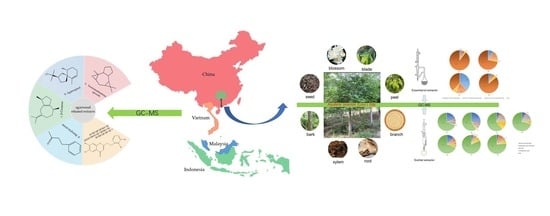
GC-MS Study of the Chemical Components of Different Aquilaria sinensis (Lour.) Gilgorgans and Agarwood from Different Asian Countries
Abstract
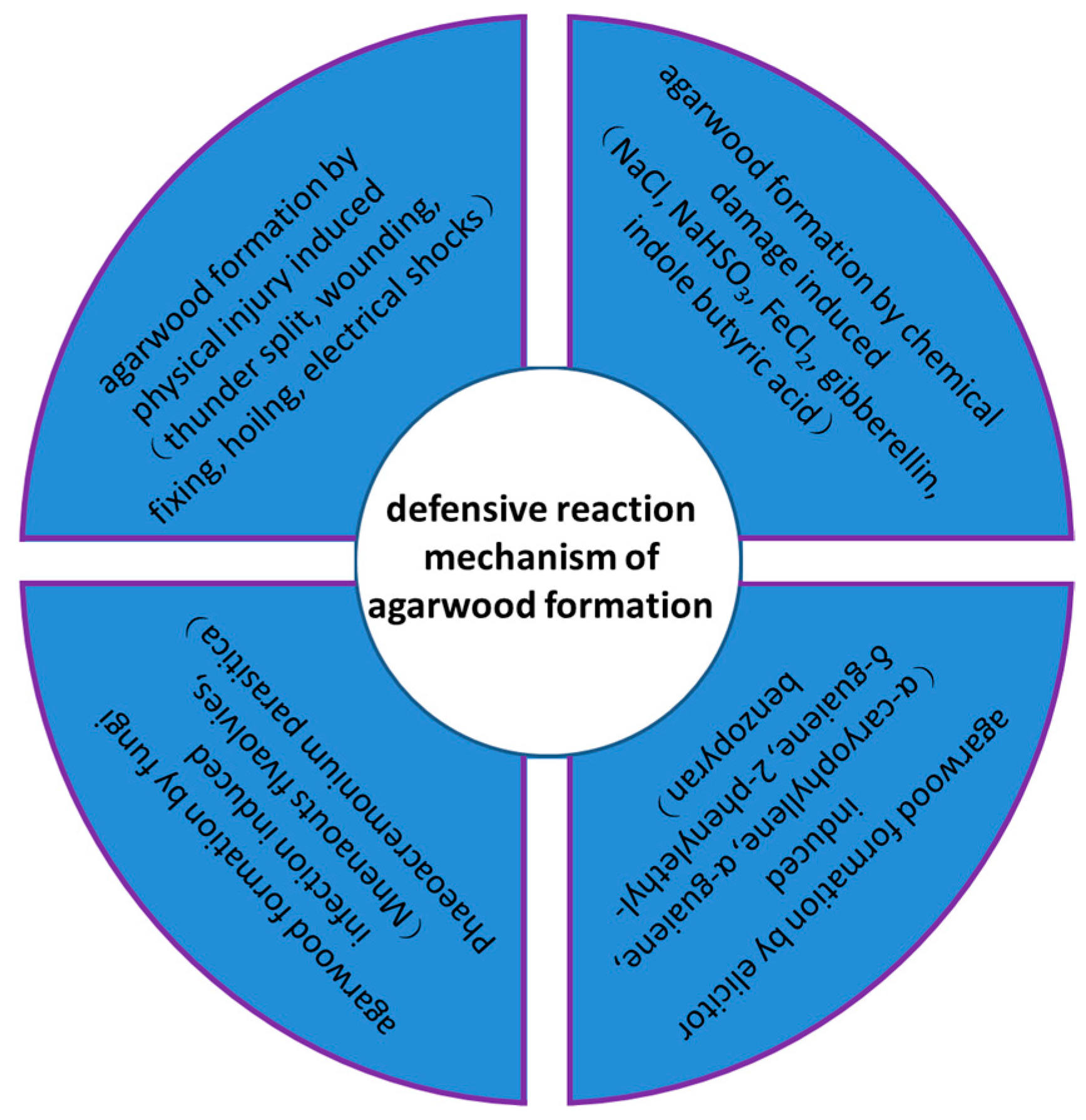
:1. Introduction
2. Results and Discussion
2.1. Moisture Analysis of A. sinensis Organs and Agarwood from Different Regions
2.2. Chemical Composition Analysis of A. sinensis
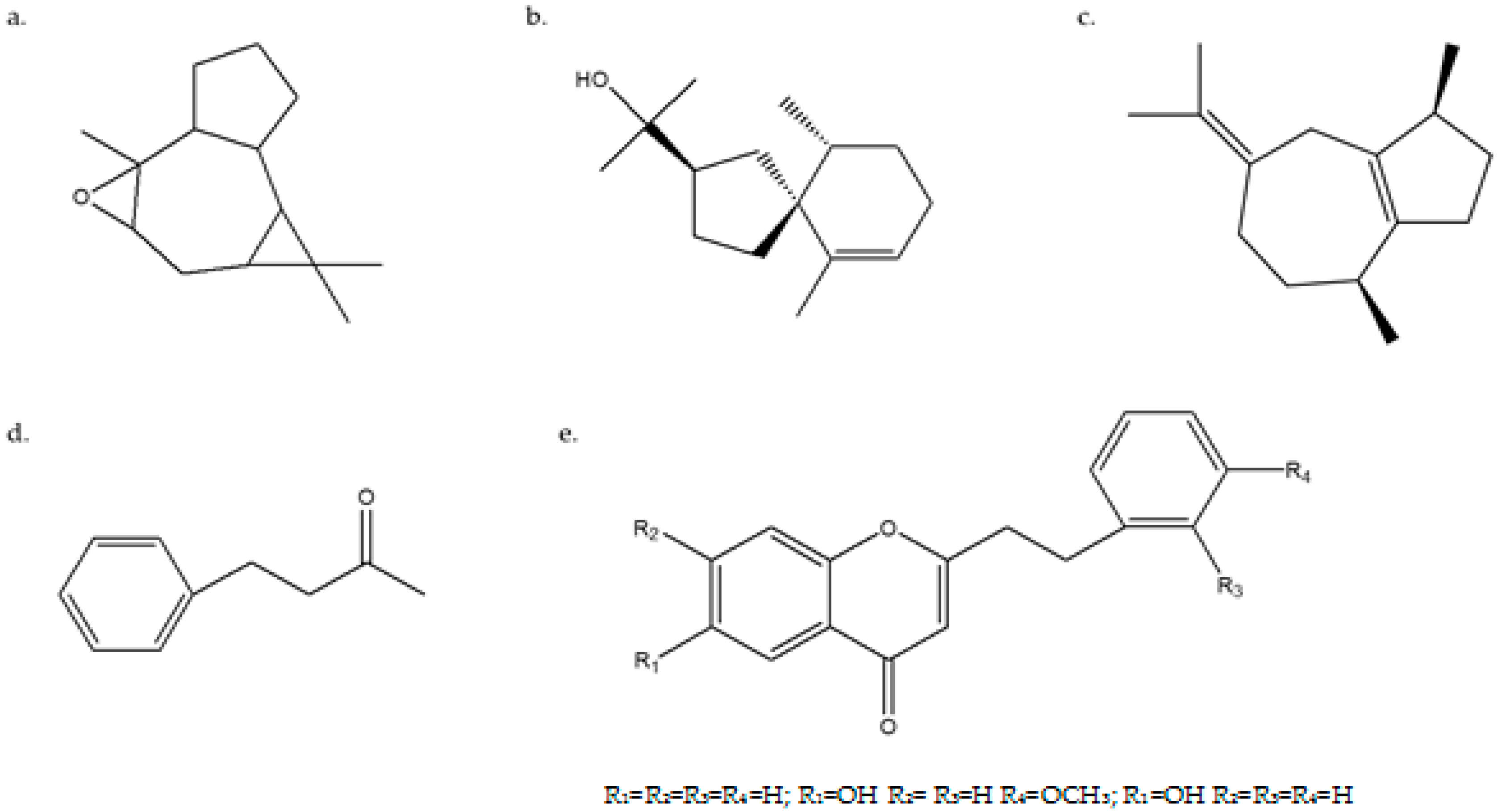
2.3. Chemical Composition Analysis of Agarwood from Different Regions
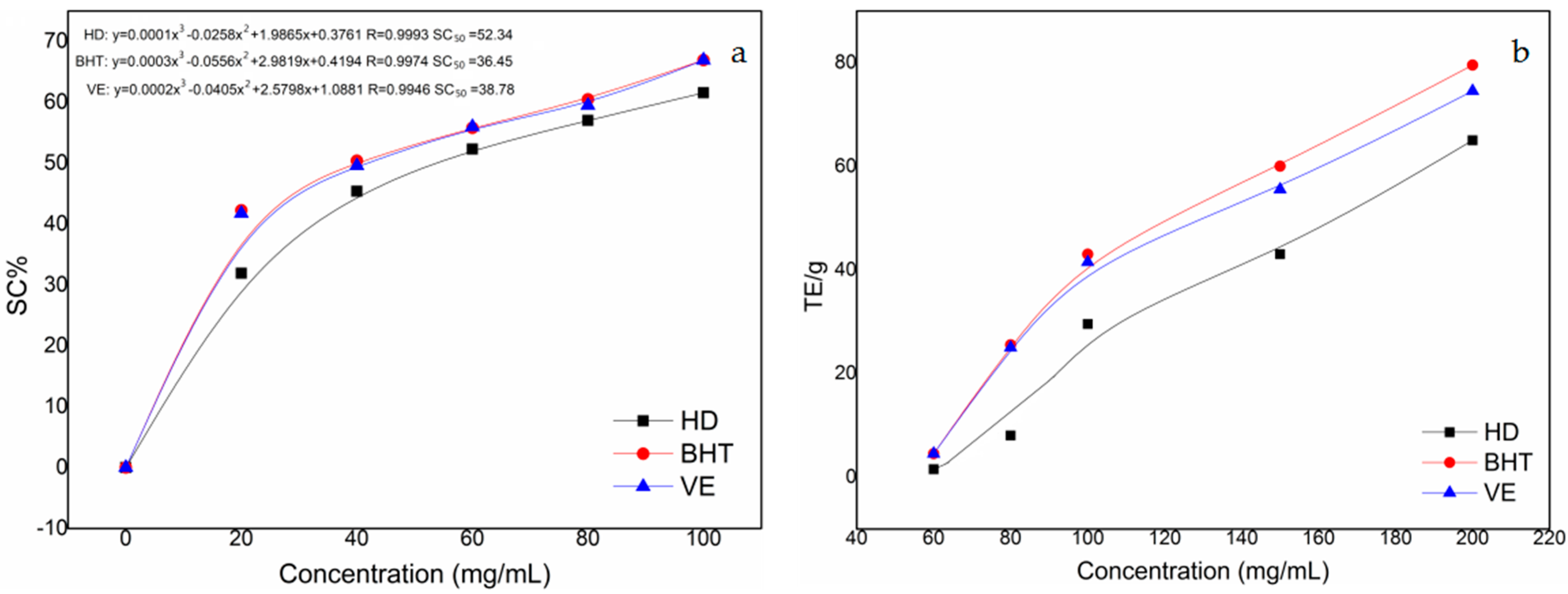
2.4. Antioxidant Activity Tests
Free Radical Scavenging Activity Assay of the Essential Oil
2.5. Antibacterial Properties
3. Materials and Methods
3.1. Materials
3.1.1. Apparatus
3.1.2. Plant Material
3.1.3. Bacterial Strains
3.1.4. Preparation of Culture Medium
3.2. Methods
3.2.1. Moisture Detection Method
3.2.2. Hydrodistillation (HD) Method for Extracting Volatile Oils
3.2.3. Soxhlet Extraction Method for Preparation of Alcohol Extracts
3.2.4. GC-MS Analysis Condition
3.2.5. Antioxidant Capacity
DPPH Radical Scavenging Activity Assay of Volatile Oils (DPPH)
Ferric Ion Reducing Antioxidant Power (FRAP) of the Volatile Oil
3.2.6. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, G.; Liu, C.Q.; Sun, W.B. Pollination and seed dispersal of Aquilaria sinensis (Lour.) Gilg (Thymelaeaceae): An economic plant species with extremely small populations in China. Plant Divers. 2016, 38, 227–232. [Google Scholar] [CrossRef]
- Xu, Y.H.; Liao, Y.C.; Zhang, Z.; Liu, J.; Sun, P.W.; Gao, Z.H.; Sui, C.; Wei, J.H. Jasmonic acid is a crucial signal transducer in heat shock induced sesquiterpene formation in Aquilaria sinensis. Sci. Rep. 2016, 6, 21843. [Google Scholar] [CrossRef] [PubMed]
- Putri, N.; Karlinasari, L.; Turjaman, M.; Wahyudi, I.; Nandika, D. Evaluation of incense-resinous wood formation in agarwood (Aquilaria malaccensis Lam.) using sonic tomography. Agric. Nat. Resour. 2017, 51, 84–90. [Google Scholar] [CrossRef]
- Groves, M.; Rutherford, C. CITES and Timber: A Guide to CITES-Listed Tree Species; Kew Publishing, Royal Botanic Kew Garden: London, UK, 2015; pp. 14–16. ISBN 9781842465929. [Google Scholar]
