Co-Amorphous Simvastatin-Nifedipine with Enhanced Solubility for Possible Use in Combination Therapy of Hypertension and Hypercholesterolemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Thermal Characterization
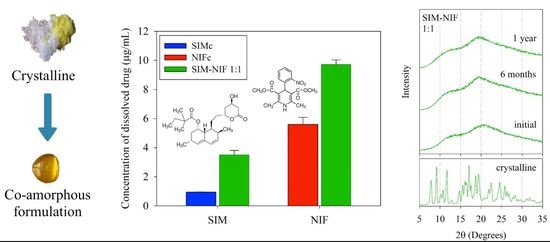
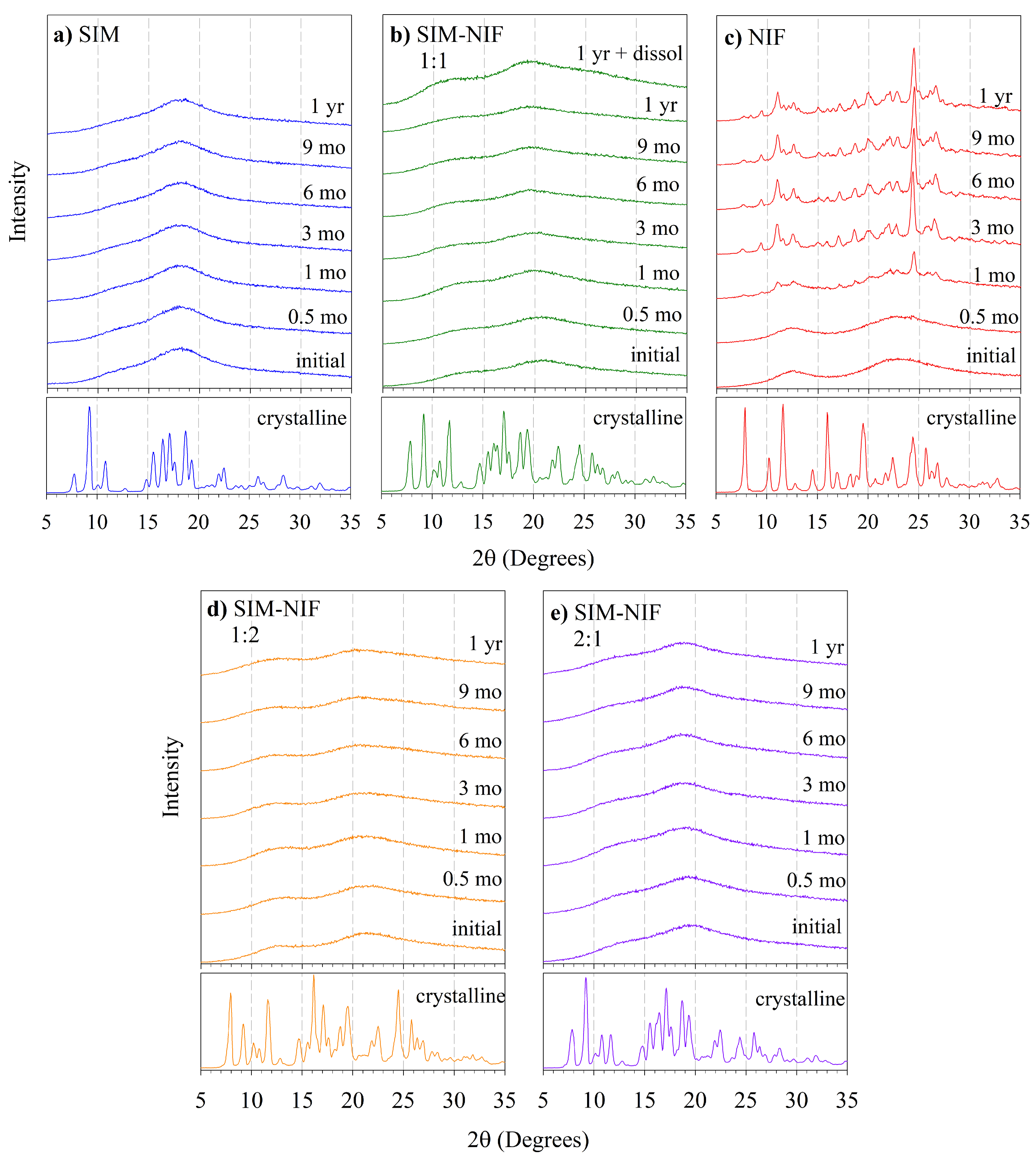
2.4. Study of Storage Stability by XRD
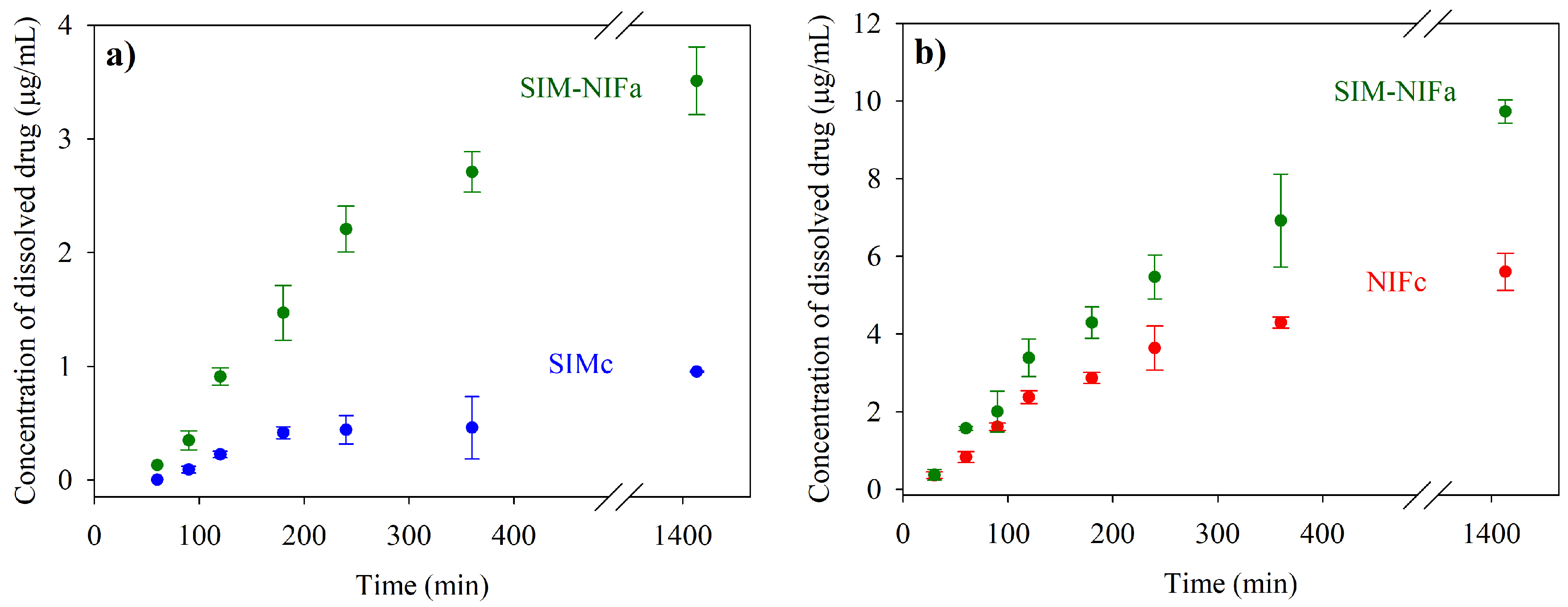
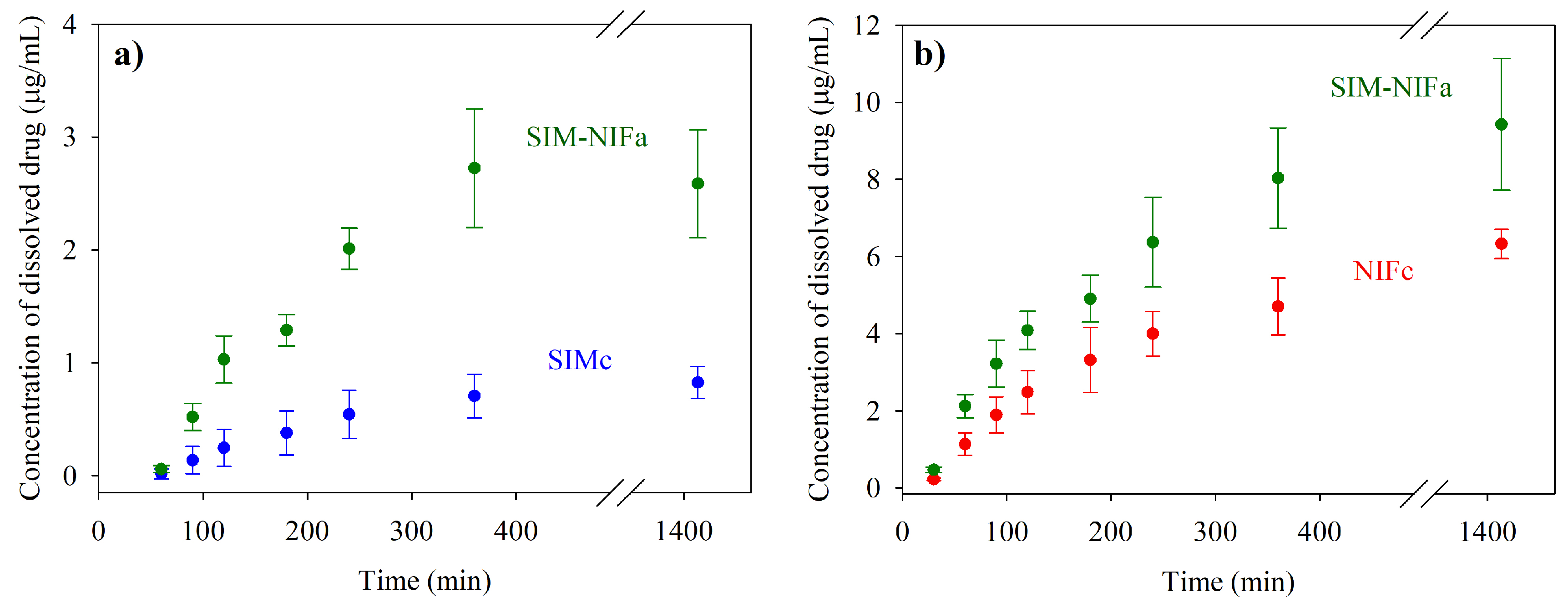
2.5. Dissolution Profiles
2.6. Dissolution Kinetics Analysis by High Performance Liquid Chromatography (HPLC)
2.7. Structural Analysis by FTIR
3. Results and Discussion
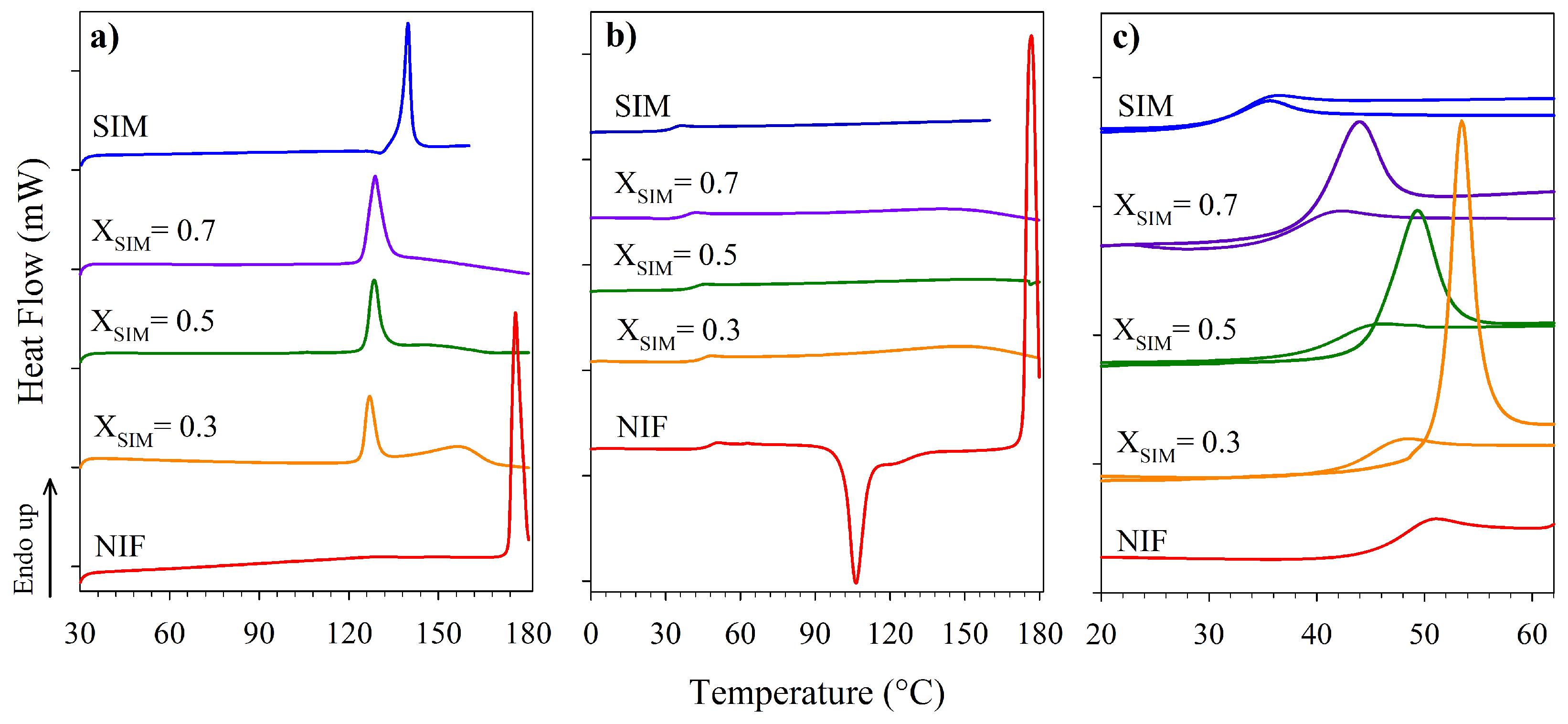
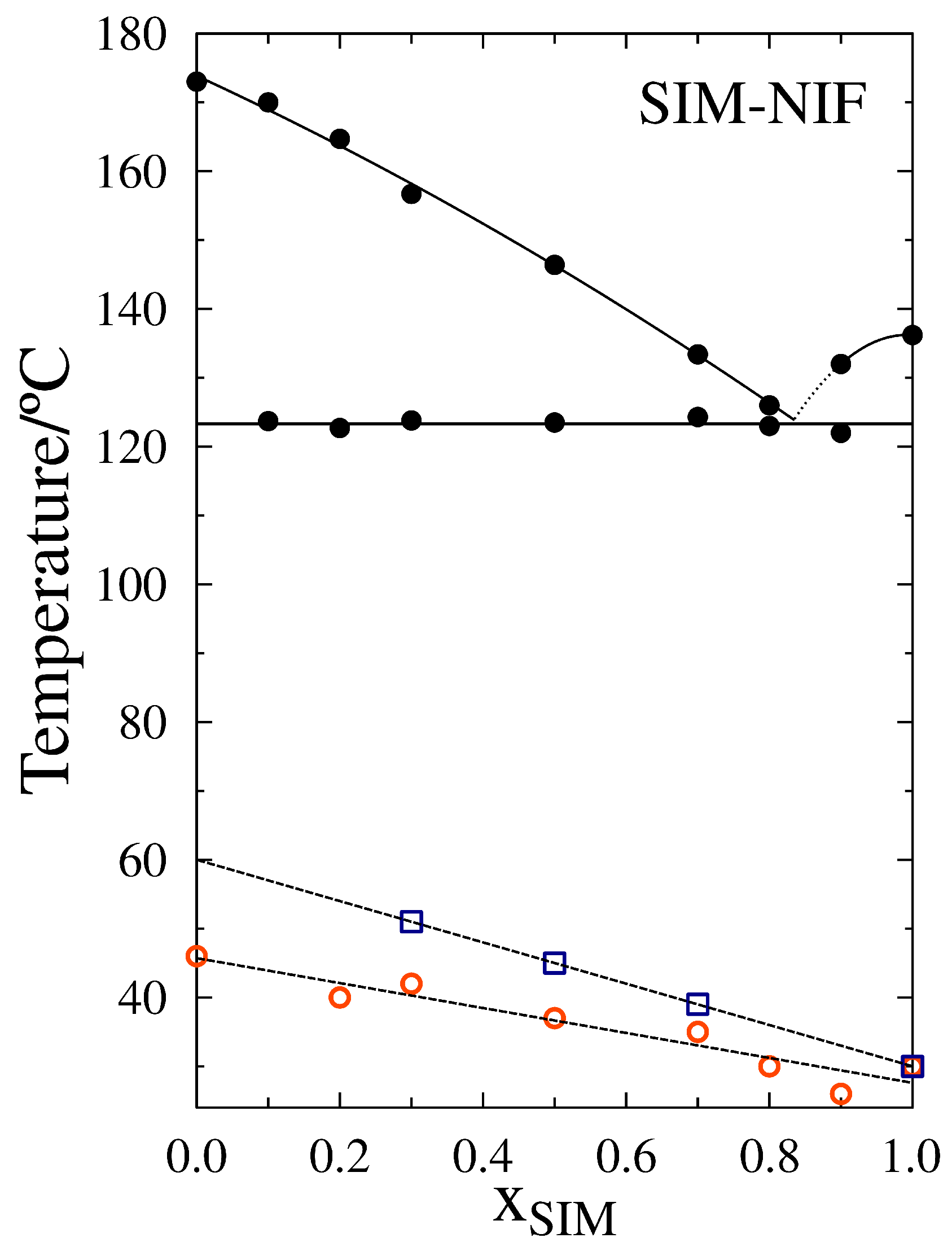
3.1. Thermal Characterization
3.2. Results of Stability of the Amorphous State by PXRD
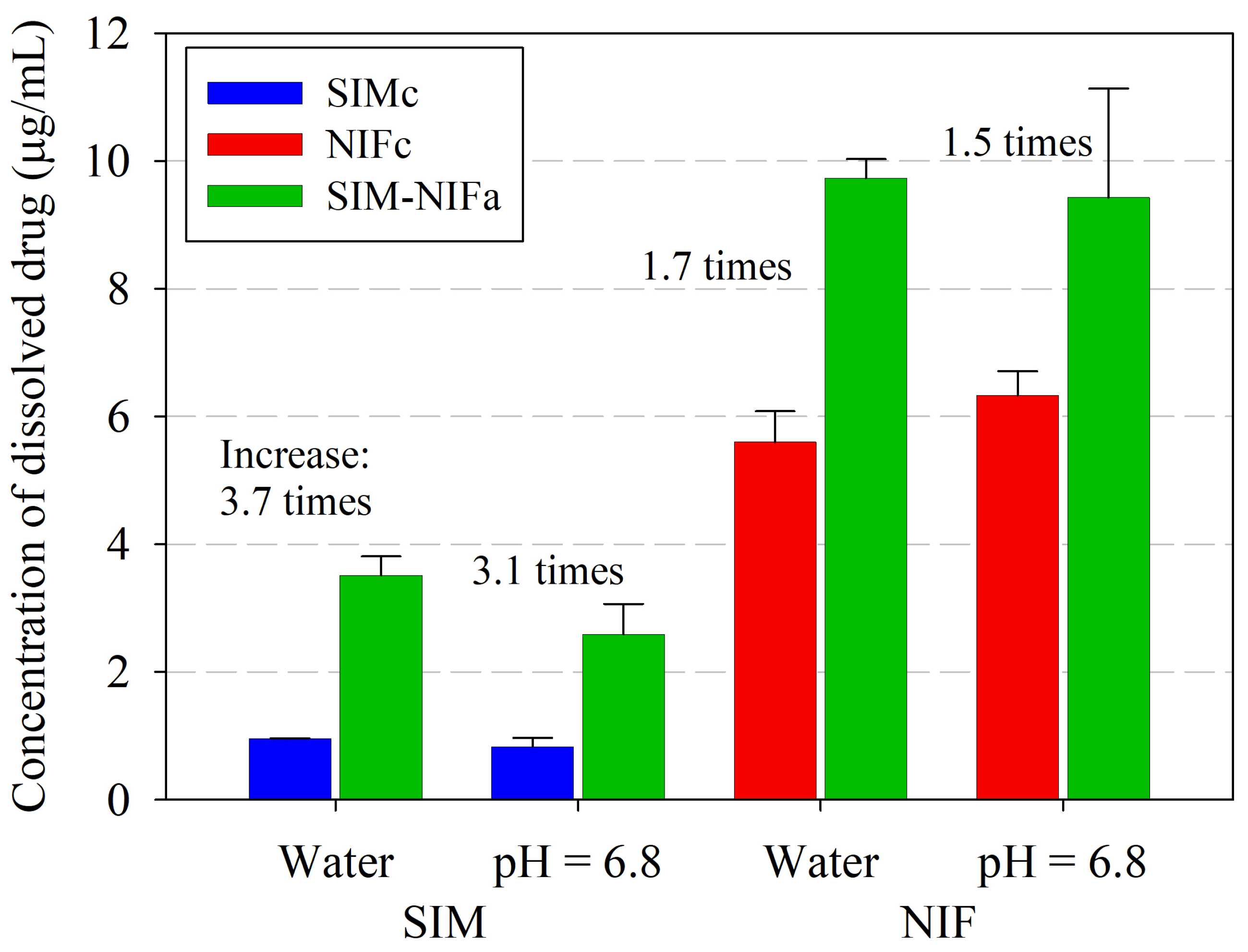
3.3. Results of Dissolution Profiles
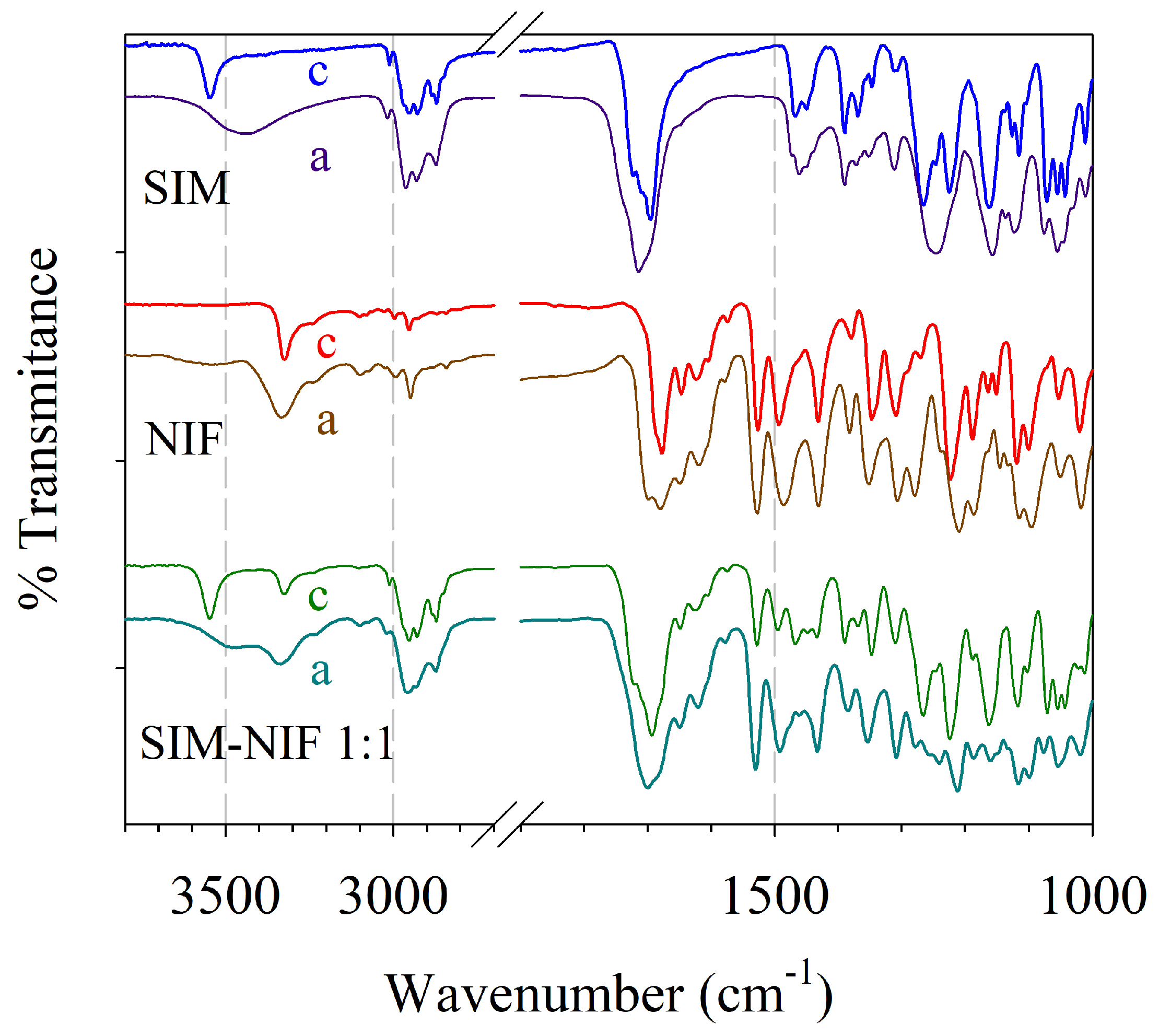
3.4. Results of Structural Analysis by FTIR
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | American Chemical Society |
| AR (ACS) | American Chemical Society Commitee on Analytical Reagents |
| HT | Hypertension |
| HCL | Hypercholesterolemia |
| SIM | Simvastatin |
| NIF | Nifedipine |
| API | Active Pharmaceutical Principle |
| DSC | Differential Scanning Calorimetry |
| HPLC | High performance liquid chromatography |
| FTIR | Fourier transform infrared spectroscopy |
| PXRD | powder X-ray diffraction |
| Tg | Glass Transition Tempeature |
| Tm | Melting Temperature |
| USP | United States Pharmacopeia. |
References
