Abstract
A series of novel alkyl amine-substituted icariside II (ICA II) derivatives were synthesized by Mannich reactions at the 6-C position (compounds 4a–d) and changing the carbon chain length at the 7-OH position (compounds 7a–h), and their in vitro antitumor activity towards human breast cancer lines (MCF-7 and MDA-MB-231) and human hepatoma cell lines (HepG2 and HCCLM3-LUC) were evaluated by the MTT assay. Compared with ICA II, most of the twelve derivatives showed good micromole level activity and a preliminary structure-activity relationship (SAR) for the anticancer activity was obtained. Compound 7g showed the most potent inhibitory activity for the four cancer cell lines (13.28 μM for HCCLM3-LUC, 3.96 μM for HepG2, 2.44 μM for MCF-7 and 4.21 μM for MDA-MB-231), which was 2.94, 5.54, 12.56 and 7.72-fold stronger than that of ICA II. The preliminary SAR showed that the introduction of a alkyl amine substituent at 6-C was not favorable for the anticancer activity, while most of the 7-O-alkylamino derivatives exhibited good antitumor activity and the anticancer activity 7-O-alkylamino derivatives were influenced by the alkyl chain length and the different terminal amine substituents. Furthermore, the effects of compound 7g on apoptosis and cell cycle of MCF-7 cells were further investigated, which showed that compound 7g triggered apoptosis and arrested the cell cycle at the G0/G1 phase in MCF-7 cells. Our findings indicate that compound 7g may be a promising anticancer drug candidate lead.
1. Introduction
Cancer is one of the major causes of death across the world, owing to its great increasing morbidity and significant mortality [1]. For example, liver cancer has been recognized as the third most common cause of cancer deaths [2]. Breast cancer is one of the common malignant tumors in the female population, ranking first in female malignant tumor mortality [3,4]. Cancer is caused by rapid, uncontrolled, and pathological proliferation of abnormally transformed cells [5]. Chemotherapy is a common method to treat malignancies, but resistance to chemotherapeutic agents, lack of selectivity and serious adverse effects, are the primary challenges of cancer therapy [6,7]. Thus, searching for novel and effective anticancer agents with minimal side effects is considered to be of great value.
With the development of Traditional Chinese Medicine (TCM), it nowadays plays an important role in anti-cancer drug discovery. TCMs can treat cancer through different molecular targets and multiple signaling pathways, such as inducing apoptosis, suppressing proliferation, restraining tumor to transfer, reversing multidrug resistance and enhancing immunity. Moreover, because they cause few adverse effects, more and more TCMs have been applied in clinical studies [8]. Epimedii folium (EF) is the dried leaves from E. sagittatum (Sieb.et Zucc.) Maxim., Epimedium brevicornu Maxim., E. koreanum Nakai, E. pubescens Maxim. EF, also named yinyanghuo, barrenwort, copper wire grass and faeries spleen, and has been used as a tonic herb for strengthening the bones and nourishing the kidneys in East Asia, particularly in China, Korea and Japan, for thousands of years [1]. Icariin (ICA), an 8-isopentenyl flavonoid glycoside (Figure 1, compound A), is the main active ingredients of EF [9]. ICA possesses a wide-range of pharmacological effects, such as antidepressant-like, hormone regulation, anti-inflammatory, neuroprotective, antioxidative, antirheumatic, and antiosteoporotic effects [10,11,12,13,14]. Moreover, ICA was reported to exhibit anticancer activity against a series of human cancer cell lines. For example, Zheng et al. found that ICA could suppress thyroid carcinoma cell (B-CPAP) proliferation by inducing high expression of intracellular ROS and reducing the expression of antioxidase [15]; ICA could induce apoptosis and arrest cell cycle at S phase in medulloblastoma cells to suppress proliferation [16]. Icariside II (ICA II), another active ingredient of EF (Figure 1, compound B), is formed by the intestinal flora from ICA by the loss of the glycosyl moiety at the C-7 position [17]. Our group has worked extensively on the development of ICA and found that 91.2% of ICA was transformed into ICA II after oral administration, and direct oral administration of ICA II increased its concentration in the body leading to faster absorption, slower metabolism and higher absolute bioavailability [18]. Compared with ICA, ICA II possesses stronger activity against inflammation, osteoporosis, cancer, and improves erectile dysfunction [19,20,21,22,23]. We have explored the anticancer activity of ICA II and ICA, and the results (see Table 1) showed that ICA II had better anticancer activities than ICA. Thus, ICA II has attracted increasing research interest. However, because the amount of natural ICA II in EF was about only 0.01% g/g, which is about 1/60–1/6 that of ICA, we also have optimized biotransformation of ICA into ICA II by β-Gglucosidase for further pharmaceutical research [24].
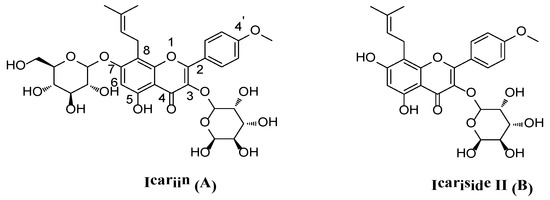
Figure 1.
The structures of icariin (A) and icariside II (B).

Table 1.
Antitumor cell proliferation activity of alkyl amine derivatives of ICA II by MTT assay.
Like many other biologically active flavonoids such as naringenin and quercetin, ICA II is a hydrophobic drug with low absorptive permeability and a high rate of efflux via apical efflux transport [25]. ICA II only showed moderate anticancer activities (see Table 1). Therefore, improving the potency against cancer of ICA II is important for its further application. A literature survey shows that most of the important classes of drugs are nitrogen-containing, and the presence of an amine moiety in drugs may increase their biological potency due to the greater number of molecular sites for electrophilic attack by cellular constituents, as well as due to the cascade effect of preferential chemosensitization [26]. Amine moieties in drugs could also enhance their physicochemical properties (e.g., water solubility) and improve the bioavailability of bioactive molecules [27]. Continuing our study on the chemistry and biology of ICA II, a nitrogen-containing hydrophilic, heterocyclic ring was introduced for the first time at the 6-C position and the 7-OH of ICA II to improve its potency against cancer.
Firstly, we established the hydrolysis process of ICA to get ICA II from our previous reports [24], and a novel series of nitrogen-containing ICA II derivatives were synthesized with a certain length of carbon chain at the 7-OH position and by the Mannich reaction at the 6-C position. Furthermore, we also evaluated the effect of the synthesized derivatives on cell proliferation against human breast cancer cell lines (MCF-7 and MDA-MB-231) and human liver cancer lines (HepG2 and HCCLM3-LUC). Lastly the effects of the most active compound 7g, on cell cycle and apoptosis of MCF-7 cells were further investigated.