- Zhang, X.L.; Liu, Y.Y.; Wei, J.H.; Yang, Y.; Zhang, Z.; Huang, J.Q.; Chen, H.Q.; Liu, Y.J. Production of high-quality agarwood in Aquilaria sinensis trees via whole-tree agarwood-induction technology. Chin. Chem. Lett. 2012, 23, 727–730. [Google Scholar] [CrossRef]
- Al-Hindi, R.R.; Aly, S.E.; Hathout, A.S.; Alharbi, M.G.; Al-Masaudi, S.; Al-Jaouni, S.K.; Harakeh, S.M. Isolation and molecular characterization of mycotoxigenic fungi in agarwood. Saudi J. Biol. Sci. 2017. [Google Scholar] [CrossRef]
- Mei, W.L.; Zuo, W.J.; Yang, D.L.; Dong, W.H.; Dai, H.F. Advances in the Mechanism, Artificial Agarwood-induction Techniques and Chemical Constituents of Artificial Agarwood Production. Chin. J. Trop. Crops 2013, 34, 2513–2520. [Google Scholar] [CrossRef]
- Chen, H.Q.; Wei, J.H.; Yang, J.S.; Zhang, Z.; Yang, Y.; Gao, Z.H.; Sui, C.; Gong, B. Chemical constituents of agarwood originating from the endemic genus Aquilaria plants. Chem. Biodivers. 2012, 9, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, P.; Tan, R.X.; Li, X.W.; Liao, B.S.; Ouyang, P.Y.; Huang, Z.H. Global Suitability Analysis of Aquilaria Sinensis (Lour.) Gilg Base on GMPGIS. World Chin. Med. 2017, 12, 979–985. [Google Scholar] [CrossRef]
- Chen, D.; Bi, D.; Song, Y.L.; Tu, P.F. Flavanoids from the stems of Aquilaria sinensis. Chin. J. Nat. Med. 2012, 10, 287–291. [Google Scholar] [CrossRef]
- Li, W.; Cai, C.H.; Guo, Z.K.; Wang, H.; Zuo, W.J.; Dong, W.H.; Mei, W.L.; Dai, H.F. Five new eudesmane-type sesquiterpenoids from Chinese agarwood induced by artificial holing. Fitoterapia 2015, 100, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; He, X.; Wu, H.Q.; Wang, L.; Zhang, W.M.; Fan, Y.F.; Li, H.H.; Liu, T.M.; Gao, X.X. Identification and characterization of a novel sesquiterpene synthase from Aquilaria sinensis: An important gene for agarwood formation. Int. J. Biol. Macromol. 2018, 108, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, C.; Li, H.F.; Sun, J.B.; Li, Y.Y.; Gu, W.; Wang, D.Y.; Liu, J.G.; Hu, Y.L. A novel neolignan glycoside from Aquilaria sinensis. Biochem. Syst. Ecol. 2014, 55, 41–45. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, H.Q.; Kong, F.D.; Zhou, L.M.; Li, W.; Dong, W.H.; Chen, Z.B.; Mei, W.L.; Dai, H.F. Dimeric sesquiterpenoid-4H-chromone derivatives from agarwood of Aquilaria crassna and their cytotoxicity. Phytochemistry 2018, 145, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.N.; Dong, W.H.; Huang, S.Z.; Li, W.; Kong, F.D.; Wang, H.; Wang, J.; Mei, W.L.; Dai, H.F. Three new sesquiterpenoids from agarwood of Aquilaria crassna. Fitoterapia 2016, 114, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mei, W.L.; Kong, F.D.; Chen, H.Q.; Li, W.; Chen, Z.B.; Dai, H.F. Four new bi-2-(2-phenylethyl)chromone derivatives of agarwood from Aquilaria crassna. Fitoterapia 2017, 119, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cai, C.H.; Dong, W.H.; Guo, Z.K.; Wang, H.; Mei, W.L.; Dai, H.F. Anti-inflammatory 2-(2-phenylethyl)chromone derivatives from Chinese agarwood. Fitoterapia 2017, 118, 49–55. [Google Scholar] [CrossRef]
- Pharmacopoeia Committee of P.R. China. Pharmacopoeia of the People’s Republic of China, 4th ed.; China Medical Science and Technology Press: Beijing, China, 2015; p. 185. ISBN 9787506773379. [Google Scholar]
- Strobl, S.; Cueva, E.; Silva, B.; Knuesting, J. Water relations and photosynthetic water use efficiency as indicators of slow climate change effects on trees in a tropical mountain forest in South Ecuador. Ecol. Indic. 2017, 83, 550–558. [Google Scholar] [CrossRef]
- Kuang, T.D.; Chen, H.Q.; Kong, F.D.; Cai, C.H.; Yang, L.; Mei, W.L.; Dai, H.F. Three new 2-(2-phenylethyl)chromone derivatives from artificial holing agarwood of Aquilaria sinensis. Phytochem. Lett. 2018, 26, 96–100. [Google Scholar] [CrossRef]
- Shao, H.; Mei, W.L.; Kong, F.D.; Dong, W.H.; Gai, C.J.; Li, W.; Zhu, G.P.; Dai, H.F. Sesquiterpenes of agarwood from Gyrinopssalicifolia. Fitoterapia 2016, 113, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Wang, F.; Yue, C.H. Chemical constituents from the petioles and leaves of Aquilaria sinensis. Biochem. Syst. Ecol. 2015, 61, 458–461. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wei, J.H.; Gao, Z.H.; Zhang, Z.; Lyu, J.C. A Review of Quality Assessment and Grading for Agarwood. Chin. Herb. Med. 2017, 9, 22–30. [Google Scholar] [CrossRef]