- Ivanovic, B.; Tadic, M. Hypercholesterolemia and Hypertension: Two Sides of the Same Coin. Am. J. Cardiovasc. Drugs 2015, 15, 403–414. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, T.M.; Morant, S.V. Prevalence and treatment of isolated and concurrent hypertension and hypercholesterolaemia in the United Kingdom. Br. J. Clin. Pharmacol. 2008, 65, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.D.; Lopez, V.; Tang, S.; Williams, G.R. Prevalence, Treatment, and Control of Combined Hypertension and Hypercholesterolemia in the United States. Am. J. Cardiol. 2006, 98, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Campos-Nonato, I.; Hernández-Barrera, L.; Rojas-Martínez, R.; Pedroza-Tobías, A.; Medina-García, C.; Barquera, S. Hipertensión arterial: Prevalencia, diagnóstico oportuno, contro y tendencias en adultos mexicanos. Salud Publica Mex. 2013, 55, 144–150. [Google Scholar] [CrossRef]
- Ramirez, E.; Laosa, O.; Guerra, P.; Duque, B.; Mosquera, B.; Borobia, A.M.; Lei, S.H.; Carcas, A.J.; Frias, J. Acceptability and characteristics of 124 human bioequivalence studies with active substances classified according to the Biopharmaceutic Classification System. Br. J. Clin. Pharmacol. 2010, 70, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Benet, L.Z.; Broccatelli, F.; Oprea, T.I. BDDCS applied to over 900 drugs. AAPS J. 2011, 13, 519–547. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, A.; Barzegar-Jalali, M.; Garjani, A.; Javadzadeh, Y.; Hamishehkar, H.; Afroozian, A.; Adibkia, K. Pharmacological and histological examination of atorvastatin-PVP K30 solid dispersions. Powder Technol. 2015, 286, 538–545. [Google Scholar] [CrossRef]
