Novel Electrochemical Sensors Based on Cuprous Oxide-Electrochemically Reduced Graphene Oxide Nanocomposites Modified Electrode toward Sensitive Detection of Sunset Yellow
Abstract
:1. Introduction
2. Results and Discussion
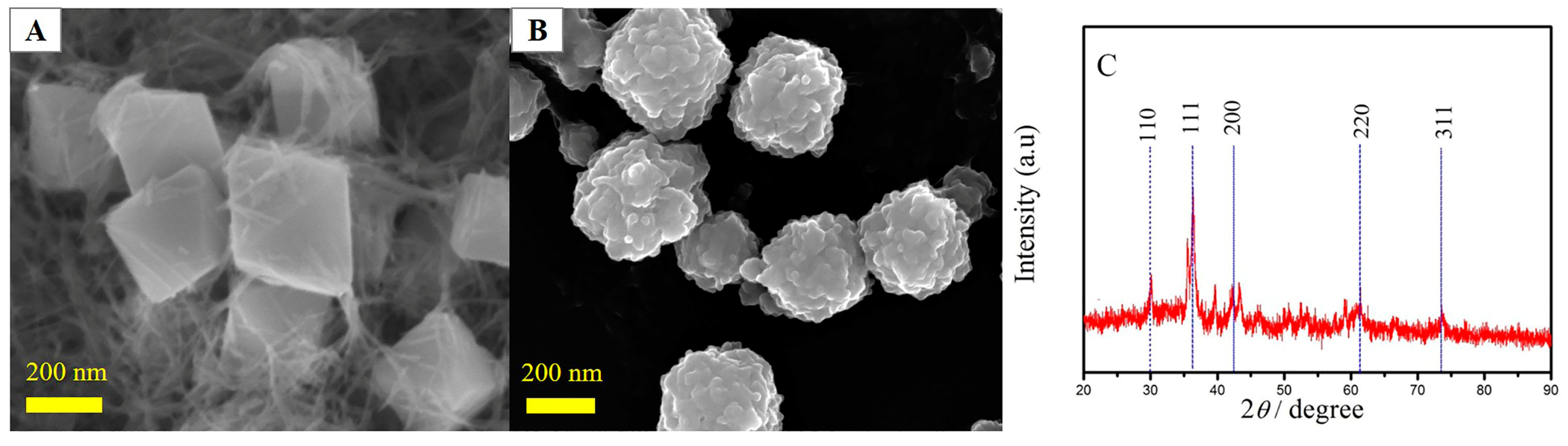
2.1. Morphology and Microstructural Characterization
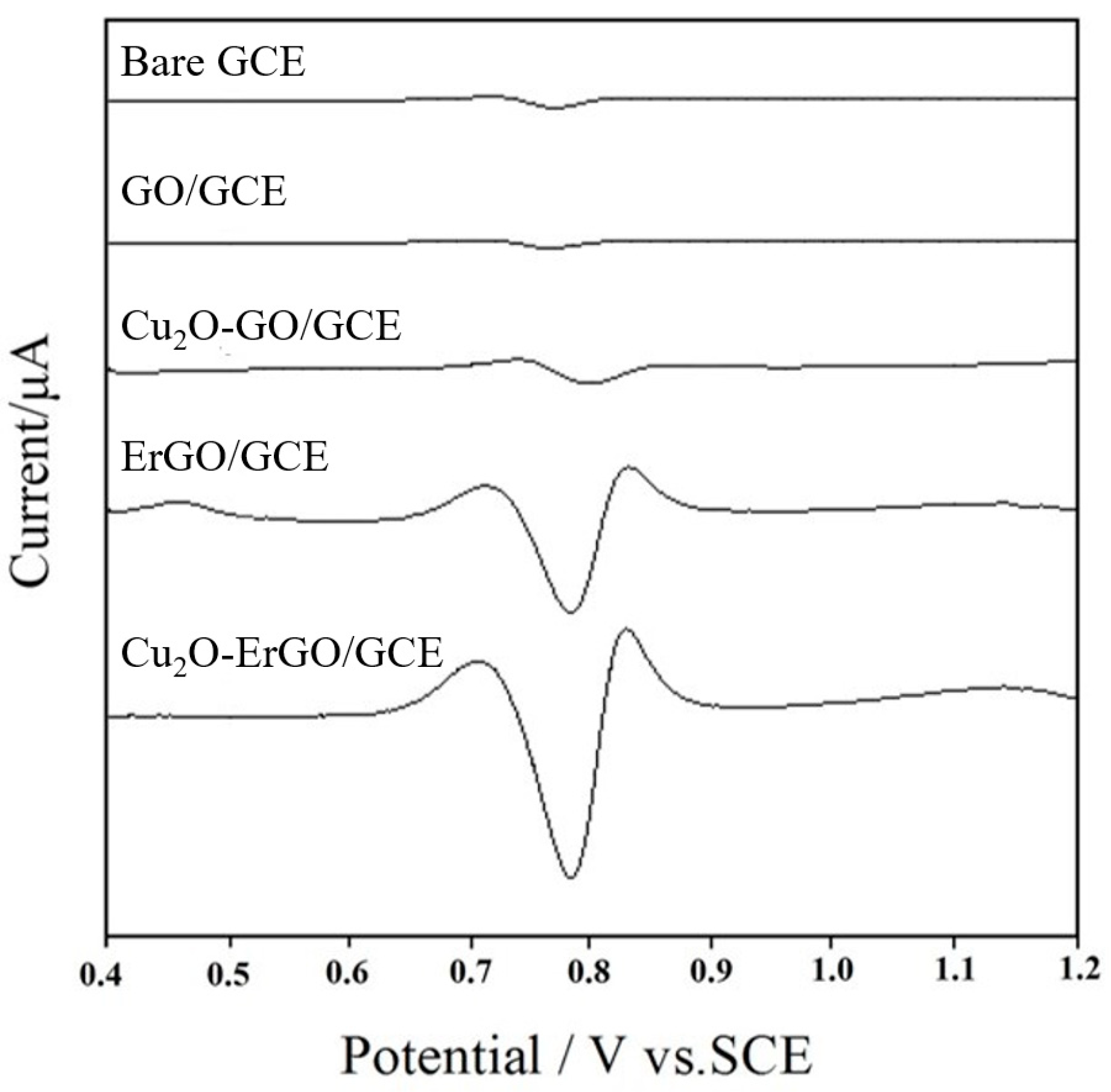
2.2. Electrochemical Behavior of Sunset Yellow on Modified Electrodes
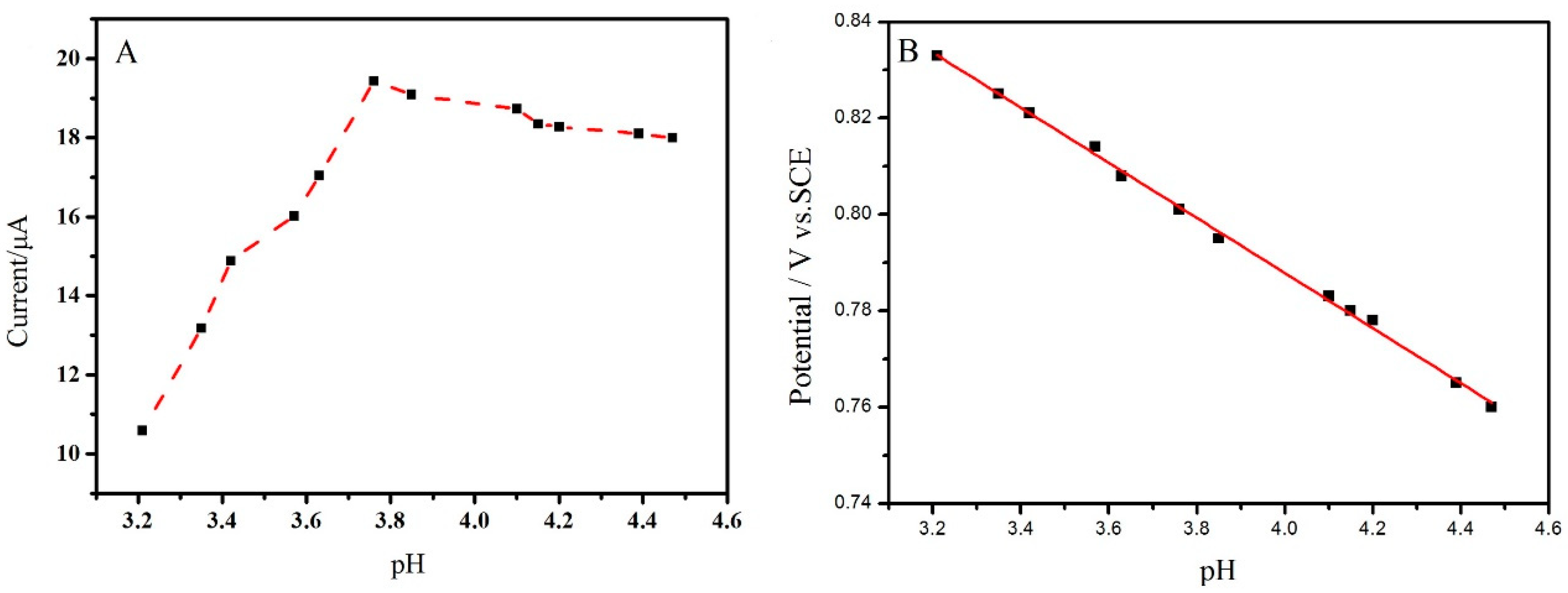
2.3. Effect of pH Value
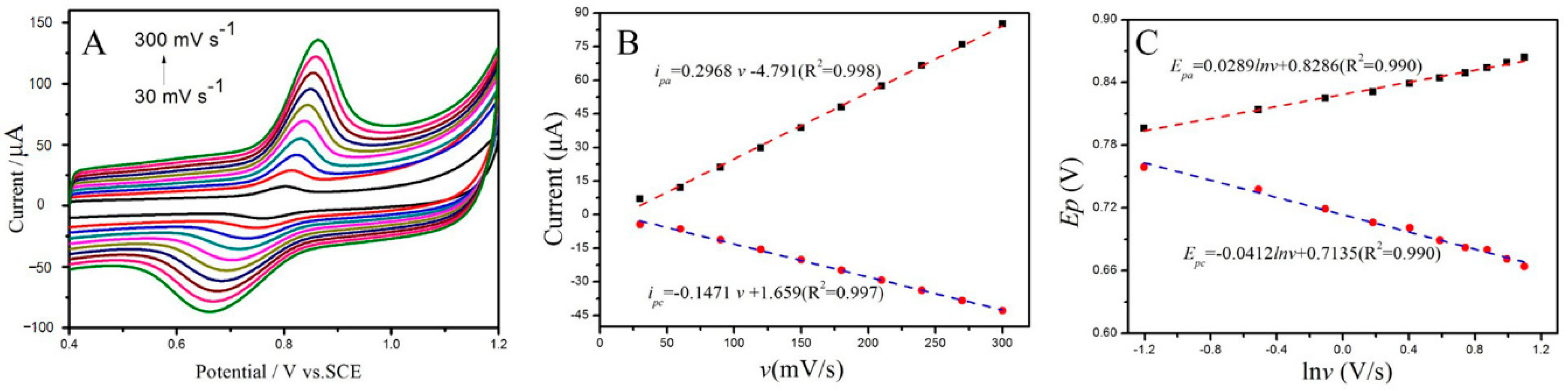
2.4. Effect of Sweep Rates
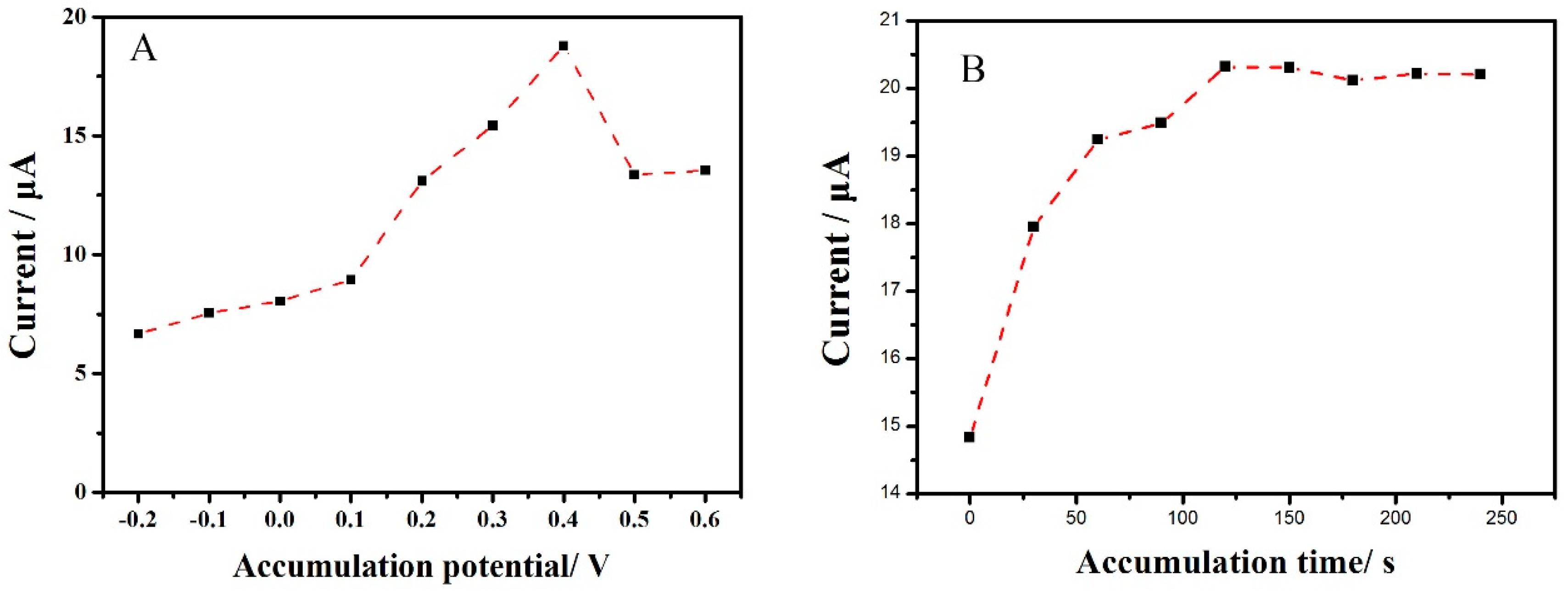
2.5. Effect of Acumualtion Parameters
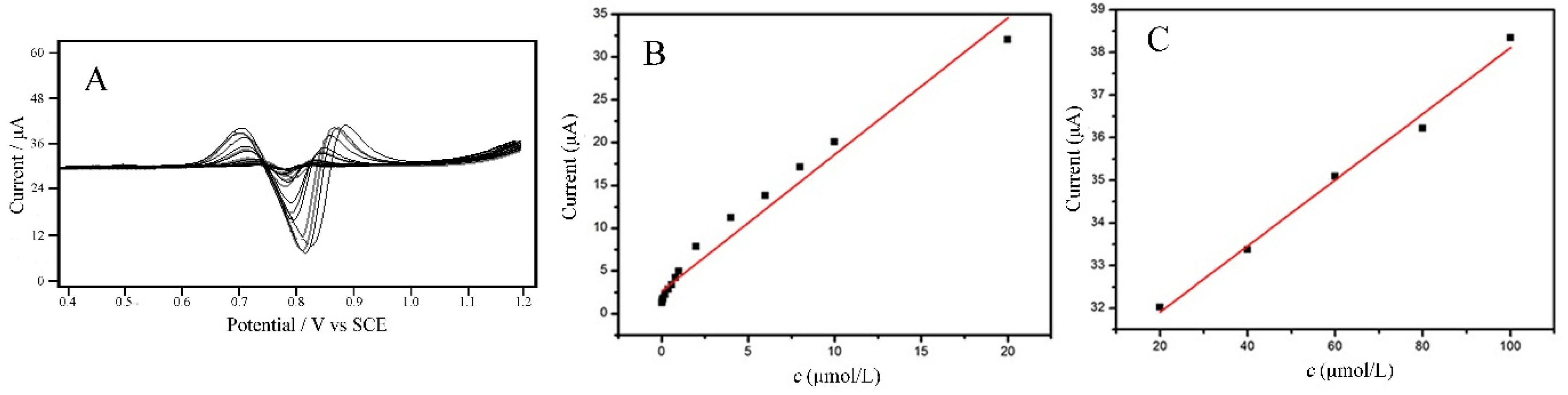
2.6. Standard Curves, Linear Range and Limit of Detection
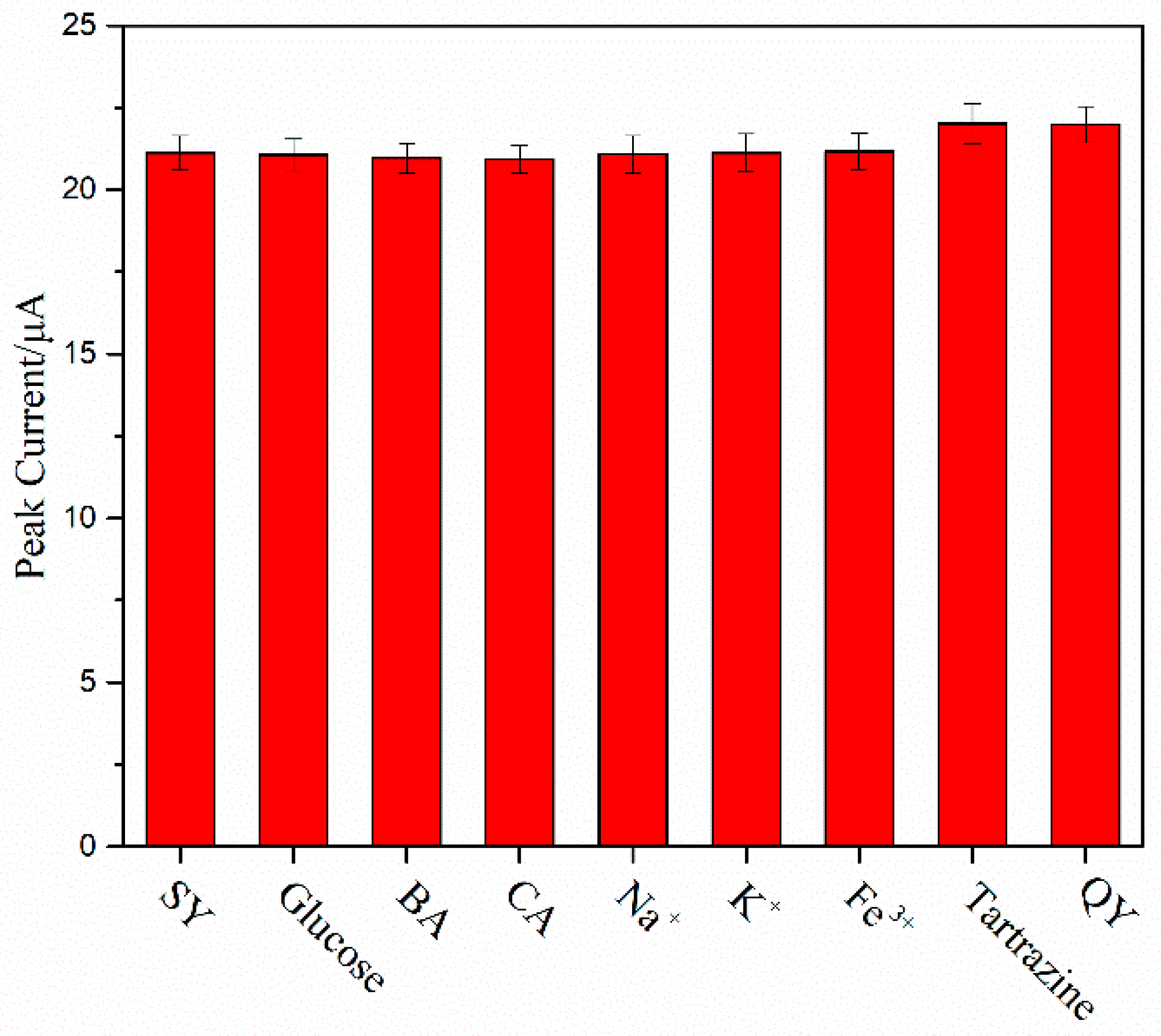
2.7. Interference and Reproducibility Investigation
2.8. Detection Sunset Yellow in Real Samples
3. Materials and Methods
3.1. Chemical and Solution
3.2. Synthesis of Cu2O Nanoparticles
3.3. Preparation Cu2O-GO Nanocomposite Dispersion
3.4. Preparation of Cu2O-ErGO/GCE
3.5. Electrochemical Measurements
3.6. Analysis of Real Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ni, Y.; Wang, Y.; Kokot, S. Simultaneous kinetic spectrophotometric analysis of five synthetic food colorants with the aid of chemometrics. Talanta 2009, 78, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Al-Degs, Y.S. Determination of three dyes in commercial soft drinks using HLA/GO and liquid chromatography. Food Chem. 2009, 117, 485–490. [Google Scholar] [CrossRef]