- Feng, J.; Yang, X.W.; Wang, R.F. Bio-assay guided isolation and identification of α-glucosidase inhibitors from the leaves of Aquilaria sinensis. Phytochemistry 2011, 72, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.; Fryer, M.; Grosso, A. Plant Uptake of Non-Ionic Organic Chemicals. Environ. Sci. Technol. 2006, 40, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.C.; Wei, Y.H.; Wei, F.L.; Liang, Y.Z. Analysis on the Transportation Mechanism and Channel of Matter in Angiosperma. J. Anhui Agric. Sci. 2011, 39, 5083–5086. [Google Scholar] [CrossRef]
- Abubacker, M.N.; Devi, P.K. In vitro antifungal potentials of bioactive compound oleic acid, 3-(octadecyloxy) propyl ester isolated from Lepidagathiscristata Willd. (Acanthaceae) inflorescence. Asian Pac. J. Trop. Med. 2014, 7, S190–S193. [Google Scholar] [CrossRef]
- Al-Marzoqi, A.H.; Hameed, I.H.; Idan, S.A. Analysis of bioactive chemical components of two medicinal plants (Coriandrum sativum and Meliaazedarach) leaves using gas chromatography-mass spectrometry (GC-MS). Afr. J. Biotechnol. 2015, 40, 2812–2830. [Google Scholar] [CrossRef]
- Kumar, L.R.G.; Chatterjee, N.S.; Tejpal, C.S.; Vishnu, K.V.; Anas, K.K.; Asha, K.K.; Anandan, R.; Mathew, S. Evaluation of chitosan as a wall material for microencapsulation of squalene by spray drying: Characterization and oxidative stability studies. Int. J. Biol. Macromol. 2017, 104, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Tajuddin, S.N.; Muhamad, N.S.; Yarmo, M.A.; Yusoff, M.M. Characterization of the chemical constituents of agarwood oils from Malaysia by comprehensive two-dimensional gas chromatography—Time-of-flight mass spectrometry. Mendeleev Commun. 2013, 23, 51–52. [Google Scholar] [CrossRef]
- Yang, D.L.; Wang, H.; Guo, Z.K.; Li, W.; Mei, W.L.; Dai, H.F. Fragrant agarofuran and eremophilane sesquiterpenes in agarwood ‘Qi-Nan’ from Aquilaria sinensis. Phytochem. Lett. 2014, 8, 121–125. [Google Scholar] [CrossRef]
- Chhipa, H.; Chowdhary, K.; Kaushik, N. Artificial production of agarwood oil in Aquilaria sp. by fungi: A review. Phytochem. Rev. 2017, 16, 835–860. [Google Scholar] [CrossRef]
