounds were dissolved in water and administered intravenously. Initial blood pressure before administration of the tested compounds in all groups was similar.
7.3.3. Statistical Analysis
Statistical calculations were carried out with the GraphPadPrism 6 program (GraphPad Software). Results are given as the arithmetic means with standard deviation (SD). The statistical significance was calculated using a one-way ANOVA and posthoc Bonfferoni Test in comparison to 0.9% NaCl. Differences were considered statistically significant at: * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.
Supplementary Materials
Supporting Information Available: MS, 1H-NMR and 13C-NMR spectra for representative final compounds.
Author Contributions
Conceptualization, M.B., P.Z. and J.S.; Methodology, P.Z., J.S.; Investigation, V.C., A.R., M.K., J.K., A.S., M.B., L.N., M.Z., P.K., E.P.; Writing-Original Draft Preparation, V.C., M.B., J.S., P.Z.; Funding Acquisition, J.S.
Funding
The studies were financially supported by the National Science Centre in Poland within the Grant No. 2011/03/B/NZ7/00724.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hannenberg, M.; Stief, C.; Gratzke, C. Prostatic α1-adrenoceptors: New concepts of function, regulation, and intracellular signaling. Neurourol. Urodyn. 2014, 33, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Hague, C.; Chen, Z.; Uberti, M.; Minneman, K.P. α1-Adrenergic receptor subtypes: non-identical triplets with different dancing partners? Life Sci. 2003, 74, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Docherty, J.R. Subtypes of functional α1-adrenoceptor. Cell. Mol. Life Sci. 2010, 67, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Kuo, H.C. Pathophysiology of benign prostate enlargement and lower urinary tract symptoms: Current concepts. Tzu Chi Med. J. 2017, 29, 65–71. [Google Scholar] [CrossRef]
- Michel, M.; Heemann, U.; Schumacher, H.; Mehlburger, L.; Goepel, M. Association of Hypertension with Symptoms of Benign Prostatic Hyperplasia. J. Urol. 2004, 172, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Biester, K.; Skipka, G.; Jahn, R.; Buchberger, B.; Rohde, V.; Lange, S. Systematic review of surgical treatments for benign prostatic hyperplasia and presentation of an approach to investigate therapeutic equivalence (non-inferiority). BJU Int. 2012, 109, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.D.; Parsons, J.K. Epidemiology and etiology of benign prostatic hyperplasia and bladder outlet obstruction. Indian J. Urol. 2014, 30, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.; Desgrandchamps, F. New Concepts and Pathophysiology of Lower Urinary Tract Symptoms in Men. Eur. Urol. Suppl. 2010, 9, 472–476. [Google Scholar] [CrossRef]
- Untergasser, G.; Madersbacher, S.; Berger, P. Benign prostatic hyperplasia: Age-related tissue-remodeling. Exp. Gerontol. 2005, 40, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, V. Is chronic prostatic inflammation a new target in the medical therapy of lower urinary tract symptoms (LUTS) due to benign prostate hyperplasia (BPH)? BJU Int. 2013, 112, 421–422. [Google Scholar] [CrossRef] [PubMed]
- Gratzke, C.; Bachmann, A.; Descazeaud, A.; Drake, M.J.; Madersbacher, S.; Mamoulakis, C.; Oelke, M.; Tikkinen, K.A.O.; Gravas, S. EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur. Urol. 2015, 67, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Nasu, K.; Moriyama, N.; Kawabe, K.; Tsujimoto, G.; Murai, M.; Tanaka, T.; Yano, J. Quantification and distribution of α1-adrenoceptor subtype mRNAs in human prostate: Comparison of benign hypertrophied tissue and non-hypertrophied tissue. Br. J. Pharmacol. 1996, 119, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Schwinn, D.A.; Roehrborn, C.G. Alpha1-adrenoceptor subtypes and lower urinary tract symptoms. Int. J. Urol. 2008, 15, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Michelotti, G.A.; Price, D.T.; Schwinn, D.A. α1-Adrenergic receptor regulation: Basic science and clinical implications. Pharmacol. Ther. 2000, 88, 281–309. [Google Scholar] [CrossRef]