- Mao, Y.; Fan, Q.; Li, J.; Yu, L.; Qu, L. A novel and green ctab-functionalized graphene nanosheets electrochemical sensor for Sudan I determination. Sens. Actuators B Chem. 2014, 203, 759–765. [Google Scholar] [CrossRef]
- Chung, K.-T.; Cerniglia, C.E. Mutagenicity of azo dyes: Structure-activity relationships. Mutat. Res. 1992, 277, 201–220. [Google Scholar] [CrossRef]
- Aguilar, F.; Charrondiere, U.R.; Dusemund, B.; Galtier, P.; Gilbert, J.; Gott, D.M.; Grilli, S.; Guertler, R.; Koenig, J.; Lambre, C.; et al. Scientific Opinion on the re-evaluation of Sunset Yellow FCF (E 110) as a food additive on request from the European Commission. EFSA J. 2009, 7, 1–44. [Google Scholar]
- Ye, X.; Du, Y.; Lu, D.; Wang, C. Fabrication of β-cyclodextrin-coated poly (diallyldimethylammonium chloride)-functionalized graphene composite film modified glassy carbon-rotating disk electrode and its application for simultaneous electrochemical determination colorants of sunset yellow and tartrazine. Anal. Chim. Acta 2013, 779, 22–34. [Google Scholar] [PubMed]
- Ghoreishi, S.M.; Behpour, M.; Golestaneh, M. Simultaneous determination of Sunset yellow and Tartrazine in soft drinks using gold nanoparticles carbon paste electrode. Food Chem. 2012, 132, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Minioti, K.S.; Sakellariou, C.F.; Thomaidis, N.S. Determination of 13 synthetic food colorants in water-soluble foods by reversed-phase high-performance liquid chromatography coupled with diode-array detector. Anal. Chim. Acta 2007, 583, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Soponar, F.; Cătălin Moţ, A.; Sârbu, C. Quantitative determination of some food dyes using digital processing of images obtained by thin-layer chromatography. J. Chromatogr. 2008, 1188, 295–300. [Google Scholar] [CrossRef] [PubMed]
