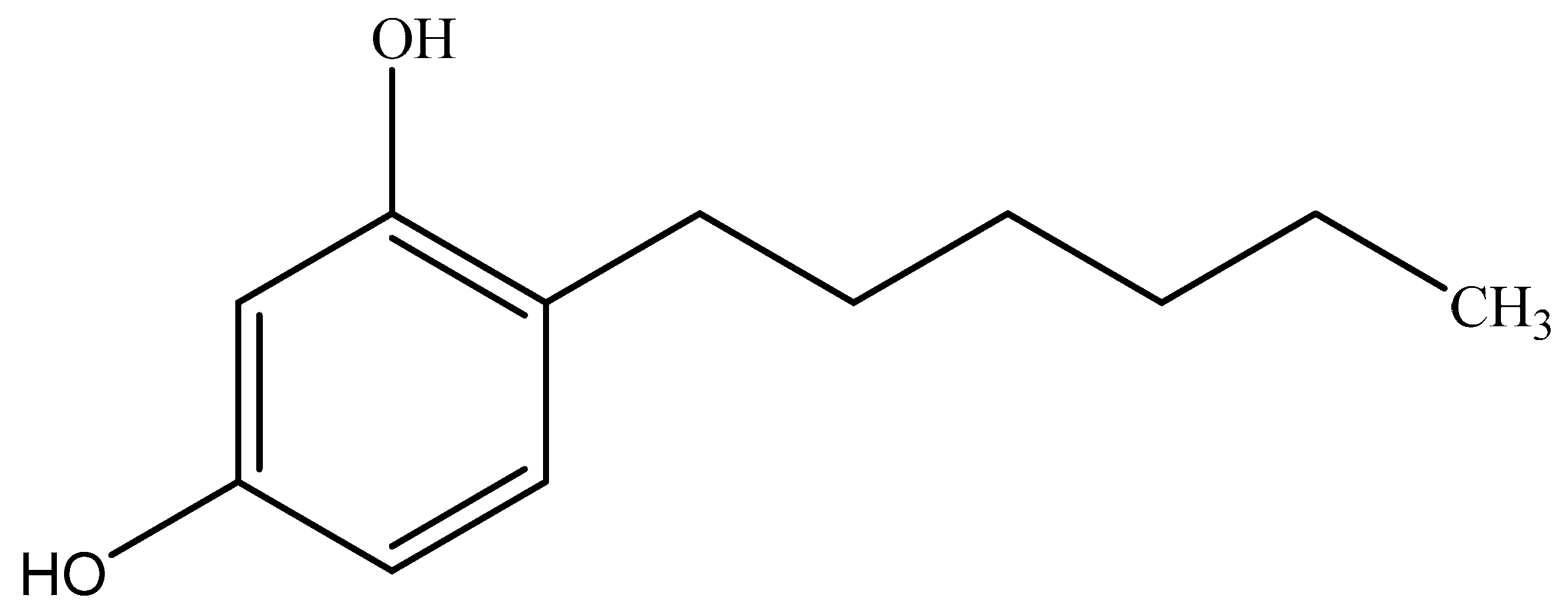
Determination of 4-Hexylresorcinol in Shrimp Samples by Solid Phase Extraction Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
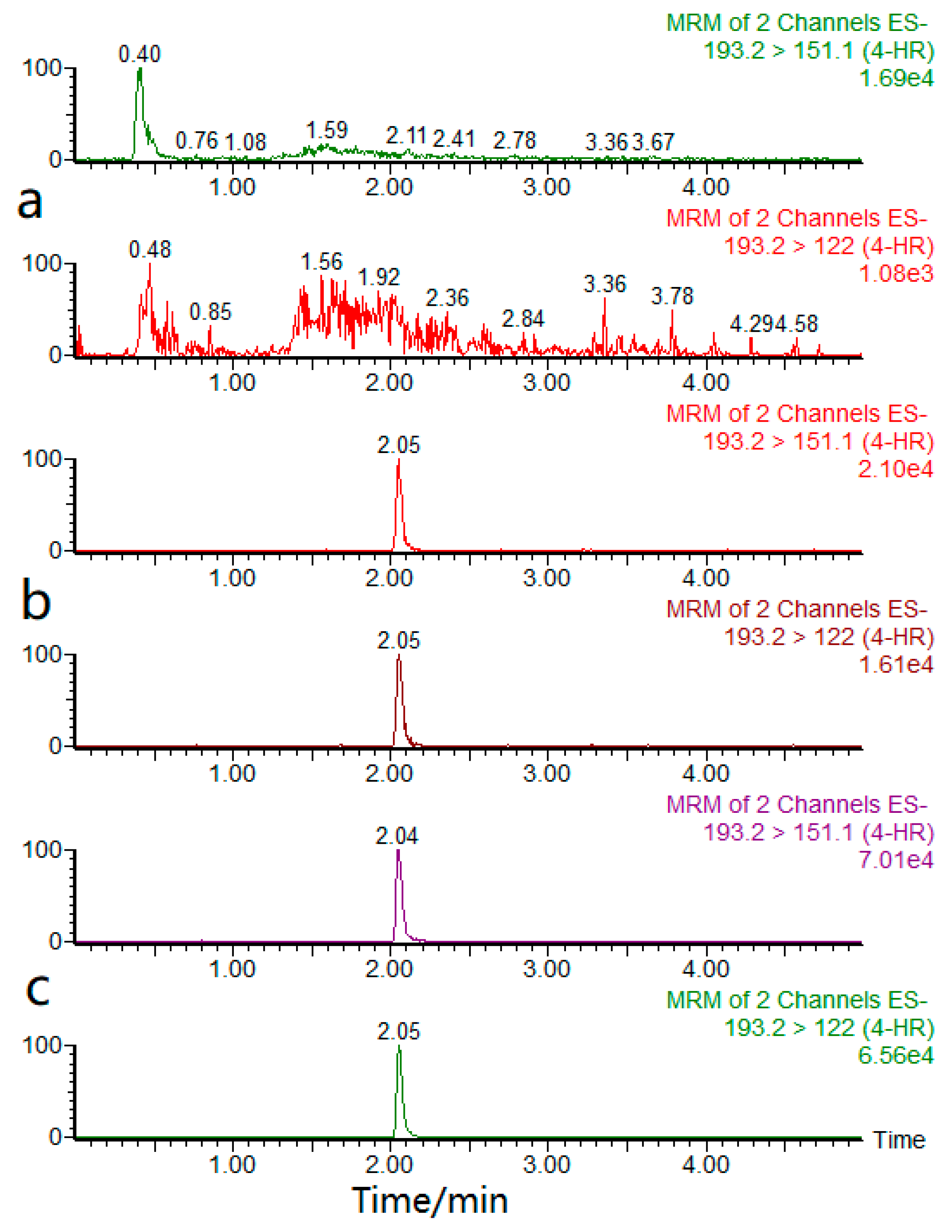
2.1. Optimization of the Chromatography Conditions
2.2. Optimization of the MS Condition
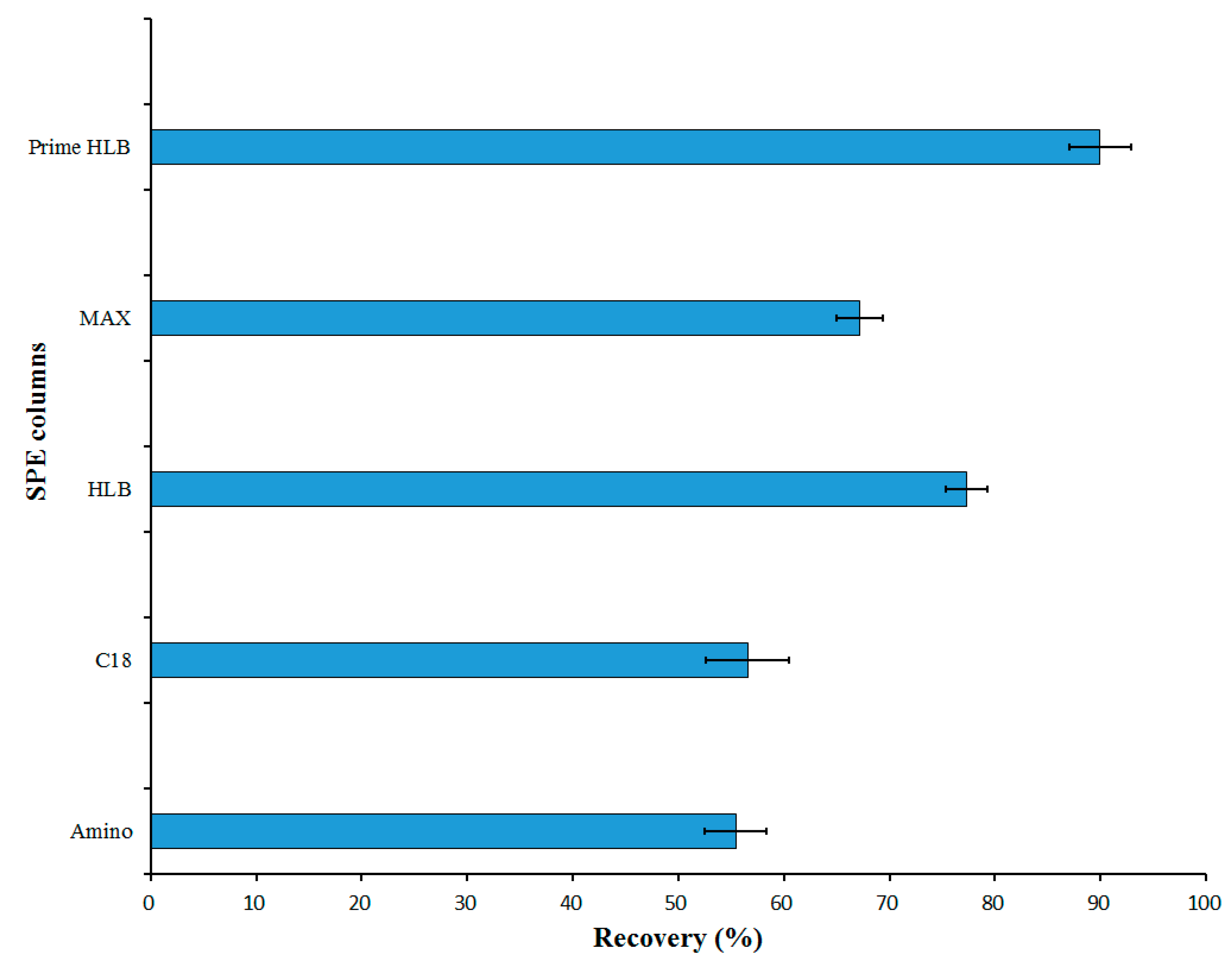
2.3. Selection of the Solid Phase Extraction Column
2.4. Optimization of the Prime HLB Solid Phase Extraction Column Conditions
2.4.1. Selection of the Type and Volume of the Washing Solution
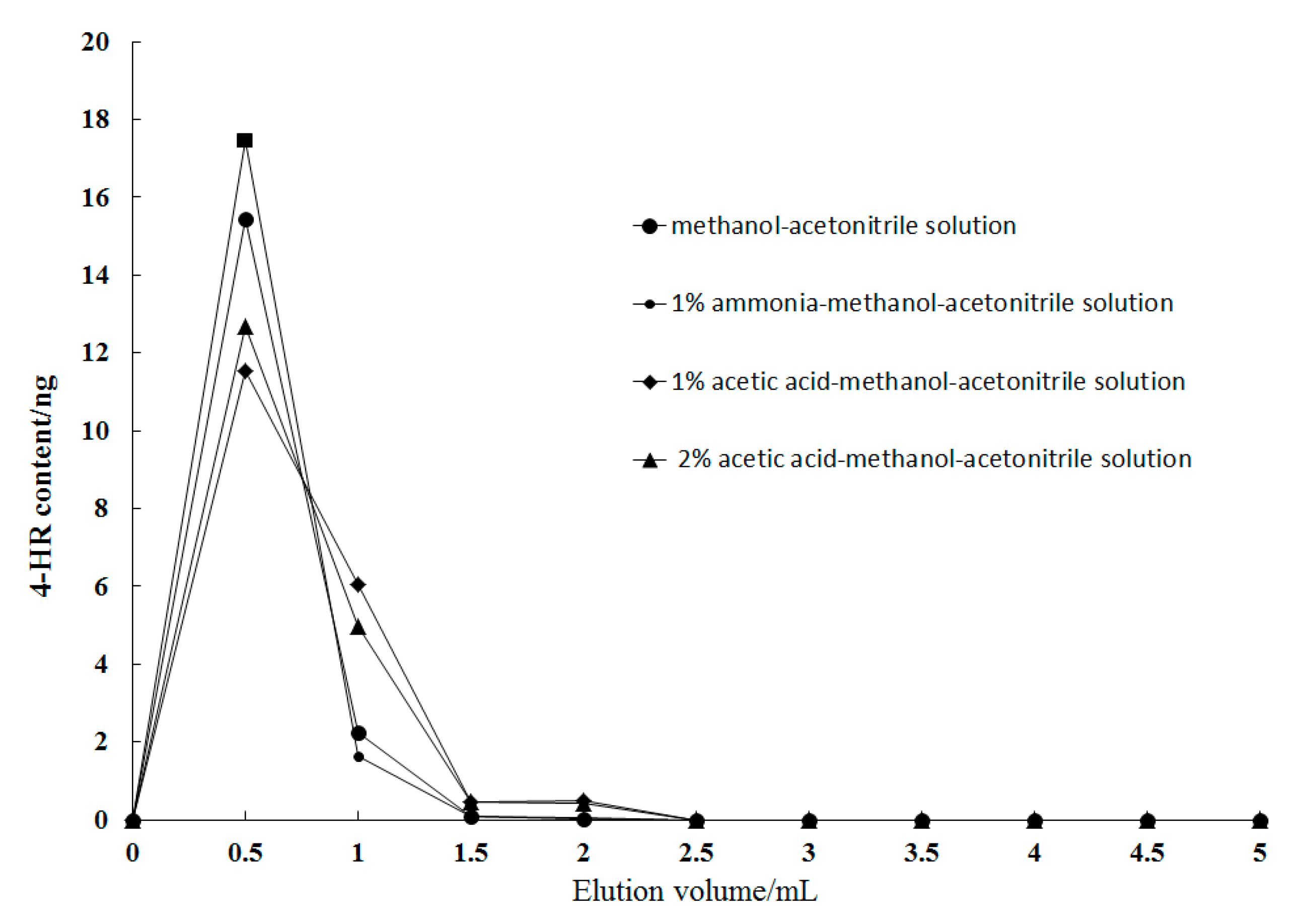
2.4.2. Selection of the Eluent
2.5. Linear Range and Detection Limit
2.6. Recovery Rate and Accuracy
2.7. Practical Sample Analysis
3. Materials and Methods
3.1. Materials and Reagents
3.2. Instruments and Equipment
3.3. Methods
3.3.1. Solution Preparation
3.3.2. Sample Extraction
3.3.3. Purification by Solid Phase Extraction Column
3.3.4. Conditions of UPLC
3.3.5. Conditions for MS
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Oujifard, A.; Seyfabadi, J.; Kenari, A.A.; Rezaei, M. Growth and apparent digestibility of nutrients, fatty acids and amino acids in pacific white shrimp, litopenaeus vannamei, fed diets with rice protein concentrate as total and partial replacement of fish meal. Aquaculture 2012, 342, 56–61. [Google Scholar] [CrossRef]
- Ouraji, H.; Abedian Kenari, A.M.; Shabanpour, B.; Shabani, A.; Sodagar, M.; Jafarpour, S.A.; Ebrahimi, G.H. Growth, survival, and fatty acid composition of indian white shrimp fenneropenaeus indicus (milne edwards) fed diets containing different levels of vitamin e and lipid. Aquacult. Int. 2011, 19, 903–916. [Google Scholar] [CrossRef]