- Castiglione, F.; Benigni, F.; Briganti, A.; Salonia, A.; Villa, L.; Nini, A.; Di Trapani, E.; Capitanio, U.; Hedlund, P.; Montorsi, F. Naftopidil for the treatment of benign prostate hyperplasia: A systematic review. Curr. Méd. Res. Opin. 2013, 30, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Wilde, M.; Mctavish, D. Tamsulosin: A Review of its Pharmacological Properties and Therapeutic Potential in the Management of Symptomatic Benign Prostatic Hyperplasia. Drugs 1996, 52, 883–898. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Aoki, Y.; Yoshikawa, T.; Hachiya, T.; Saito, T.; Takahashi, S. Silodosin versus naftopidil for the treatment of benign prostatic hyperplasia: A multicenter randomized trial. Int. J. Urol. 2013, 20, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Ukimura, O.; Kanazawa, M.; Fujihara, A.; Kamoi, K.; Okihara, K.; Miki, T. Naftopidil versus tamsulosin hydrochloride for lower urinary tract symptoms associated with benign prostatic hyperplasia with special reference to the storage symptom: A prospective randomized controlled study. Int. J. Urol. 2008, 15, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Zajdel, P.; Kurczab, R.; Grychowska, K.; Satala, G.; Pawlowski, M.; Bojarski, A.J. The multiobjective based design, synthesis and evaluation of the arylsulfonamide/amide derivatives of aryloxyethyl- and arylthioethyl-piperidines and pyrrolidines as a novel class of potent 5-HT(7) receptor antagonists. Eur. J. Med. Chem. 2012, 56, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Rak, A.; Canale, V.; Marciniec, K.; Żmudzki, P.; Kotańska, M.; Knutelska, J.; Siwek, A.; Stachowicz, G.; Bednarski, M.; Nowiński, L.; et al. Arylsulfonamide derivatives of (aryloxy)ethyl pyrrolidines and piperidines as α1-adrenergic receptor antagonist with uro-selective activity. Bioorg. Med. Chem. 2016, 24, 5582–5591. [Google Scholar] [CrossRef] [PubMed]
- Zajdel, P.; Canale, V.; Partyka, A.; Marciniec, K.; Kurczab, R.; Satala, G.; Siwek, A.; Jastrzebska-Wiesek, M.; Wesolowska, A.; Kos, T.; et al. Arylsulfonamide derivatives of (aryloxy)ethylpiperidines as selective 5-HT7 receptor antagonists and their psychotropic properties. Med. Chem. Commun. 2015, 6, 1272–1277. [Google Scholar] [CrossRef]
- Canale, V.; Kurczab, R.; Partyka, A.; Satała, G.; Lenda, T.; Jastrzębska-Więsek, M.; Wesołowska, A.; Bojarski, A.J.; Zajdel, P. Towards new 5-ht7 antagonists among arylsulfonamide derivatives of (aryloxy)ethyl-alkyl amines: Multiobjective based design, synthesis, and antidepressant and anxiolytic properties. Eur. J. Med. Chem. 2016, 108, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Maj, J.; Klimek, V.; Nowak, G. Antidepressant drugs given repeatedly increase binding to alpha 1-adrenoceptors in the rat cortex. Eur. J. Pharmacol. 1985, 119, 113–116. [Google Scholar] [CrossRef]
- Cheng, Y.; Prusoff, W.H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [PubMed]
- Zagorska, A.; Bucki, A.; Kolaczkowski, M.; Siwek, A.; Gluch-Lutwin, M.; Starowicz, G.; Kazek, G.; Partyka, A.; Wesolowska, A.; Sloczynska, K.; et al. Synthesis and biological evaluation of 2-fluoro and 3-trifluoromethyl-phenyl-piperazinylalkyl derivatives of 1H-imidazo[2,1-f]purine-2,4(3H,8H)-dione as potential antidepressant agents. J. Enzyme Inhib. Med. Chem. 2016, 31, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Available online: www.eurofins.com (accessed on 13 August 2018).
- Vanda, D.; Soural, M.; Canale, V.; Chaumont-Dubel, S.; Satała, G.; Kos, T.; Funk, P.; Fülöpová, V.; Lemrová, B.; Koczurkiewicz, P.; et al. Novel non-sulfonamide 5-HT6 receptor partial inverse agonist in a group of imidazo[4,5-b]pyridines with cognition enhancing properties. Eur. J. Med. Chem. 2018, 144, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Palea, S.; Lluel, P.; Barras, M.; Duquenne, C.; Galzin, A.M.; Arbilla, S. Involvement of 5-hydroxytryptamine (HT)7 receptors in the 5-HT excitatory effects on the rat urinary bladder. BJU Int. 2004, 94, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; de Groat, W.C. Role of 5-HT1A receptors in control of lower urinary tract function in anesthetized rats. Am. J. Physiol. Ren. Physiol. 2010, 298, F771–F778. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Li, P.; Gallegos, R.; Uttamsingh, V.; Xia, C.Q.; Miwa, G.T.; Balani, S.K.; Gan, L.S. Comparison of intrinsic clearance in liver microsomes and hepatocytes from rats and humans: Evaluation of free fraction and uptake in hepatocytes. Drug Metab. Dispos. 2006, 34, 1600–1605. [Google Scholar] [CrossRef] [PubMed]
- Kajbaf, M.; Ricci, R.; Zambon, S.; Fontana, S. Contribution of rat intestinal metabolism to the xenobiotics clearance. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zareba, P.; Dudek, M.; Lustyk, K.; Siwek, A.; Starowicz, G.; Bednarski, M.; Nowinski, L.; Razny, K.; Sapa, J.; Malawska, B.; et al. alpha-Adrenoceptor antagonistic and hypotensive properties of novel arylpiperazine derivatives of pyrrolidin-2-one. Bioorg. Med. Chem. 2015, 23, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.K.; Solanki, A.; Shirsath, S.V. Comparative in vitro intrinsic clearance of Imipramine in multiplespecies liver microsomes: Human, rat, mouse and dog. J. Drug Metab. Toxicol. 2012, 3, 126. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 2–18 are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).