- De León-Rodríguez, L.M.; Basuil-Tobias, D.A. Testing the possibility of using UV–vis spectrophotometric techniques to determine non-absorbing analytes by inclusion complex competition in cyclodextrins. Anal. Chim. Acta 2005, 543, 282–290. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, X.; Qiao, M.; Zhu, J.; Liu, S.; Yang, J.; Hu, X. Determination of sunset yellow in soft drinks based on fluorescence quenching of carbon dots. Spectrochim. Acta A 2016, 167, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Ryvolová, M.; Táborský, P.; Vrábel, P.; Krásenský, P.; Preisler, J. Sensitive determination of erythrosine and other red food colorants using capillary electrophoresis with laser-induced fluorescence detection. J. Chromatogr. 2007, 1141, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, J. Facile synthesis of Au supported on ionic liquid functionalized reduced graphene oxide for simultaneous determination of Sunset yellow and Tartrazine in drinks. Sens. Actuators B Chem. 2015, 216, 578–585. [Google Scholar] [CrossRef]
- Rovina, K.; Siddiquee, S.; Shaarani, S.M. Highly sensitive electrochemical determination of sunset yellow in commercial food products based on CHIT/GO/MWCNTs/AuNPs/GCE. Food Control 2017, 82, 66–73. [Google Scholar] [CrossRef]