- Li, W.; Cai, C.H.; Dong, W.H.; Guo, Z.K.; Wang, H.; Mei, W.L.; Dai, H.F. 2-(2-Phenylethyl)chromone derivatives from Chinese agarwood induced by artificial holing. Fitoterapi 2014, 98, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Qiao, L.R.; Xie, D.; Yuan, Y.H.; Chen, N.H.; Dai, J.G.; Guo, S.X. 2-(2-Phenylethyl)chromones from Chinese eaglewood. Phytochemistry 2012, 76, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.X.; Xie, M.R.; Liu, S.F.; Guo, X.L.; Chen, X.Y.; Zhong, Z.J.; Wang, L.; Zhang, W.M. Chromatographic fingerprint analysis of metabolites in natural and artificial agarwood using gas chromatography–mass spectrometry combined with chemometric methods. J. Chromatogr. B 2014, 967, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.X.; Zhu, Z.X.; Pang, D.R.; Li, Y.T.; Huang, Z.; Shi, S.P.; Zheng, J.; Zhang, Q.; Zhao, Y.F.; Tu, P.F.; et al. Anti-neuroinflammatory sesquiterpenes from Chinese eaglewood. Fitoterapia 2015, 106, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Chen, H.Q.; Yang, Y.; Zhang, Z.; Wei, J.H.; Meng, H. Whole-tree agarwood-inducing technique: An efficient novel technique for producing high-quality agarwood in cultivated Aquilaria sinensis trees. Molecules 2013, 18, 3086–3106. [Google Scholar] [CrossRef] [PubMed]
- Nobuchi, T.; Somkid, S. Preliminary observation of A. quliariacrassna wood associated with the formation of aloeswoodbult. Kyoto Univ. For. 1991, 63, 226–235. [Google Scholar]
- Chong, S.P.; Osman, M.F.; Bahari, N.; Nuri, E.A.; Zakaria, R.; Abdul-Rahim, K. Agarwood inducement technology: A method for producing oil grade agarwood in cultivated Aquilaria malaccensis Lamk. J. Agrobiotechnol. 2015, 6, 1–16. [Google Scholar]
- Qi, S.Y.; Lin, L.D.; Hu, H.C. The chromone compounds formation in A. quilariasinensis. Chin. Tradit. Herb. Drugs 2000, 31, 658–659. [Google Scholar]
- Michiho, I.; Ken-Ichiro, O.; Toru, Y. Induction of ses-quiterpenoid production by methyl jasmonate in A. quilariasinensis cell suspension culture. J. Essent. Oil Res. 2005, 17, 175–180. [Google Scholar] [CrossRef]