- Homayouni, A.; Sadeghi, F.; Nokhodchi, A. Preparation and characterization of celecoxib dispersions in Soluplus®: Comparison of spray drying and conventional methods. Iran J. Pharm. Res. 2015, 14, 35–50. [Google Scholar]
- Rizwanullah, M.; Amin, S.; Ahmad, J. Improved pharmacokinetics and antihyperlipidemic efficacy of rosuvastatin-loaded nanostructured lipid carriers. J. Drug Target 2017, 25, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Villar, A.M.S.; Naveros, B.C.; Campmany, A.C.C.; Trenchs, M.A.; Rocabert, C.B.; Bellowa, L.H. Design and optimization of self-nanoemulsifying drug delivery systems (SNEDDS) for enhanced dissolution of gemfibrozil. Int. J. Pharm. 2012, 431, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Taupitz, T.; Dressman, J.B.; Klein, S. New formulation approaches to improve solubility and drug release from fixed dose combinations: Case examples pioglitazone/glimepiride and ezetimibe/simvastatin. Eur. J. Pharm. Biopharm. 2013, 84, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Yu, L.X.; Lee, H.L.; Yang, C.Y.; Lue, C.S.; Chou, C.H. Biowaiver extension potential to BCS Class III high solubility-low permeability drugs: Bridging evidence for metformin immediate-release tablet. Eur. J. Pharm. Sci. 2004, 22, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, R.; Löbmann, K.; Strachan, C.J.; Grohganz, H.; Rades, T. Emerging trends in the stabilization of amorphous drugs. Int. J. Pharm. 