- Rosa, R.; Nunes, M.L. Nutritional quality of red shrimp, aristeusantennatus (risso), pink shrimp, parapenaeus longirostris (lucas), and norway lobster, nephrops norvegicus (Linnaeus). J. Sci. Food Agric. 2004, 84, 89–94. [Google Scholar] [CrossRef]
- Ayisi, C.L.; Hua, X.M.; Apraku, A.; Afriyie, G.; Beatrice Kyei, B.A. Recent Studies Toward the Development of Practical Diets for Shrimp and Their Nutritional Requirements. HAYATI J. Biosci. 2017, 24, 109–117. [Google Scholar] [CrossRef]
- Benjakul, S.; Visessanguan, W.; Tanaka, M. Properties of phenoloxidase isolated from the cephalothorex of kuruma prawn (Penaeus japonicus). J. Food Biochem. 2005, 29, 470–485. [Google Scholar] [CrossRef]
- Montero, P.; Martínez-Álvarez, O.; Zamorano, J.P.; Alique, R.; Gómez-Guillén, M.C. Melanosis inhibition and 4-hexylresorcinol residual levels in deepwater pink shrimp (Parapenaeus longirostris) following various treatments. Eur. Food Res. Technol. 2006, 223, 16–21. [Google Scholar] [CrossRef]
- Mcevily, A.J.; Radha, I.; Akiva, G. Compositions and Methods for Inhibiting Browning in Foods Using Resorcinol Derivatives. U.S. Patent 5,059,438, 22 October 1991. [Google Scholar]
- Frankos, V.H.; Schmitt, D.F.; Haws, L.C.; Mcevily, A.J.; Iyengar, R.; Miller, S.A.; Munro, L.C.; Clydesdale, F.M.; Forbes, A.L.; Sauer, R.M. Generally recognized as safe (GRAS) evaluation of 4-hexylresorcinol for use as a processing aid for prevention of melanosis in shrimp. Regul. Toxicol. Pharmacol. 1991, 14, 202–212. [Google Scholar] [CrossRef]