- Wang, J.; Yang, B.; Wang, H.; Yang, P.; Du, Y. Highly sensitive electrochemical determination of Sunset Yellow based on gold nanoparticles/graphene electrode. Anal. Chim. Acta 2015, 893, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Thirumalraj, B.; Rajkumar, C.; Chen, S.M.; Veerakumar, P.; Perumal, P.; Liu, S.B. Carbon aerogel supported palladium-ruthenium nanoparticles for electrochemical sensing and catalytic reduction of food dye. Sens. Actuators B Chem. 2018, 257, 48–59. [Google Scholar] [CrossRef]
- Wang, J.; Yang, B.; Zhang, K.; Bin, D.; Shiraishi, Y.; Yang, P.; Du, Y. Highly sensitive electrochemical determination of Sunset Yellow based on the ultrafine Au-Pd and reduced graphene oxide nanocomposites. J. Colloid Interface Sci. 2016, 481, 229–235. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Preparation of Cu2O-reduced graphene nanocomposite modified electrodes towards ultrasensitive dopamine detection. Sensors 2018, 18, 199. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Chen, D.; Deng, P.; Liang, J. Fabrication of amine-modified magnetite-electrochemically reduced graphene oxide nanocomposite modified glassy carbon electrode for sensitive dopamine determination. Nanomaterials 2018, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Li, G.; Liu, X.; Liu, J.; Deng, P.; Chen, D. Morphologically tunable MnO2 nanoparticles fabrication, modelling and their influences on electrochemical sensing performance toward dopamine. Catalysts 2018, 8, 323. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J.; Chen, D. Sensitive and selective detection of Tartrazine based on TiO2-electrochemically reduced graphene oxide composite-modified electrodes. Sensors 2018, 18, 1911. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, M.; Li, G.; He, Q.; Liu, J.; Li, F. Spherical α-MnO2 supported on N-KB as efficient electrocatalyst for oxygen reduction in Al–Air battery. Materials 2018, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, K.; Liu, J.; He, Q.; Li, G.; Li, F. Efficiently enhancing electrocatalytic activity of α-MnO2 nanorods/N-doped ketjenblack carbon for oxygen reduction reaction and oxygen evolution reaction using facile regulated hydrothermal treatment. Catalysts 2018, 8, 138. [Google Scholar] [CrossRef]
- Dorraji, P.S.; Jalali, F. Electrochemical fabrication of a novel ZnO/cysteic acid nanocomposite modified electrode and its application to simultaneous determination of sunset yellow and tartrazine. Food Chem. 2017, 227, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Sun, J.; Meng, W.; Song, L.; Zhang, Y. Electrochemical sensor based on graphene and mesoporous TiO2 for the simultaneous determination of trace colourants in food. Food Chem. 2013, 141, 3731–3737. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, X.; Zhao, C.; Qian, X. One-pot hydrothermal synthesis of ZnO/RGO/ZnO@Zn sensor for sunset yellow in soft drinks. Talanta 2018, 179, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Kondo, T.; Komoda, M.; Ikeda, S.; Kondo, J.N.; Domen, K.; Hara, M.; Shinohara, K.; Tanaka, A. Cu2O as a photocatalyst for overall water splitting under visible light irradiation. Chem. Commun. 1998, 3, 357–358. [Google Scholar] [CrossRef]
- Shao, F.; Sun, J.; Gao, L.; Luo, J.; Liu, Y.; Yang, S. High Efficiency Semiconductor-liquid junction solar cells based on Cu/Cu2O. Adv. Funct. Mater. 2012, 22, 3907–3913. [Google Scholar] [CrossRef]