2013, 453, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Dengale, S.J.; Grohganz, H.; Rades, T.; Löbmann, K. Recent advances in co-amorphous drug formulations. Adv. Drug Deliv. Rev. 2015, 100, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.M.; Videa, M.; López Silva, T.; Castro, S.; Caballero, A.; Lara-Díaz, V.J.; Castorena-Torres, F. Two-phase amorphous-amorphous solid drug dispersion with enhanced stability, solubility and bioavailability resulting from ultrasonic dispersion of an immiscible system. Eur. J. Pharm. Biopharm. 2017, 119, 243–252. [Google Scholar]
- Korhonen, O.; Pajula, K.; Laitinen, R. Rational excipient selection for co-amorphous formulations. Expert Opin. Drug Deliv. 2017, 14, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.M.; Videa, M.; Gonzalez, N.; Ramirez, H.; Castro, S. Long term stability of new co-amorphous drug binary systems: Study of glass transitions as a function of composition and shelf time. Molecules 2016, 21, 1712. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.M.; Videa, M.; López-Silva, G.A.; De Los Reyes, C.A.; Cruz-Angeles, J.; González, N. Stabilization of amorphous paracetamol based systems using traditional and novel strategies. Int. J. Pharm. 2014, 477, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Shayanfar, A.; Jouyban, A. Drug-drug coamorphous systems: Characterization and physicochemical properties of coamorphous atorvastatin with carvedilol and glibenclamide. J. Pharm. Innov. 2013, 8, 218–228. [Google Scholar] [CrossRef]
- Knapik, J.; Wojnarowska, Z.; Grzybowska, K.; Jurkiewicz, K.; Tajber, L.; Paluch, M. Molecular Dynamics and Physical Stability of Coamorphous Ezetimibe and Indapamide Mixtures. Mol. Pharm. 2015, 12, 3610–3619. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, G. Solubility enhancement of simvastatin: A review. Acta Pol. Pharm. Drug Res. 2012, 69, 581–590. [Google Scholar]