- Iyengar, R.; Bohmont, C.W.; Mcevily, A.J. 4 -hexylresorcinol and prevention of shrimp blackspot: Residual analyses. J. Food Comp. Anal. 1991, 4, 148–157. [Google Scholar] [CrossRef]
- Alvarezparrilla, E.; Ladela, R.; Rodrigogarcia, J.; Escobedo-Gonzalez, R.; Mercado-Mercado, G.; Moyers-Montoya, E.; Vázquez-Flores, A.; González-Aguilar, G.A. Dual effect of β-cyclodextrin (β-CD) on the inhibition of apple polyphenol oxidase by 4-hexylresorcinol (HR) and methyl jasmonate (MJ). Food Chem. 2007, 101, 1346–1356. [Google Scholar] [CrossRef]
- Qiu, F.Y.; Ding, L.; Chen, X.M. UPLC-MS/MS determination of 4-hexylresorcinol in Coprinus comatus. Phys. Test. Chem. Anal. 2016, 52, 514–517. [Google Scholar]
- Wang, Y. Determination of 4-Hexylresorcinol in fresh pleurotus eryngii by ultra performance liquid chromatography tandem mass spectrometry. Chin. J. Anal. Lab. 2015, 954–957. [Google Scholar]
- LóPezcaballero, M.E.; MartíNezáLvarez, O.; GóMezguilléN, M.C.; Montero, P. Quality of Norway lobster (Nephrops norwegicus) treated with a 4-hexylresorcinol-based formulation. Eur. Food Res. Technol. 2006, 222, 425–431. [Google Scholar] [CrossRef]
- Dai, X.Y.; Zhang, M.X.; Wei, X.Y.; Hider, R.C.; Zhou, T. Novel multifunctional hydroxypyridinone derivatives as potential shrimp preservatives. Food Bioprocess Technol. 2016, 9, 1079–1088. [Google Scholar] [CrossRef]