2. Results and Discussion
2.1. Chemistry
The target nitrogen-containing compounds 4a–d and 7a–h were synthesized as shown in Scheme 1 Compounds 4a–d were synthesized by the Mannich condensation of ICA II (1), formaldehyde (2), and secondary amines 3a–d in a single-step process with good yields. Due to the intramolecular hydrogen bond between 5-OH and 4-ketone, the reactivity of the 5-OH was lower than that of the 7-OH position [28]. Alkylation of ICA II (1) at the 7-OH position with dihalogenated hydrocarbons 5a–e was catalyzed by K2CO3 to afford the intermediates 6a–e in 50–65% yields. Then nucleophilic substitution between secondary amines and the halogenated hydrocarbons was accomplished to get the final products 7a–h in 70–85% yields. The structures of all synthesized compounds were characterized via 1H-NMR, 13C-NMR and MS data. In the 1H-NMR spectra of compounds 4a–d, a signal at δ 3.8–4.1 indicated the presence of an aminomethyl group at the C-6 of the ICA II scaffold, while in the 1H-NMR spectra of compounds 7a–h, the signals at δ 2.5–2.9 and δ 1.6–1.7 indicated the presence of the alkyl amine and the alkyl chain, respectively.
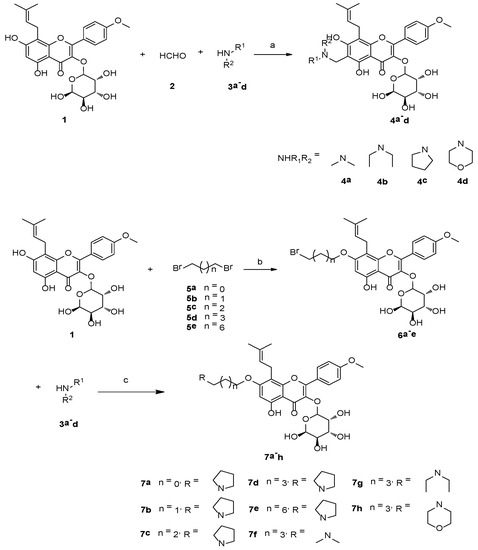
Scheme 1.
Reagents and conditions: (a) CH3OH, HCl, reflux, 3 h; (b) CH3CN, K2CO3, reflux, 12 h; (c) CH3CN, K2CO3, reflux, 12 h.
2.2. Cell Viability Analysis and Discussion on the Preliminary Structure-Activity Relationship (SAR)
All the novel alkylamino derivatives of ICA II were evaluated for their anti-proliferative activity against human breast cancer cell lines (MCF-7 and MDA-MB-231) and human liver cancer lines (HepG2 and HCCLM3-LUC) by MTT assays. All the cells were incubated with different concentrations of ICA II derivatives for 48 h, and doxorubicin and ICA II were further used as reference compounds. The anticancer activity of the tested compounds was described as the concentration of drug inhibiting 50% cell growth (IC50) and is summarized in Table 1.
The dose-response curves of representative compound 7d (HCCLM3-LUC cells and HepG2 cells), and compound 7g (MCF-7 cells and MDA-MB-231 cells) are shown in Figure 2. Among the target compounds, most 7-O-alkylamino derivatives 7a–h exhibited comparable or lower IC50 values compared with ICA II against all the four cancer cell lines. Namely, these compounds possessed a broad spectrum of anticancer ability, and some compounds possessed IC50 values below 10 μM particularly. Compound 7g showed the most potent inhibitory activity for the four cancer cell lines (13.28 μM for HCCLM3-LUC, 3.96 μM for HepG2, 2.44 μM for MCF-7 and 4.21 μM for MDA-MB-231), which was 2.94, 5.54, 12.56 and 7.72-fold stronger than those of the reference compound ICA II respectively. The Mannich base compounds 4a–d displayed lower cytotoxic activities than ICA II, suggesting that the introduction of an alkyl amine substituent at 6-C was not suitable for their anticancer activity.
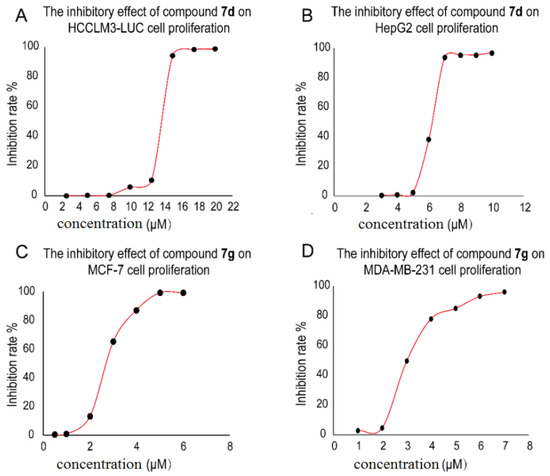
Figure 2.
The dose-response curves for MTT assay of compound 7d (A,B) on HCCLM3-LUC cells and HepG2 cells, compound 7g (C,D) on MCF-7 cells and MDA-MB-231 cells.
For 7-O-alkylamino derivatives, to determine the effect of alkyl chain length on activity, compounds 7a–e with different carbon atoms were firstly synthesized. The anti-proliferation activity of compounds for all the four cell lines changed significantly with increasing alkyl chain (CH2)n (n from 2 to 8). For example, from the IC50 values of 7a–e for MDA-MB-231, compound 7a (16.85 μM) containing a two carbon spacer had lower inhibitory activity than the other compounds (except for compound 7c) with longer carbon chain lengths. This suggested that the longer linker length seemed to be favorable for the anticancer activity, but when the alkyl chain length changed from 3 to 8, the anti-proliferation activity of compounds was similar. Based on the above, we chose the alkyl chain with n = 3 for further study. With the optimal linker in hand, we planned to introduce different terminal amine substituents to investigate the possible effects on anticancer activity.
With a cyclic amine group (a morpholinyl or pyrrolidyl group) at the terminal position of 7-O-alkylamino derivatives, compounds 7d and 7h (with IC50 at 7.14–15.31 μM) exhibited good inhibition, which were 2.6–2.9 fold (HCCLM3-LUC), 2.9–3.1 fold (HepG2), 2.1–2.8-fold (MCF-7) and 3.2–3.4-fold (MDA-MB-231) more potent than ICA II. Interestingly, it was found that the anticancer activities of compound 7g containing a N,N-diethylamino group was higher than that of the N-containing heterocyclic compounds 7d and 7h. However, the N,N-methylamino substitution in the compound 7f (with IC50 at 19.92–46.82 μM) exhibited lower activities than substitutions with pyrrolyl (7d), N,N-diethylamino (7g) or piperidyl (7h) groups, respectively. This may be due to the different volumes of the side chains. In addition, it was worth mentioning that nearly all the derivatives possessed better selectivity against breast cancer cell lines than liver cancer cell lines, although the anticancer activity SAR needs further research.