- Keratichewanun, S.; Yoshihashi, Y.; Sutanthavibul, N.; Terada, K.; Chatchawalsaisin, J. An Investigation of Nifedipine Miscibility in Solid Dispersions Using Raman Spectroscopy. Pharm. Res. 2015, 2458–24573. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, J.; Feng, Z.; Fan, M.; Han, J.; Yang, Z. Simvastatin combined with nifedipine enhances endothelial cell protection by inhibiting ROS generation and activating Akt phosphorylation. Acta Pharmacol. Sin. 2010, 31, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.L. Nifedipine. Anal. Profiles Drug Subst. 1990, 18, 221–288. [Google Scholar]
- Löbmann, K.; Strachan, C.; Grohganz, H.; Rades, T.; Korhonen, O.; Laitinen, R. Co-amorphous simvastatin and glipizide combinations show improved physical stability without evidence of intermolecular interactions. Eur. J. Pharm. Biopharm. 2012, 81, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Graeser, K.; Patterson, J.; Strachan, C.; Gordon, K.; Rades, T. Physicochemical Properties of Two Differently Prepared Amorphous Forms of Simvastatin. Cryst. Growth Des. 2008, 8, 128–135. [Google Scholar] [CrossRef]
- Grooff, D.; De Villiers, M.M.; Liebenberg, W. Thermal methods for evaluating polymorphic transitions in nifedipine. Thermochim. Acta 2007, 454, 33–42. [Google Scholar] [CrossRef]