- Ying, Y.U. Determination of 4-hexylresorcinol residue in crustacea by gas chromatography-mass spectrometry. J. Fujian Fish. 2015, 37, 227–232. [Google Scholar]
- Shokry, R.F.; Bebawy, L.I.; Elghobashy, M.R.; Abbas, S.S. Comparative stability-indicating chromatographic methods for determination of 4-hexylresorcinol in pharmaceutical formulation and shrimps. J. Pharm. Biomed. 2017, 145, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, M.; Matsumoto, H.; Kasuya, Y.; Yasuda, K. Determination of 4-hexylresorcinol residues in prawns and crabs. Shokuhin seigaku zasshi. J. Food Hyg. Soc. Jpn. 2005, 46, 282–285. [Google Scholar] [CrossRef]
- SelçUk, A.; Özden, Ö. A rapid hplc method for determination of 4-hexylresorcinol residues in shrimp. J. Fish. Sci. Com. 2014, 8, 99–108. [Google Scholar]
- Yang, W.G.; Ha, J.H.; Kim, S.G.; Chae, W.S. Spectroscopic determination of alkyl resorcinol concentration in hydroxyapatite composite. J. Anal. Sci. Technol. 2016, 7, 9. [Google Scholar] [CrossRef]
- Jonker, K.M.; Dekker, C.P. Determination of 4-hexylresorcinol in shrimp by liquid chromatography with fluorescence detection. J. Aoac. Int. 2000, 83, 241–244. [Google Scholar] [PubMed]
- Kim, Y.H.; Kim, J.M.; Lee, J.S.; Gang, S.R.; Lim, H.S.; Kim, M.; Lee, O.H. Development and validation of an analytical method for the determination of 4-hexylresorcinol in food. Food Chem. 2016, 190, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Deng, Q.; Xie, J. Determination of residues of 4-hexylresorcinol in shrimp and crab by UPLC-MS/MS. Chin. Meas. Test. 2015, 41, 60–63. [Google Scholar]