2.3. Cell Morphological Assessment
The anti-proliferative activity of compound 7g was further confirmed by cell morphological assessment. Apoptosis can induce characteristic morphological changes in cells, such as nuclear shrinkage, DNA degradation, chromatin condensation, apoptotic body formation, cytoskeletal disintegration, which can be observed through fluorescence microscopy after by Hoechst 33258 staining of cell nuclei [29]. MCF-7 cells were exposed to different concentrations of compound 7g (1–4 μM) for 48 h, and untreated cells served as control. As shown in Figure 3, the nuclei of the cells in the control were round and showed a homogeneous blue fluorescence, whereas the treated cells showed nucleus shrinkage, chromatin condensation and nuclear fragmentation as a strong blue-white fluorescence when treated with different concentrations of compound 7g for 48 h. Different from intact, round and large nuclei, multiple apoptotic bodies with irregular shapes and sizes appeared in apoptotic cells (red arrows in the figure). In addition, the number of cells with shrunk nucleii was gradually increased with compound 7g (1–4 μM), and the cytoskeleton was disintegrated at the 3 or 4 μM concentration of compound 7g. These results all indicated that compound 7g could induce apoptosis in MCF-7 cells.
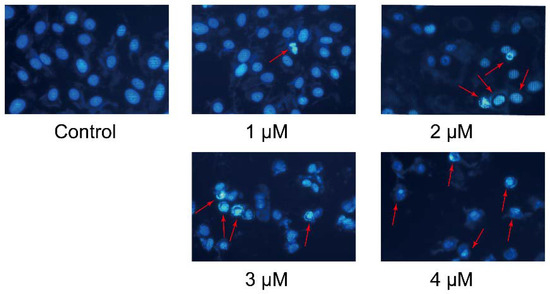
Figure 3.
Fluorescent micrographs of Hoechst 33258 stained nuclear patterns when MCF-7 cells treated with compound 7g at 1–4 μM for 48 h (400×). The red arrows indicate the apoptotic cells.
2.4. Cell Cycle Distribution Analysis
The cell cycle is the basic process of cell life activities, and the main characteristic of tumors is that the cell cycle is out of control, which maintains unrestrained cell proliferation and uncontrolled cell division. Therefore, cell cycle arrest has been appreciated as an important route for cancer therapy [30]. To investigate the effect of compound 7g on the cell cycle of proliferating cancer cells MCF-7 cells were incubated with compound 7g at different concentrations (1–4 μM) for 48 h, and the resulting cell cycle distribution was analyzed using flow cytometry after propidium iodide (PI) staining. As shown in Figure 4, the percentages of cells at G2 phase and S phase were reduced from 26.61% to 3.53% and 13.19% to 0.98%, respectively, when treated with compound 7g (1–4 μM), as compared with the 30.46% (G2 phase) and 18.72% (S phase), respectively, in the control. The percentage of cells in G0/G1 phase increased from 57.22% to 92.94%. However, no significant changes of the G0/G1 values (54.55% to 59.17%), G2 values (24.03% to 23.32%) and S values (18.76% to 14.26%) obtained were observed with ICA II. The increased percentage of cells at the G0/G1 phase and the cytotoxic activity suggested that compound 7g, could arrest cell cycle in the G0/G1 phase of MCF-7 cells, and compound 7g demonstrated stronger anticancer activity than ICA II, which agrees with the cell viability analysis results.
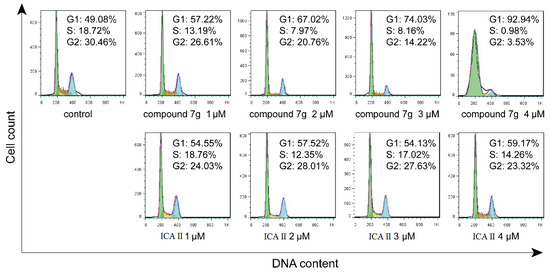
Figure 4.
Effect of compound 7g and ICA II on cell cycle distribution on MCF-7 cells. Cell cycle distribution after treatment with compound 7g and ICA II at 1–4 μM for 48 h.
2.5. Assessment of Apoptotic Analysis
To investigate the bioactivity of compound 7g against MCF-7 cells, the cells were treated with compound 7g at different concentrations (1–4 μM). Treated cells were labeled using Annexin V-APC and PI. The rates of cell apoptosis are shown in Figure 5. Treatment with low concentrations of compound 7g (1 or 2 μM) caused no significant apoptosis compared with control. However, 3 or 4 μM concentrations of compound 7g induced a high degree of apoptosis in MCF-7 cells at both the early and late stages. The percentage of late apoptotic cells (upper right quadrant) was increased from 2.97% to 22.4%, and the percentage of early apoptotic cells (lower right quadrant) was increased from 1.63% to 13.5%. This result indicated that compound 7g could induce both early and late apoptosis in MCF-7 cells in a dose-dependent manner, and cytotoxicity to MCF-7 cells occurred at micromolar concentrations.
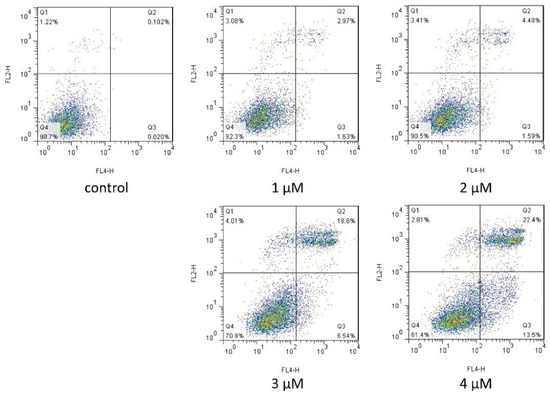
Figure 5.
Compound 7g induced apoptosis in MCF-7 cells. After treatment with compound 7g at different concentrations (1–4 μM), the apoptotic rates of MCF-7 cells were determined by Annexin V-APC and PI double staining using flow cytometry.
3. Conclusions
In conclusion, various alkyl amide derivatives of ICA II were synthesized and evaluated for their anticancer activity against four different cancer cell lines. Most of the selected compounds possessed good in vitro antitumor activity with a broad spectrum. The SAR showed the most 7-O-alkylamino derivatives showed good antitumor activity against all the four cancer cell lines, while the Mannich base compounds displayed lower cytotoxic activities. Moreover, the optimal length of the alkyl chain and different terminal amine substituents of 7-O-alkylamino derivatives markedly affected the antitumor activities. Among the target compounds, compound 7g exhibited the most significant anti-proliferative activity for the four tested cancer cell lines (13.28 μM for HCCLM3-LUC, 3.96 μM for HepG2, 2.44 μM for MCF-7 and 4.21 μM for MDA-MB-231). Compound 7g induction of MCF-7 cells apoptosis was confirmed in an Annexin V-APC/PI staining apoptotic assay and Hoechst 33258 staining analysis. In the cell cycle distribution, compound 7g could arrest the cell population at the G0/G1 phase. The results indicated that 7g exhibited enhanced anticancer activity against human breast cancer cells compared with ICA II. Thus, the novel 7-O-alkylamino derivative of ICA II, compound 7g, could be a promising antitumor agent for breast cancer and is worthy of further development.