- Gniado, K.; MacFhionnghaile, P.; McArdle, P.; Erxleben, A. The natural bile acid surfactant sodium taurocholate (NaTC) as a coformer in coamorphous systems: Enhanced physical stability and dissolution behavior of coamorphous drug-NaTc systems. Int. J. Pharm. 2018, 535, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Zhu, L.; Yu, L. Crystallization of organic glasses: Effects of polymer additives on bulk and surface crystal growth in amorphous nifedipine. Pharm. Res. 2011, 28, 2458–2466. [Google Scholar] [CrossRef] [PubMed]
- Hušák, M.; Kratochvíl, B.; Jegorov, A.; Brus, J.; Maixner, J.; Rohlíček, J. Simvastatin: Structure solution of two new low-temperature phases from synchrotron powder diffraction and ss-NMR. Struct. Chem. 2010, 21, 511–518. [Google Scholar] [CrossRef]
- Grooff, D.; Liebenberg, W.; De Villiers, M.M. Preparation and Transformation of True Nifedipine Polymorphs: Investigated with Differential Scanning Calorimetry and X-Ray Diffraction Pattern Fitting Methods. J. Pharm. Sci. 2011, 100, 1944–1957. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, H.; Zhang, J.; Gao, Y. Physicochemical characteristics of coamporphous simvastatin-gliclazide. J. China Pharm. Univ. 2015, 46, 301–308. [Google Scholar]
- Zhang, F.; Aaltonen, J.; Tian, F.; Saville, D.J.; Rades, T. Influence of particle size and preparation methods on the physical and chemical stability of amorphous simvastatin. Eur. J. Pharm. Biopharm. 2008, 71, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.K.; Moore, W.D.; Petts, C.R. Simvastatin. Anal. Profiles Drug Subst. Excip. 1993, 22, 359–388. [Google Scholar]
- Chan, K.L.A.; Fleming, O.S.; Kazarian, S.G.; Vassou, D.; Chryssikos, G.D.; Gionis, V. Polymorphism and devitrification of nifedipine under controlled humidity: A combined FT-Raman, IR and Raman microscopic investigation. J. Raman Spectrosc. 2004, 35, 353–359. [Google Scholar] [CrossRef]