- Meng, H.; Yang, W.; Ding, K.; Feng, L.; Guan, Y. Cu2O nanorods modified by reduced graphene oxide for NH3 sensing at room temperature. J. Mater. Chem. A 2014, 3, 1174–1181. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, Z.; Shen, X.; Zhu, G.; Wang, J.; Yue, X. Facile growth of Cu2O hollow cubes on reduced graphene oxide with remarkable electrocatalytic performance for non-enzymatic glucose detection. New J. Chem. 2017, 41, 9223–9229. [Google Scholar] [CrossRef]
- Wang, A.; Li, X.; Zhao, Y.; Wei, W.; Chen, J.; Hong, M. Preparation and characterizations of Cu2O/reduced graphene oxide nanocomposites with high photo-catalytic performances. Powder Technol. 2014, 261, 42–48. [Google Scholar] [CrossRef]
- Tran, P.D.; Batabyal, S.K.; Pramana, S.S.; Barber, J.; Wong, L.H.; Loo, S.C. A cuprous oxide-reduced graphene oxide (Cu2O-rGO) composite photocatalyst for hydrogen generation: employing rGO as an electron acceptor to enhance the photocatalytic activity and stability of Cu2O. Nanoscale 2012, 4, 3875–3878. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Duan, K.; Xue, M.; Du, Y.; Wang, C. Photoelectrocatalytic analysis and electrocatalytic determination of hydroquinone by using a Cu2O-reduced graphene oxide nanocomposite modified rotating ring-disk electrode. Analyst 2016, 141, 4772–4781. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Duan, H.; Wang, Y.; Bu, Y.; Luo, C. Based on magnetic graphene oxide highly sensitive and selective imprinted sensor for determination of sunset yellow. Talanta 2016, 147, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Li, C.; Li, X.; Huang, H. Simultaneous detection of sunset yellow and tartrazine using the nanohybrid of gold nanorods decorated graphene oxide. J. Electroanal. Chem. 2016, 780, 296–302. [Google Scholar] [CrossRef]
- Jampasa, S.; Siangproh, W.; Duangmal, K.; Chailapakul, O. Electrochemically reduced graphene oxide-modified screen-printed carbon electrodes for a simple and highly sensitive electrochemical detection of synthetic colorants in beverages. Talanta 2016, 160, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Tkalya, E.E.; Ghislandi, M.; With, G.D.; Koning, C.E. The use of surfactants for dispersing carbon nanotubes and graphene to make conductive nanocomposites. Curr. Opin. Colloid Interface Sci. 2012, 17, 225–232. [Google Scholar] [CrossRef]
- Songyang, Y.; Yang, X.; Xie, S.; Hao, H.; Song, J. Highly-sensitive and rapid determination of sunset yellow using functionalized montmorillonite-modified electrode. Food Chem. 2015, 173, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Lu, L.; Leng, J.; Yu, Y.; Wang, W.; Jiang, M.; Bai, L. An enhanced electrochemical platform based on graphene oxide and multi-walled carbon nanotubes nanocomposite for sensitive determination of Sunset Yellow and Tartrazine. Food Chem. 2016, 190, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Vladislavić, N.; Buzuk, M.; Rončević, I.Š.; Brinić, S. Electroanalytical Methods for Sunset Yellow Determination—A Review. Int. J. Electrochem. Sci. 2018, 13, 7008–7019. [Google Scholar] [CrossRef]