- Qi, S.Y.; He, M.L.; Lin, L.D. Production of 2-(2-phenylethyl) chromones in cell suspension cultures of A. quilariasinensis. Plant Cell Tissue Organ Cult. 2005, 11, 217–221. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Y.; Wu, Y.N.; Xia, F.G.; Wu, Z.Q. Quickly verifying the antioxidant contribution of the individual composition in natural antioxidants by HPLC-free radical scavenging detection. LWT 2018, 96, 461–468. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007, 103, 1003–1008. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Permeability of the outer membrane of bacteria. Angew. Chem. Int. Ed. 1979, 18, 337–420. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, G.; Simsek, B.; Kuleasan, H. Antioxidant activities of Saturejacilicica essential oil in butter and in vitro. Food Eng. 2007, 79, 1391–1396. [Google Scholar] [CrossRef]
- Luo, W.; Zhao, M.M.; Yang, B.; Shen, G.L.; Rao, G.H. Identification of bioactive compounds in Phyllenthusemblica L. fruit and their free radical scavenging activities. Food Chem. 2009, 114, 499–504. [Google Scholar] [CrossRef]
- Rota, M.C.; Carraminana, J.J.; Burillo, J.; Herrera, A. In vitro antimicrobial activity of essential oils from aromatic plants against selected foodborne pathogens. J. Food Prot. 2004, 67, 1252–1256. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
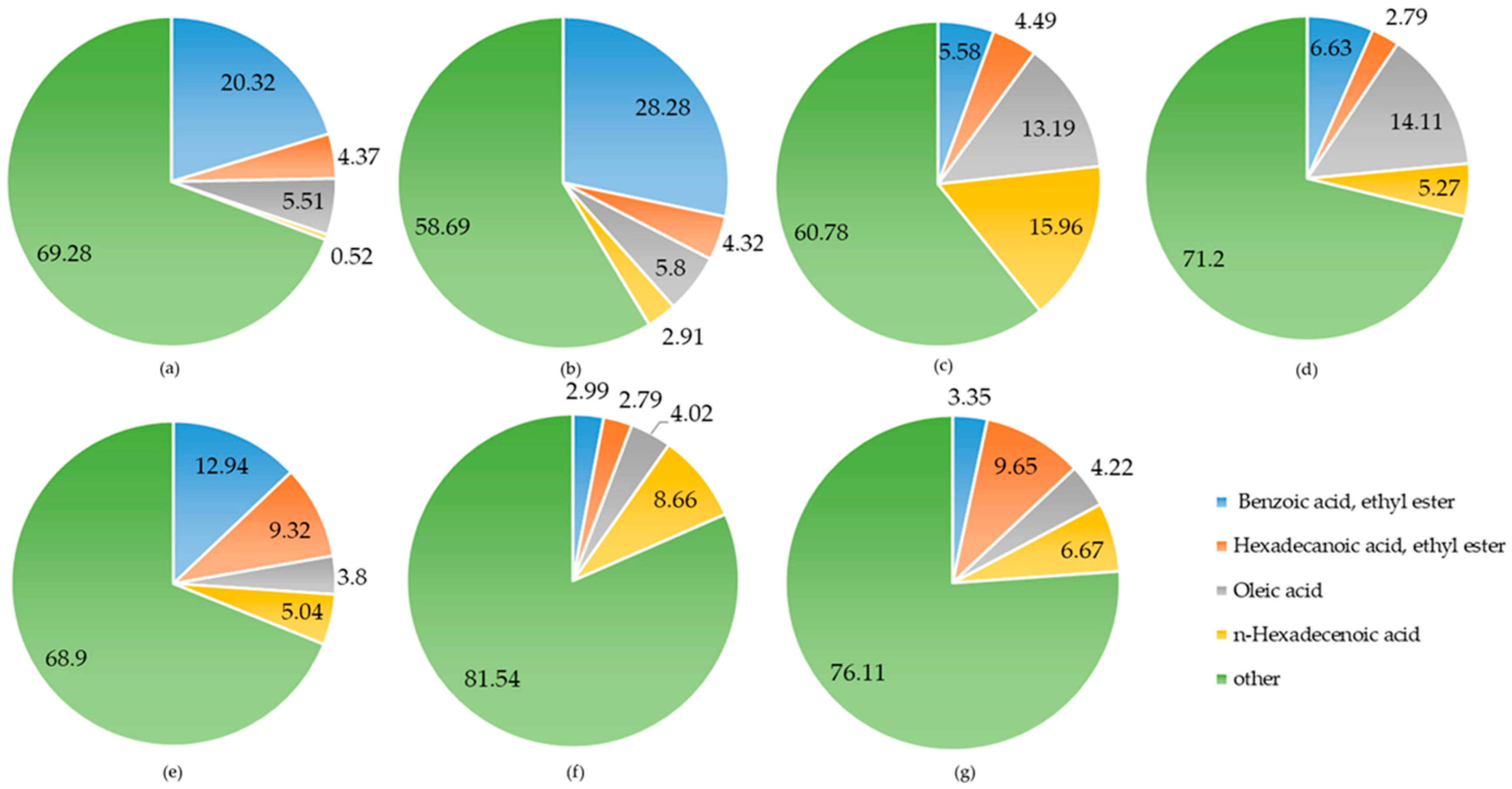
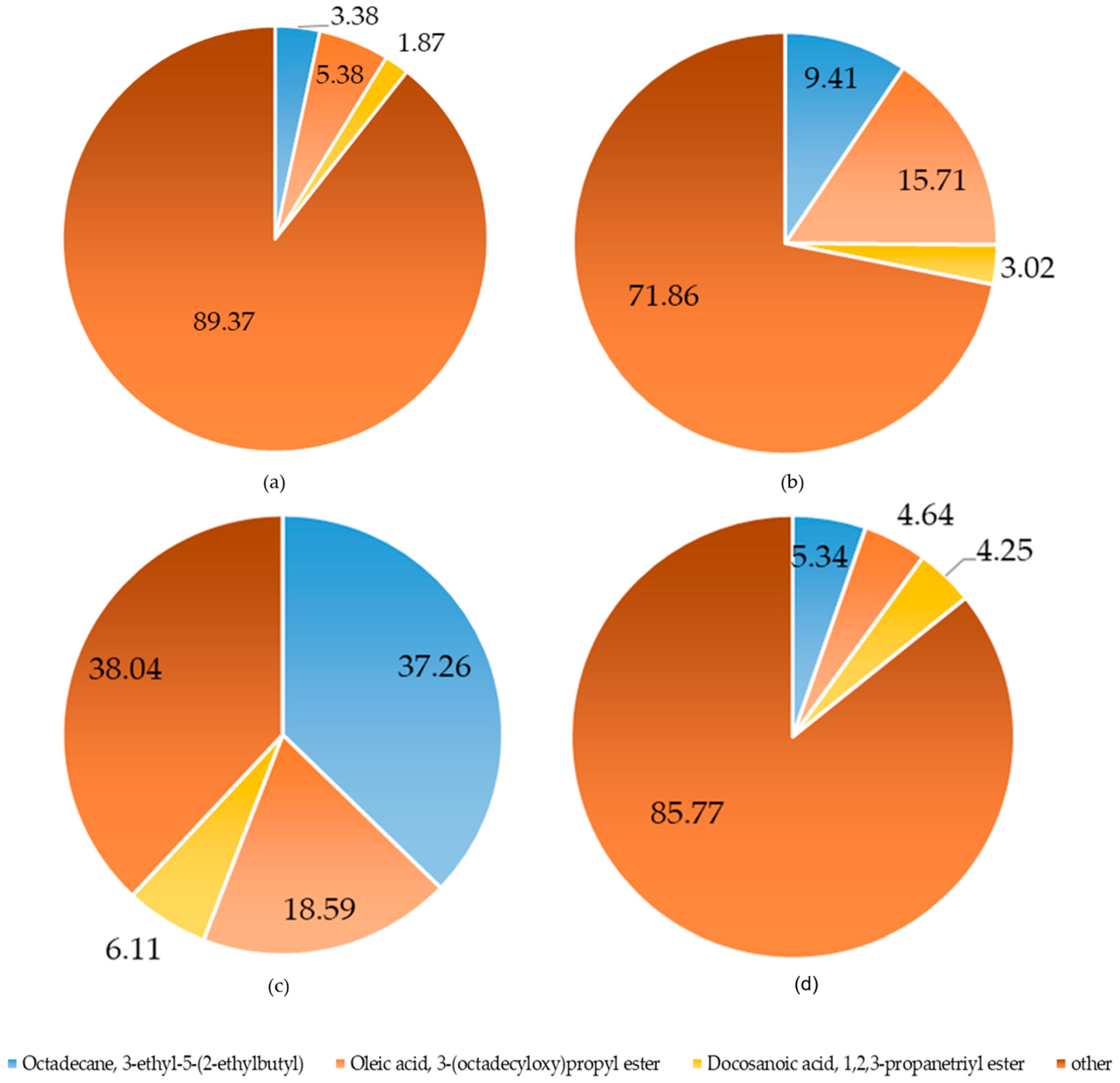
| No. | Origin | Hydrodistillation Extraction (%) | Soxhlet Extraction (%) | Moisture Percentage (%) |
|---|---|---|---|---|
| 1 | China | ND | 2.28 ± 0.16 | 7.99 ± 0.13 |
| 2 | Malaysia | ND | 21.47 ± 0.90 | 9.22 ± 0.16 |
| 3 | Indonesia | ND | 10.51 ± 0.36 | 11.27 ± 0.31 |
| 4 | Vietnam | ND | 11.5 ± 0.45 | 14.73 ± 0.08 |
| 5 * | blossom | <0.05 | 21.51 ± 1.05 | 71.98 ± 0.83 |
| 6 * | seed | <0.1 | 11.01 ± 0.43 | 79.42 ± 1.27 |
| 7 * | peel | <0.05 | 25.24 ± 1.10 | 81.2 ± 0.68 |
| 8 * | blade | <0.05 | 20.30 ± 1.33 | 63.88 ± 0.66 |
| 9 * | branch | ND | 3.48 ± 0.21 | 63.23 ± 0.24 |
| 10 * | xylem | ND | 2.39 ± 0.28 | 51.95 ± 0.03 |
| 11 * | bark | ND | 6.85 ± 0.26 | 70.07 ± 0.23 |
| 12 * | root | ND | 3.81 ± 0.26 | 46.48 ± 0.36 |
| A. sinensis Organ | Main Chemical Components | Molecular Formular | Molecular Weight | CAS Number | RA% | |
|---|---|---|---|---|---|---|
| Volatile Component | Alcohol Extracts | |||||
| blossom | Benzoic acid, 2-hydroxy-, phenylmethyl ester n-Hexadecanoic acid | C14H12O3 C16H32O2 | 228 256 | 118-58-1 57-10-3 | 16.59 -- | -- 15.96 |