- Alqurshi, A.; Chan, K.L.A.; Royall, P.G. In-situ freeze-drying - forming amorphous solids directly within capsules: An investigation of dissolution enhancement for a poorly soluble drug. Sci. Rep. 2017, 7, 2910. [Google Scholar] [CrossRef] [PubMed]
- Grohganz, H.; Priemel, P.A.; Löbmann, K.; Nielsen, L.H.; Laitinen, R.; Mullertz, A.; van den Mooter, G.; Rades, T. Refining stability and dissolution rate of amorphous drug formulations. Expert Opin. Drug Deliv. 2014, 11, 977–989. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Amorphous samples prepared were consumed during characterization. All compounds were purchased from commercial sources and are not currently available from the authors. |
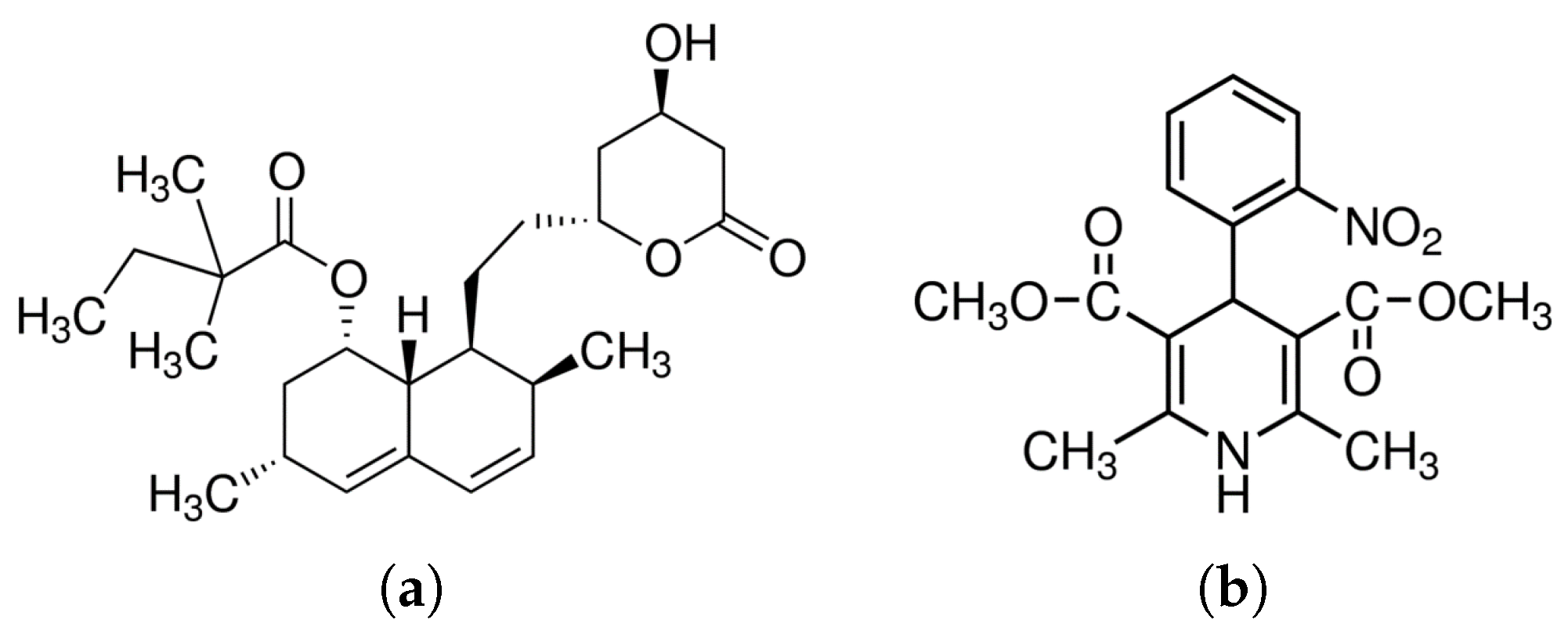
| API | Illnes | Solubility | Permeability | BCS | Reference |
|---|---|---|---|---|---|
| Pravastatin | HCL | High | High | I | [5] |
| Glimepiride | HCL | Low | High | II | [6] |
| Atorvastatin | HCL | Low | High | II | [7] |
| Simvastatin | HCL | Low | High | II | [5] |
| Fenofibrate | HCL | Low | High | II | [8] |
| Rosuvastatin | HCL | Low | High | II | [9] |
| Gemfibrozil | HCL | Low | High | II | [10] |
| Ezetimibe | HCL | Low | High | II | [11] |
| Metoprolol | HT | High | High | I | [6] |
| Telmisartan | HT | Low | High | II | [6] |
| Losartan | HT | High | Low | III | [12] |
| Irbesartan | HT | Low | High | II | [5] |
| Sample | T/C | Tm/C | Tg/C |
|---|---|---|---|
| SIM | 136 | 30 | |
| X | 122 | 132 | 26 |
| X | 123 | 126 | 30 |