4. Experimental Section
4.1. Materials and Methods
All chemicals (reagent grade) used were purchased from Sino Pharm Chemical Reagent Co., Ltd. (Shanghai, China). Reaction progress was monitored using analytical thin layer chromatography (TLC) on precoated silica gel GF254 (Qingdao Haiyang Chemical Plant, Qingdao, China) plates and the spots were detected under UV light (254 nm). Melting point was measured on an XT-4 micromelting point instrument (Agilent, Shanghai, China) and are uncorrected. 1H-NMR (400 MHz) and 13C-NMR spectra (100 MHz) were measured on an AVANCE III spectrometer (Bruker, Germany) at 25 °C and referenced to TMS. Chemical shifts are reported in ppm (δ) using the residual solvent line as internal standard. Splitting patterns are designed as s, singlet; d, doublet; t, triplet; m, multiplet. Mass spectra were obtained on a MS Agilent 1100 Series LC/MSD Trap mass spectrometer (ESI-MS, Agilent, China).
4.2. General Experimental Procedure for Mannich Base Derivatives 4a–d
A mixture of 37% aqueous formaldehyde (1.8 mL, 0.22 mmol) and secondary amine (0.22 mmol) in 10 mL of methanol and 0.02 mL of 15% HCl (aq) was stirred at 80 °C until complete homogenization. The solution obtained was added slowly to a solution of icariside II (98 mg, 0.19 mmol) in methanol, and the reaction mixture was refluxed for 1–3 h. After the reaction was complete, the mixture was rotary evaporated to distill off the methanol, then water was added, and the aqueous phase was extracted three times with EtOAc and dried over anhydrous Na2SO4. The solvent was removed under the reduced pressure. The residue was crystallized from EtOAc/petroleum ether (PE) to afford compounds 4a–d as yellow crystals in 70–85% yields.
6-((Dimethylamino)methyl)-5,7-dihydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-3-(((3R,4R,5R,6S)-3,4,5,6-tetrahydroxytetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one (4a). Yield 70%; yellow solid; m.p. 80.0–81.8 °C; ESI/MS m/z: 574.2 [M + H]+; 1H-NMR (400 MHz, CDCl3) δ 12.74 (s, 1H), 7.84 (d, J = 8.7 Hz, 2H), 7.02 (d, J = 8.8 Hz, 2H), 5.50 (s, 1H), 5.32 (s, 1H), 5.22 (s, 1H), 3.88 (s, 3H), 3.84 (s, 2H), 3.62 (m, 7H), 3.48–3.42 (m, 2H), 3.31 (dd, J = 13.7, 7.6 Hz, 1H), 2.41 (s, 6H), 1.76 (s, 3H), 1.70 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 178.4, 164.1, 161.7, 157.1, 156.7, 153.8, 134.9, 131.8, 130.6, 130.6, 123.0, 122.6, 114.0, 114.0, 106.5, 104.2, 103.4, 101.7, 72.5, 71.5, 70.60, 70.3, 55.5, 50.9, 44.1, 44.1, 25.8, 18.0, 17.1.
6-((Diethylamino)methyl)-5,7-dihydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-3-(((3R,4R,5R,6S)-3,4,5,6-tetrahydroxytetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one (4b). Yield 79%; yellow solid; m.p. 155.0–157.1 °C; ESI/MS m/z: 602.3 [M + H]+; 1H-NMR (400 MHz, CDCl3) δ 12.75 (s, 1H), 7.85 (d, J = 8.7 Hz, 2H), 7.02 (d, J = 8.7 Hz, 2H), 5.50 (s, 1H), 5.24 (s, 1H), 4.46 (s, 1H), 3.94 (m, 2H), 3.91 (m, 6H), 3.88 (s, 3H), 3.46 (m, 3H), 3.35–3.24 (m, 1H), 2.73 (dd, J = 14.0, 6.9 Hz, 4H), 1.75 (s, 3H), 1.70 (s, 3H), 1.17 (t, J = 7.1 Hz, 6H). 13C-NMR (100 MHz, CDCl3) δ 178.3, 161.9, 161.6, 157.0, 156.6, 153.8, 134.8, 131.6, 130.6, 130.6, 123.1, 122.7, 113.9, 113.9, 106.6, 103.4, 103.5, 101.6, 72.5, 71.6, 70.6, 70.2, 55.5, 49.1, 46.4, 46.4, 25.8, 18.0, 17.1, 10.8, 10.8.
5,7-Dihydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-6-(pyrrolidin-1-ylmethyl)-3-(((3R,4R,5R,6S)-3,4,5,6-tetrahydroxytetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one (4c). Yield 85%; yellow solid; m.p. 115.7–116.5 °C; ESI/MS m/z: 600.2 [M + H]+; 1H-NMR (400 MHz, CDCl3) δ 12.77 (s, 1H), 7.85 (d, J = 8.7 Hz, 2H), 7.02 (d, J = 8.8 Hz, 2H), 5.51 (s, 1H), 5.23 (s, 1H), 4.45 (s, 1H), 4.02 (s, 2H), 3.89 (s, 3H), 3.46 (t, J = 6.8 Hz, 4H), 3.32 (m, 6H), 2.78 (m, 4H), 1.93 (s, 4H), 1.76 (s, 3H), 1.71 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 178.4, 164.7, 161.6, 157.0, 156.3, 153.8, 134.8, 131.7, 130.6, 130.6, 123.1, 122.7, 113.9, 113.9, 106.6, 104.0, 104.0, 101.6, 72.6, 71.6, 70.6, 70.2, 55.5, 53.4, 53.4, 50.8, 25.8, 23.7, 23.7, 18.0, 17.1.
5,7-Dihydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-6-(morpholinomethyl)-3-(((3R,4R,5R,6S)-3,4,5,6-tetrahydroxytetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one (4d). Yield 82%; yellow solid; m.p. 155.6–158.7 °C; ESI/MS m/z: 616.3 [M + H]+; 1H-NMR (400 MHz, CDCl3) δ 12.77 (s, 1H), 7.85 (d, J = 8.8 Hz, 2H), 7.03 (d, J = 8.8 Hz, 2H), 5.50 (s, 1H), 5.22 (d, J = 6.8 Hz, 1H), 4.46 (s, 1H), 3.88 (s, 3H), 3.87 (m, 3H), 3.79 (m, 3H), 3.74–3.69 (m, 2H), 3.52–3.41 (m, 5H), 3.38–3.27 (m, 2H), 2.74–2.63 (m, 3H), 2.53 (s, 2H), 1.76 (s, 3H), 1.71 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 178.5, 163.1, 161.7, 157.4, 156.9, 153.7, 134.9, 131.9, 130.7, 130.7, 122.9, 122.4, 114.0, 114.0, 106.5, 104.5, 102.7, 101.7, 72.5, 71.5, 70.6, 70.3, 66.7, 66.7, 55.5, 53.5, 52.7, 52.7, 25.8, 18.0, 17.1.