| seed | 10-Octadecenoic acid, methyl ester Squalene | C19H36O2 C30H50 | 296 410 | 13481-95-3 111-02-4 | 21.30 -- | -- 36.66 |
| peel | Benzoic acid, 2-hydroxy-, phenylmethyl ester Benzoic acid, ethyl ester | C14H12O3 C9H10O2 | 228 150 | 118-58-1 93-89-0 | 21.50 -- | -- 28.28 |
| blade | Octadecane, 3-ethyl-5-(2-ethylbutyl)-n-Hexadecanoic acid | C26H54 C16H32O2 | 366 256 | 55282-12-7 57-10-3 | 13.34 -- | -- 8.66 |
| branch | 4-((1E)-3-Hydroxy-1-propenyl)-2-methoxyphenol | C10H12O3 | 180 | 458-35-5 | -- | 15.07 |
| xylem | 8-Naphthol, 1-(benzyloxy)- | C17H14O2 | 250 | 326875-68-7 | -- | -- 18.18 |
| bark | 9-Octadecenoic acid, 1,2,3-propanetriyl ester | C57H104O6 | 884 | 537-39-3 | -- | 6.74 |
| root | Benzoic acid, ethyl ester | C9H10O2 | 150 | 93-89-0 | -- | 20.32 |
| Main Chemical Components (RA%) | Molecular Weight | Malaysia | China | Indonesia | Vietnam |
|---|---|---|---|---|---|
| Isoaromadendrene epoxide (C15H24O) | 220 | 0.28% | 1.14% | 0.12% | 4.50% |
| Agarospirol (C15H26O) | 222 | 0.11% | 0.13% | 0.49% | 0.66% |
| β-Guaiene (C15H24) | 204 | 0.08% | 0.07% | 0.21% | 0.21% |
| Benxylacatone (C10H12O) | 148 | 0.21% | 0.18% | 0.30% | 0.15% |
| 2-(2phenylethyl)chromone (C17H14O2) | 250 | 1.78% | 1.65% | 0.07% | 0.03% |
| 6-hydroxy-2-[2-(4-methoxyl-phenyl)ethyl]chromone (C18H16O4) | 0.56% | 0.49% | 0.23% | 0.21% | |
| 6-hydroxy-2-(2-phenylethyl)chromone (C17H15O3) | 0.43% | 0.79% | 0.77% | 0.17% |
| Concentration(mg/mL) | E. coli | B. subtilis | S. aureus | |||
|---|---|---|---|---|---|---|
| Average OD (cm) | Inhibition Ratio (%) | Average OD (cm) | Inhibition Ratio (%) | Average OD (cm) | Inhibition Ratio (%) | |
| 0 | 2.03 | 0 | 2.05 | 0 | 2.04 | 0 |
| 0.2 | 2.15 | 5.58 ± 0.22 | 2.44 | 15.98 ± 0.32 | 2.43 | 16.05 ± 0.33 |
| 0.4 | 2.34 | 13.25 ± 0.43 | 2.72 | 24.63 ± 0.82 | 2.77 | 26.35 ± 0.81 |
| 0.6 | 2.56 | 20.70 ± 1.07 | 3.12 | 34.29 ± 1.30 | 3.36 | 39.29 ± 1.21 |
| 0.8 | 2.84 | 28.52 ± 1.31 | 3.57 | 42.58 ± 1.42 | 3.76 | 45.74 ± 1.24 |
| 1.0 | 3.09 | 34.30 ± 1.52 | 3.89 | 47.30 ± 1.33 | 4.01 | 49.13 ± 1.65 |
| 1.2 | 3.53 | 42.49 ± 1.88 | 4.39 | 53.30 ± 1.82 | 4.48 | 54.46 ± 1.75 |
| 1.4 | 3.81 | 46.72 ± 2.07 | 4.66 | 56.01 ± 2.31 | 4.82 | 57.68 ± 1.97 |