| X | 124 | 133 | 35 |
| X | 124 | 146 | 37 |
| X | 124 | 157 | 42 |
| X | 123 | 165 | 40 |
| X | 124 | 170 | 42 |
| NIF | 174 | 46 |
| Drug | Sample | Deionized Water | Phosphate Buffer pH = 6.8 |
|---|---|---|---|
| Concentration of Dissolved Drug at 24 h (g/mL) | Concentration of Dissolved Drug at 24 h (g/mL) | ||
| SIM | SIMc | ||
| SIM–NIFa | |||
| NIF | NIFc | ||
| SIM–NIFa |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Jiménez, C.; Cruz-Angeles, J.; Videa, M.; Martínez, L.M. Co-Amorphous Simvastatin-Nifedipine with Enhanced Solubility for Possible Use in Combination Therapy of Hypertension and Hypercholesterolemia. Molecules 2018, 23, 2161. https://doi.org/10.3390/molecules23092161
Martínez-Jiménez C, Cruz-Angeles J, Videa M, Martínez LM. Co-Amorphous Simvastatin-Nifedipine with Enhanced Solubility for Possible Use in Combination Therapy of Hypertension and Hypercholesterolemia. Molecules. 2018; 23(9):2161. https://doi.org/10.3390/molecules23092161
Chicago/Turabian StyleMartínez-Jiménez, Cecilia, Jorge Cruz-Angeles, Marcelo Videa, and Luz María Martínez. 2018. "Co-Amorphous Simvastatin-Nifedipine with Enhanced Solubility for Possible Use in Combination Therapy of Hypertension and Hypercholesterolemia" Molecules 23, no. 9: 2161. https://doi.org/10.3390/molecules23092161
APA StyleMartínez-Jiménez, C., Cruz-Angeles, J., Videa, M., & Martínez, L. M. (2018). Co-Amorphous Simvastatin-Nifedipine with Enhanced Solubility for Possible Use in Combination Therapy of Hypertension and Hypercholesterolemia. Molecules, 23(9), 2161. https://doi.org/10.3390/molecules23092161