4.3. General Experimental Procedure for the 7-O-Alkylamino Derivatives 7a–h
Excess potassium carbonate was added as the catalyst to a solution of the ICA II (0.50 mmol, 1 equiv) in acetonitrile (10 mL). After stirring for 10 min, di-halogenated hydrocarbon (2.50 mmol, 5 equiv) was added, and the resulting mixture was stirred at 80 °C for 12 h. The reaction was monitored by thin-layer chromatography. After completion of the reaction, the solid was filtered, and the solution was concentrated in vacuo. The crude product was purified by chromatography on silica gel (PE/EtOAc) to give compounds 6a–e with 50–65% yields. Excess potassium carbonate was added as the catalyst to compounds 6a–e (0.20 mmol, 1 equiv) in acetonitrile (10 mL). After stirring for 10 min, the desired secondary amine (0.40 mmol, 2 equiv) was added, and the reaction mixture was stirred at 80 °C for 12 h. The solid was removed by filtration, and the solution was concentrated in vacuo. The crude product was purified by chromatography on silica gel (PE/EtOAc) to give the compounds 7a–h as yellow solid in 55–75% yields.
5-Hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-7-(2-(pyrrolidin-1-yl)ethoxy)-3-(((3R,4R,5R,6S)-3,4,5,6-tetrahydroxytetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one (7a). Yield 60%; yellow solid; m.p. > 250 °C; ESI/MS m/z: 614.2 [M + H]+; 1H-NMR (400 MHz, CDCl3) δ 12.54 (s, 1H), 7.81 (d, J = 8.3 Hz, 2H), 7.00 (d, J = 8.2 Hz, 2H), 5.47 (s, 1H), 5.11 (s, 1H), 4.46 (s, 1H), 4.30 (s, 2H), 3.86 (s, 3H), 3.37 (m, 7H), 3.15 (s, 3H), 2.88 (m, 6H), 1.92 (s, 4H), 1.72 (s, 3H), 1.67 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 178.6, 161.8, 161.8, 160.2, 157.5, 153.6, 135.1, 131.8, 130.7, 130.7, 122.7, 122.4, 122.4, 114.0, 114.0, 107.8, 105.7, 101.9, 95.7, 72.4, 71.5, 70.4, 67.0, 55.5, 54.8, 54.8, 50.8, 25.7, 23.5, 23.5, 18.1, 17.2.
5-Hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-7-(3-(pyrrolidin-1-yl)propoxy)-3-(((3R,4R,5R,6S)-3,4,5,6-tetrahydroxytetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one (7b). Yield 55%; yellow solid; m.p. 70.8–71.3 °C; ESI/MS m/z: 628.3 [M + H]+; 1H-NMR (400 MHz, DMSO-d6) δ 12.65 (s, 1H), 7.87 (d, J = 8.9 Hz, 2H), 7.14 (d, J = 8.9 Hz, 2H), 5.27 (s, 1H), 5.13 (s, 1H), 4.16 (d, J = 5.6 Hz, 2H), 4.00 (s, 1H), 3.86 (s, 3H), 3.48 (m, 9.3 Hz, 10H), 2.69 (s, 3H), 2.60 (s, 4H), 1.98 (d, J = 6.3 Hz, 2H), 1.74 (s, 3H), 1.69 (s, 3H), 1.62 (s, 3H). 13C-NMR (100 MHz, DMSO-d6) δ 178.7, 165.4, 162.30 161.9, 161.0, 160.1, 157.6, 134.9, 131.7, 131.0, 131.0, 122.8, 122.5, 114.6, 114.6, 107.6, 105.0, 102.5, 96.3, 71.6, 71.2, 70.8, 70.5, 56.0, 53.9, 53.9, 52.4, 28.0, 25.9, 23.5, 21.7, 18.3, 17.9.
5-Hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-7-(4-(pyrrolidin-1-yl)butoxy)-3-(((3R,4R,5R,6S)-3,4,5,6-tetrahydroxytetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one (7c). Yield 70%; yellow solid; m.p. 92.7–94.2 °C; ESI/MS m/z: 642.3 [M]+; 1H-NMR (400 MHz, DMSO-d6) δ 12.51 (s, 1H), 7.85 (dd, J = 17.7, 8.9 Hz, 2H), 7.16–7.08 (m, 2H), 5.28 (s, 1H), 5.16 (dd, J = 13.3, 6.7 Hz, 1H), 4.12 (t, J = 6.0 Hz, 1H), 4.00 (s, 1H), 3.85 (d, J = 3.4 Hz, 4H), 3.36 (m, 10H), 2.46–2.38 (m, 3H), 2.15–1.98 (m, 6H), 1.79 (dd, J = 14.1, 6.0 Hz, 1H), 1.67 (d, J = 3.6 Hz, 5H), 1.62 (s, 5H). 13C-NMR (100 MHz, DMSO-d6) δ 177.6, 166.4, 161.5, 159.4, 156.1, 154.3, 130.9, 130.7, 130.7, 123.6, 123.3, 122.5, 114.6, 114.4, 114.4, 106.5, 102.3, 100.4, 99.8, 71.6, 70.8, 70.6, 69.0, 62.3, 62.3, 55.9, 54.0, 25.9, 25.3, 23.5, 22.0, 21.8, 18.3, 17.9.
5-Hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-7-((5-(pyrrolidin-1-yl)pentyl)oxy)-3-(((3R,4R,5R,6S)-3,4,5,6-tetrahydroxytetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one (7d). Yield 75%; yellow solid; m.p. 140.4–142.9 °C; ESI/MS m/z: 656.3 [M + H]+; 1H-NMR (400 MHz, DMSO-d6) δ 12.54 (s, 1H), 7.87 (d, J = 8.4 Hz, 2H), 7.12 (d, J = 8.6 Hz, 2H), 5.29 (s, 1H), 5.13 (s, 1H), 4.06 (s, 3H), 3.85 (s, 4H), 3.50 (m, 10H), 2.44–2.31 (m, 5H), 1.72 (s, 7H), 1.68 (s, 3H), 1.65 (s, 3H), 1.62 (s, 3H). 13C-NMR (100 MHz, DMSO-d6) δ 175.7, 165.2, 162.2, 161.7, 160.8, 157.2, 150.8, 135.4, 131.5, 130.8, 122.8, 122.0, 114.6, 114.6, 106.3, 102.7, 100.0, 97.5, 95.8, 72.4, 70.8, 70.6, 69.0, 56.1, 55.9, 54.1, 54.1, 28.6, 25.9, 24.8, 24.1, 23.5, 21.7, 18.2, 18.0.