- Li, X.Q.; Chao, J.; Sun, Y.Y.; Yang, M.L.; Chu, X.G. Analysis of synthetic antioxidants and preservatives in edible vegetable oil by HPLC/TOF-MS. Food Chem. 2009, 113, 692–700. [Google Scholar]
- Eeckhaut, A.V.; Lanckmans, K.; Sarre, S.; Smolders, I.; Michotte, Y. Validation of bioanalytical LC-MS/MS assays: Evaluation of matrix effects. J. Chromatogr. B 2009, 877, 2198–2207. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, X.; Xiao, C.; Wang, Z.; Wang, J.; Xiao, H.; Cui, L.; Xiang, Q.; Yue, T. HPLC determination of aflatoxin M1, in liquid milk and milk powder using solid phase extraction on OASIS HLB. Food Control 2012, 28, 131–134. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
| Samples | Spiked (μg/kg) | Mean Recovery/% | Intraday RSD (n = 6)/% | Interday RSD (n = 5)/% |
|---|---|---|---|---|
| Parapenaeopsis hardwichii | 0.8 | 83.36 | 4.88 | 5.43 |
| 4.0 | 84.01 | 5.13 | 4.82 | |
| 8.0 | 85.41 | 6.10 | 5.95 | |
| Fenneropenaeus chinensis | 0.8 | 82.53 | 5.29 | 6.86 |
| 4.0 | 88.23 | 3.57 | 5.04 | |
| 8.0 | 83.25 | 5.14 | 5.35 | |
| Palaemon gravieri | 0.8 | 82.46 | 6.34 | 6.13 |
| 4.0 | 85.83 | 4.31 | 5.53 | |
| 8.0 | 90.62 | 5.57 | 6.72 | |
| Acetes chinensis | 0.8 | 81.35 | 5.36 | 5.76 |
| 4.0 | 86.82 | 3.79 | 6.29 | |
| 8.0 | 82.33 | 4.66 | 6.51 | |
| Exopalaemon annadalei | 0.8 | 81.62 | 6.32 | 6.87 |
| 4.0 | 94.68 | 5.26 | 5.73 | |
| 8.0 | 85.19 | 5.81 | 5.54 |
| Time/min | Flow Rate/mL·min−1 | Water/% | Acetonitrile/% | Gradient Curve |