| 1.6 | 4.07 | 50.12 ± 2.44 | 4.97 | 58.75 ± 2.82 | 5.30 | 61.51 ± 2.65 |
| 1.8 | 4.40 | 53.86 ± 2.47 | 5.12 | 59.96 ± 2.34 | 5.54 | 63.18 ± 2.57 |
| 2.0 | 4.83 | 57.97 ± 3.44 | 5.23 | 60.80 ± 3.82 | 5.74 | 64.46 ± 3.01 |
| Origin | Geographical Location Information |
|---|---|
| China | Guangdong, China is located between latitude 20°13′~25°31′ and longitude 109°39′~117°19′; subtropical monsoon climate, annual average precipitation is 1300~2500 mm. |
| Malaysia | Malaysia is located between 1~7° north latitude and 97–120° east longitude, tropical rainforest climate and tropical monsoon climate, no obvious four seasons, average temperature is 26~30 °C, abundant rainfall. |
| Indonesia | Indonesia is located between 12° S–7° N and 96° E~140° E, tropical rainforest climate, annual average temperature is 25~27 °C, no four seasons, abundant precipitation, annual precipitation is 1600~2200 mm. |
| Vietnam | Vietnam is located at 8°30′~23°22′ north latitude and 102°10′~109°30′ east longitude, tropical monsoon climate, average annual rainfall is 1500~2000 mm. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.-R.; Li, W.; Luo, S.; Zhao, X.; Ma, C.-H.; Liu, S.-X. GC-MS Study of the Chemical Components of Different Aquilaria sinensis (Lour.) Gilgorgans and Agarwood from Different Asian Countries. Molecules 2018, 23, 2168. https://doi.org/10.3390/molecules23092168
Wang M-R, Li W, Luo S, Zhao X, Ma C-H, Liu S-X. GC-MS Study of the Chemical Components of Different Aquilaria sinensis (Lour.) Gilgorgans and Agarwood from Different Asian Countries. Molecules. 2018; 23(9):2168. https://doi.org/10.3390/molecules23092168
Chicago/Turabian StyleWang, Meng-Ru, Wei Li, Sha Luo, Xin Zhao, Chun-Hui Ma, and Shou-Xin Liu. 2018. "GC-MS Study of the Chemical Components of Different Aquilaria sinensis (Lour.) Gilgorgans and Agarwood from Different Asian Countries" Molecules 23, no. 9: 2168. https://doi.org/10.3390/molecules23092168
APA StyleWang, M.-R., Li, W., Luo, S., Zhao, X., Ma, C.-H., & Liu, S.-X. (2018). GC-MS Study of the Chemical Components of Different Aquilaria sinensis (Lour.) Gilgorgans and Agarwood from Different Asian Countries. Molecules, 23(9), 2168. https://doi.org/10.3390/molecules23092168