5-Hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-7-((8-(pyrrolidin-1-yl)octyl)oxy)-3-(((3R,4R,5R,6S)-3,4,5,6-tetrahydroxytetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one (7e). Yield 63%; yellow solid; m.p. 139.4–141.4 °C; ESI/MS m/z: 670.3 [M + H]+; 1H-NMR (400 MHz, DMSO-d6) δ 12.65 (s, 1H), 7.87 (d, J = 8.9 Hz, 2H), 7.13 (d, J = 9.0 Hz, 2H), 5.28 (d, J = 1.2 Hz, 1H), 5.13 (t, J = 7.0 Hz, 1H), 4.10 (t, J = 6.1 Hz, 2H), 4.00 (d, J = 1.4 Hz, 1H), 3.86 (s, 3H), 3.49 (m, 10H), 2.69 (s, 4H), 2.60 (d, J = 5.9 Hz, 2H), 1.74 (s, 7H), 1.68 (s, 3H), 1.62 (s, 3H), 1.47 (dd, J = 14.1, 7.1 Hz, 4H), 1.30 (s, 5H). 13C-NMR (100 MHz, DMSO-d6) δ 178.7, 162.4, 161.8, 160.1, 157.6, 153.3, 134.9, 131.6, 130.9, 130.9, 122.8, 122.5, 122.5, 114.6, 114.6, 107.5, 105.0, 102.5, 96.2, 71.6, 71.2, 70.8, 70.5, 69.1, 56.0, 55.5, 53.7, 29.2, 29.0, 29.0, 27.6, 27.1, 25.9, 25.9, 23.4, 21.7, 18.2, 17.9.
7-((5-(Dimethylamino)pentyl)oxy)-5-hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-3-(((3R,4R,5R,6S)-3,4,5,6-tetrahydroxytetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one (7f). Yield 72%; yellow solid; m.p. 164.6–166.8 °C; ESI/MS m/z: 630.3 [M + H]+; 1H-NMR (400 MHz, CDCl3) δ 12.47 (s, 1H), 7.85 (d, J = 8.8 Hz, 2H), 7.02 (d, J = 8.9 Hz, 2H), 6.34 (s, 1H), 5.50 (s, 1H), 5.15 (t, J = 6.8 Hz, 1H), 4.47 (s, 1H), 4.03 (t, J = 6.3 Hz, 2H), 3.88 (s, 3H), 3.79–3.72 (m, 4H), 3.52–3.39 (m, 4H), 3.33 (dd, J = 9.4, 6.2 Hz, 1H), 2.43 (s, 6H), 2.44–2.37 (m, 2H), 1.90–1.83 (m, 2H), 1.73 (s, 3H), 1.68 (s, 3H), 1.60 (d, J = 7.2 Hz, 2H), 1.53 (d, J = 6.5 Hz, 2H). 13C-NMR (100 MHz, CDCl3) δ 178.7, 162.3, 161.8, 160.2, 157.6, 153.6, 135.0, 131.6, 130.7, 130.7, 122.8, 122.4, 118.2, 114.0, 114.0, 107.8, 105.4, 101.8, 95.6, 72.5, 71.5, 70.3, 66.9, 58.9, 55.5, 53.7, 53.7, 29.0, 26.2, 25.8, 23.8, 18.0, 17.1.
7-((5-(Diethylamino)pentyl)oxy)-5-hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-3-(((3R,4R,5R,6S)-3,4,5,6-tetrahydroxytetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one (7g). Yield 64%; yellow solid; m.p. 134.3–135.9 °C; ESI/MS m/z: 658.2 [M + H]+; 1H-NMR (400 MHz, CDCl3) δ 12.53 (s, 1H), 7.84 (d, J = 8.7 Hz, 2H), 7.02 (d, J = 8.7 Hz, 2H), 6.33 (s, 1H), 5.50 (s, 1H), 5.11 (s, 1H), 4.43 (s, 1H), 4.04 (s, 2H), 3.90 (s, 3H), 3.57–3.28 (m, 8H), 3.18 (d, J = 7.0 Hz, 5H), 3.06 (s, 2H), 1.92 (s, 4H), 1.71 (s, 3H), 1.68 (s, 3H), 1.61 (s, 2H), 1.41 (t, J = 7.1 Hz, 6H). 13C-NMR (100 MHz, CDCl3) δ 178.6, 161.9, 161.8, 160.2, 157.5, 153.5, 135.0, 132.8, 130.7, 130.7, 122.8, 122.4, 121.9, 114.0, 114.0, 107.7, 104.2, 101.7, 95.6, 72.6, 70.6, 70.3, 67.5, 55.5, 51.3, 50.9, 46.4, 28.7, 25.8, 23.5, 23.3, 18.0, 17.1, 8.6, 8.6.
5-Hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-7-((5-morpholinopentyl)oxy)-3-(((3R,4R,5R,6S)-3,4,5,6-tetrahydroxytetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one (7h). Yield 71%; yellow solid; m.p. 227.2–229.5 °C; ESI/MS m/z: 672.2 [M + H]+; 1H-NMR (400 MHz, DMSO-d6) δ 12.66 (s, 1H), 7.87 (d, J = 8.9 Hz, 2H), 7.14 (d, J = 9.0 Hz, 2H), 5.14 (t, J = 7.0 Hz, 1H), 5.01 (d, J = 4.3 Hz, 1H), 4.13 (t, J = 6.2 Hz, 2H), 4.00 (s, 1H), 3.86 (s, 3H), 3.37–3.34 (m, 10H), 3.19–3.02 (m, 4H), 2.77 (s, 6H), 1.79 (dd, J = 14.1, 6.6 Hz, 2H), 1.68 (d, J = 12.0 Hz, 5H), 1.63 (s, 3H), 1.52–1.42 (m, 2H). 13C-NMR (100 MHz, DMSO-d6) δ 178.7, 162.3, 161.9, 160.1, 157.6, 153.4, 134.9, 131.7, 131.0, 131.0, 122.8, 122.5, 114.6, 114.6, 107.5, 105.0, 102.5, 96.3, 71.6, 71.2, 70.8, 70.5, 68.7, 56.9, 56.9, 56.0, 56.0, 42.6, 42.6, 28.5, 26.0, 23.8, 22.9, 18.3, 17.9.