|---|---|---|---|---|
| 0.00 | 0.3 | 90.0 | 10.0 | Initial |
| 0.50 | 0.3 | 90.0 | 10.0 | 6 |
| 1.50 | 0.3 | 10.0 | 90.0 | 6 |
| 4.00 | 0.3 | 10.0 | 90.0 | 6 |
| 4.50 | 0.3 | 90.0 | 10.0 | 6 |
| 5.00 | 0.3 | 90.0 | 10.0 | 6 |
| Compound Name | Parent (m/z) | Daughter (m/z) | Capillary Pressure/kV | Cone Pressure/V | Collision Energy/eV |
|---|---|---|---|---|---|
| 4-HR quantitative ion pair | 193.2 | 151.1 | 2.7 | 30 | 17 |
| 4-HR qualitative ion pair | 193.2 | 122.0 | 2.7 | 30 | 20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Y.; Gao, X.; Xia, W. Determination of 4-Hexylresorcinol in Shrimp Samples by Solid Phase Extraction Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry. Molecules 2018, 23, 2173. https://doi.org/10.3390/molecules23092173
Hao Y, Gao X, Xia W. Determination of 4-Hexylresorcinol in Shrimp Samples by Solid Phase Extraction Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry. Molecules. 2018; 23(9):2173. https://doi.org/10.3390/molecules23092173
Chicago/Turabian StyleHao, Yunbin, Xuehui Gao, and Wenshui Xia. 2018. "Determination of 4-Hexylresorcinol in Shrimp Samples by Solid Phase Extraction Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry" Molecules 23, no. 9: 2173. https://doi.org/10.3390/molecules23092173
APA StyleHao, Y., Gao, X., & Xia, W. (2018). Determination of 4-Hexylresorcinol in Shrimp Samples by Solid Phase Extraction Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry. Molecules, 23(9), 2173. https://doi.org/10.3390/molecules23092173