4.4. Cell Lines and Cell Culture Conditions
All cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Human breast cancer cells (MCF-7 and MDA-MB-231) and human hepatocellular carcinoma cell lines (HCCLM3-LUC) were cultured in DMEM (HyClone, Logan, UT, USA) medium, supplemented with 10% fetal bovine serum (FBS, Gibco, Life Technologies, New York, NY, USA) and antibiotics (100 mg/mL streptomycin and 100 units/mL penicillin (Gibco, Life Technologies). Human liver carcinoma cell lines (HepG2) were cultured in MEM (HyClone) medium, supplemented with 10% fetal bovine serum (FBS) and antibiotics. Human cancer cells were maintained in humidified atmosphere with 5% CO2 and 95% air at 37 °C in a carbon dioxide incubator (SANYO, CO2 incubator, Osaka, Japan).
4.5. Cell Viability Inhibition Assay
The cytotoxicity of compounds 4a–d and 7a–g formulation against human breast cancer cells (MCF-7 and MDA-MB-231) and human hepatic carcinoma cells (HepG2 and HCCLM3-LUC) were evaluated by measuring the cell viability with CCK-8 kit (Beyotime Biotechnology Co., Ltd., Shanghai, China). Four cancer cells were seeded into 96-well plates at 5 × 103 cells per well in 100 µL RPMI 1640 medium at 37 °C under a 5% CO2 atmosphere for 12 h, after cancer cells were treated with various concentrations of ICA II derivatives from 0 to 100 μM for 48 h. For comparison purpose, doxorubicin, and ICA II were used as reference compounds. 100 µL free medium containing 10 µL CCK-8 was added to each well according to the manufacturer’s protocol and cells were incubated for a further 1 h. Then, the absorbance was measured using a micro-plate reader (ThermoFisher, Varioskan Flash, Finland) at 450 nm wavelength. Untreated cells in the wells were used as blank controls. The results are shown as the average cell viability = ([ODtreat − ODblank]/[ODcontrol − ODblank] × 100%). The cytotoxic activity was expressed as the half maximal inhibitory concentration (IC50) value [22,23]. Every sample was repeated in triplicate wells.
4.6. Cell Morphological Assessment
To detect morphological evidence of apoptosis, cells were visualized following DNA staining with the fluorescent dye Hoechst 33,258. The MCF-7 cells were plated in glass-bottom culture dish at a density of 1 × 104 cells and were incubated overnight. The cells were treated with 1–4 μM compound 7g for 48 h. The cells of each dish were stained with Hoechst 33,258 (Beyotime Biotechnology Co., Ltd., Shanghai, China) in a dark room for 5 min, the cells were washed with PBS and then were observed by a fluorescent microscope (Axio Observer A1, Zeiss, Japan).
4.7. Cell Cycle Distribution Analysis
To investigate the effect of the most promising compound 7g on cell division cycle of proliferating cancer cells MCF-7 cells were plated at 1 × 106 cells per 6 cm dish and cultured overnight. The cells were incubated with compound 7g at 1–4 μM for 48 h. The cells of each well were harvested, washed twice with PBS and fixed with 70% EtOH at 4 °C overnight. Then, the fixed cells were washed twice with PBS to removed EtOH and harvested. The cells were resuspended in 500 μL of staining buffer containing propidium iodide (PI) and RNase. The mixture was incubated in a dark room for 30 min at 37 °C, then analyzed using a FACS XCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA) and the cell cycle analyzed using the FlowJo 7.6 software (Tree Star, Ashland, OR, USA).
4.8. Assessment of Apoptotic Analysis
The MCF-7 cells were plated at the concentration of 1 × 106 cells into 6-well plates. After overnight growth, cells were treated with 1–4 μM compound 7g for 48 h. The cells of each dish were trypsinized, washed twice with PBS. The cells were resuspended in 500 μL of binding buffer containing propidium iodide (PI) and Annexin-V-APC, and incubated in a dark room for 30 min at room temperature, then subjected to cell apoptotic analysis using a FACSCalibur flow cytometer. Annexin V-APC and PI emission were detected in the FL4 and FL2 channels of flow cytometry, respectively. Cells in the lower left quadrant, lower right quadrant, upper right quadrant represent normal cells, early apoptosis and late apoptosis or necrosis, respectively. The data were analyzed using the Flowjo 7.6 software.
Author Contributions
J.-S.L., Y.D. and T.Z. conceived and designed the experiments; T.W. and X.-M.W. performed the synthesis work; T.L. and L.L. performed the anticancer activity assays; T.W. and Y.-N.K. performed the experiments, analyzed the data and wrote the original draft; Y.D. and J.-S.L. revised the manuscript and supervised the project.
Funding
This work was supported by the programs of the National Natural Science Foundation of China [grant numbers 81403175 and 81274200]; Project of the Shanghai Committee of Science and Technology [grant number 13401900301]; Youth Talent Sail Plan from Shanghai Committee of Science and Technology [grant number 14YF1411300 and 18YF1423600]; Project of the Shanghai Municipal Commission of Health and Family planning [grant number 20134Y053, 2017YQ072 and 201740152]; Research Fund for the Doctoral Program of Shanghai [grant number B201703]; Research Fund of Shanghai University of Traditional Chinese Medicine [grant number 2016YSN21]; Project of Guangxi Key Laboratory of Brain and Cognitive Neuroscience [grant number GKLBCN-20170105-02]; Undergraduate innovation project from Shanghai University of traditional Chinese Medicine [grant number 2017SHUTCM121] and Xinglin Scholar Plan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Poustchi, H.; Sepanlou, S.; Esmaili, S.; Mehrabi, N.; Ansarymoghadam, A. Hepatocellular carcinoma in the world and the Middle East. Middle East J. Dig. Dis. 2010, 2, 31–41. [Google Scholar] [PubMed]
- Bugano, D.D.; Conforti-Froes, N.; Yamaguchi, N.H.; Baracat, E.C. Genetic polymorphisms, the metabolism of estrogens and breast cancer: A review. Eur. J. Gynaecol. Oncol. 2008, 29, 313–320. [Google Scholar] [PubMed]
- Gao, Y.; Chen, L.; Zhang, Z.; Chen, Y.; Li, Y. Reversal of multidrug resistance by reduction-sensitive linear cationic click polymer/iMDR1-pDNA complex nanoparticles. Biomaterials 2011, 32, 1738–1747. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Pan, Y.; Wang, H.; Li, H.; Peng, Q.; Wei, D.; Chen, C.; Zheng, J. Synthesis and evaluation of new pyrazoline derivatives as potential anticancer agents in HepG-2 cell line. Molecules 2017, 22, 467. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, W.; Shari, Y.; Liu, F.; Ku, X.; Yang, Y.; Zhang, Q.; Zhang, Y.; Kuang, H.; Wang, Z.; Wang, S. Design, synthesis and anticancer activity of novel nopinone-based thiosemicarbazone derivatives. Bioorg. Med. Chem. Lett. 2017, 27, 2360–2363. [Google Scholar] [CrossRef] [PubMed]
- Galanski, M.; Arion, V.B.; Jakupec, M.A.; Keppler, B.K. Recent developments in the field of tumor-inhibiting metal complexes. Curr. Pharm. Des. 2003, 9, 2078–2089. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Lai, Z.; Lin, J. Anticancer properties of Traditional Chinese Medicine. Comb. Chem. High Throughput Screen. 2017, 20, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.L.; Li, W.K.; Yu, J.G.; Xiao, P.G. Brevicornin, a new flavone from Epimedium brevicornum. Phytochemistry 1996, 41, 991–992. [Google Scholar] [CrossRef]
- Liu, B.; Xu, C.; Wu, X.; Liu, F.; Du, Y.; Sun, J.; Tao, J.; Dong, J. Icariin exerts an antidepressant effect in an unpredictable chronic mild stress model of depression in rats and is associated with the regulation of hippocampal neuroinflammation. Neuroscience 2015, 294, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.B.; Yang, Q.T. The testosterone mimetic properties of icariin. Asian J. Androl. 2006, 8, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, M.; Hong, M.; Diao, S.; Liu, A.; Huang, Y.; Yu, Q.; Peng, Z. Icariin exerts estrogen-like activity in ameliorating EAE via mediating estrogen receptor β, modulating HPA function and glucocorticoid receptor expression. Am. J. Transl. Res. 2016, 8, 1910–1918. [Google Scholar] [PubMed]
- Liu, P.; Jin, X.; Lv, H.; Li, J.; Xu, W.; Qian, H.; Yin, Z. Icaritin ameliorates carbon tetrachloride-induced acute liver injury mainly because of the antioxidative function through estrogen-like effects. In Vitro Cell. Dev. Biol. Anim. 2014, 50, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xiong, Y.; Zhu, X.; Gao, H.; Yin, S.; Wang, J.; Chen, G.; Wang, C.; Xiang, L.; Wang, P.; et al. Icariin improves osteoporosis, inhibits the expression of PPARγ, C/EBPα, FABP4 mRNA, N1ICD and jagged1 proteins, and increases Notch2 mRNA in ovariectomized rats. Exp. Ther. Med. 2017, 13, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.M.; Liu, X.Z.; Li, Q.L.; Wang, J.F.; Tan, Z.; Ge, M.H. The bisphenol A-enhanced activity of thyroid carcinoma cell line B-CPAP is inhibited by Icarrin. Chin. J. Otorhinolaryngol. Head Neck Surg. 2017, 52, 458–462. [Google Scholar]
- Sun, Y.; Sun, X.H.; Fan, W.J.; Jiang, X.M.; Li, A.W. Icariin induces S-phase arrest and apoptosis in medulloblastoma cells. Cell Mol. Biol. 2016, 62, 123–129. [Google Scholar] [PubMed]
- Xia, Q.; Xu, D.; Huang, Z.; Liu, J.; Wang, X.; Wang, X.; Liu, S. Preparation of icariside II from icariin by enzymatic hydrolysis method. Fitoterapia 2010, 81, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Zhang, Y.; Zhang, T.; Lu, L.; Ding, Y.; Zhao, Y. Comparative pharmacokinetics study of Icariin and Icariside II in rats. Molecules 2015, 20, 21274–21286. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Gu, F.; Zhang, Y.; Liu, T.; Guo, P.; Huang, Y. Icariside II promotes osteogenic differentiation of bone marrow stromal cells in beagle canine. Int. J. Clin. Exp. Pathol. 2015, 8, 4367–4377. [Google Scholar] [PubMed]
- Gao, J.; Deng, Y.; Yin, C.; Liu, Y.; Zhang, W.; Shi, J.; Gong, Q. Icariside II, a novel phosphodiesterase 5 inhibitor, protects against H2O2-induced PC12 cells death by inhibiting mitochondria-mediated autophagy. J. Cell Mol. Med. 2017, 21, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.Y.; Zhou, F.; Hui, Y.; Xu, Y.D.; Lei, H.E.; Pu, J.X.; Xin, Z.C. Effects of Icariside II on corpus cavernosum and major pelvic ganglion neuropathy in streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2014, 15, 23294–23306. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xie, M.; Jiang, N.; Huang, F.; Zhang, X.; Li, R.; Lu, J.; Liao, S.; Liu, Y. Icarisid II inhibits the proliferation of human osteosarcoma cells by inducing apoptosis and cell cycle arrest. Tumour Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.D.; Zhang, C.; Shi, Y.M.; Xia, Y.Z.; Guo, C.; Yang, L.; Kong, L.Y. Icariside II-induced mitochondrion and lysosome mediated apoptosis is counterbalanced by an autophagic salvage response in hepatoblastoma. Cancer Lett. 2015, 366, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Yang, J.; Zhang, T.; Yang, Y.S.; Ding, Y. Optimized biotransformation of Icariin into Icariside II by β-Glucosidase from trichoderma viride using central composite design method. BioMed Res. Int. 2016. [Google Scholar] [CrossRef]
- Yan, H.; Song, J.; Jia, X.; Zhang, Z. Hyaluronic acid-modified didecyldimethylammonium bromide/d-a-tocopheryl polyethylene glycol succinate mixed micelles for delivery of baohuoside I against non-small cell lung cancer: In vitro and in vivo evaluation. Drug Deliv. 2017, 24, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.; Field, J.; Murphy, J.; Abdelrahim, M.; Schönherr, H.; Sparrow, J.; Ellestad, G.; Nakanishi, K.; Zask, A. Synthesis of antioxidants for prevention of age-related macular degeneration. J. Nat. Prod. 2013, 76, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Song, S.; Shen, M.; Tang, Y.; Shi, Z.; Tang, H.; Shi, Q.; Fu, Y.; Duan, J. Mannich bases of scutellarein as thrombin-inhibitors: Design, synthesis, biological activity and solubility. Bioorg. Med. Chem. 2012, 20, 6919–6923. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, P.; Shi, J.F.; Jiang, Z.H.; Wei, X.Y. Synthesis and cancer cell growth inhibitory activity of icaritin derivatives. Eur. J. Med. Chem. 2015, 100, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Li, Y. β-elemene decreases cell invasion by upregulating E-cadherin expression in MCF-7 human breast cancer cells. Oncol. Rep. 2013, 30, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, N.; Dai, Q.; Wei, L.; Zhao, Q.; Li, Z.; He, Q.; Dai, Y.; Guo, Q. GL-V9, a newly synthetic flavonoid derivative, induces mitochondrial-mediated apoptosis and G2/M cell cycle arrest in human hepatocellular carcinoma HepG2 cells. Eur. J. Pharmacol. 2011, 670, 13–21. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 4a–d and 7a–h are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).