- Chao, M.; Ma, X. Convenient electrochemical determination of sunset yellow and tartrazine in food samples using a poly(L-phenylalanine)-modified glassy carbon electrode. Food Anal. Method 2015, 8, 130–138. [Google Scholar] [CrossRef]
- Arvand, M.; Zamani, M.; Sayyar Ardaki, M. Rapid electrochemical synthesis of molecularly imprinted polymers on functionalized multi-walled carbon nanotubes for selective recognition of sunset yellow in food samples. Sens. Actuators B Chem. 2017, 243, 927–939. [Google Scholar] [CrossRef]
- Yu, L.; Shi, M.; Yue, X.; Qu, L. A novel and sensitive hexadecyltrimethyl ammonium bromide functionalized graphene supported platinum nanoparticles composite modified glassy carbon electrode for determination of sunset yellow in soft drinks. Sens. Actuators B Chem. 2015, 209, 1–8. [Google Scholar] [CrossRef]
- Qin, C.; Guo, W.; Liu, Y.; Liu, Z.; Qiu, J.; Peng, J. A novel electrochemical sensor based on graphene oxide decorated with silver nanoparticles–molecular imprinted polymers for determination of sunset yellow in soft drinks. Food Anal. Method 2017, 10, 1–9. [Google Scholar] [CrossRef]
- Sierra-Rosales, P.; Toledo-Neira, C.; Squella, J.A. Electrochemical determination of food colorants in soft drinks using MWCNT-modified GCEs. Sens. Actuators B Chem. 2017, 240, 1257–1264. [Google Scholar] [CrossRef]
- Yu, L.; Zheng, H.; Shi, M.; Jing, S.; Qu, L. A novel electrochemical sensor based on poly (diallyldimethylammonium chloride)-dispersed graphene supported palladium nanoparticles for simultaneous determination of sunset yellow and tartrazine in soft drinks. Food Anal. Method 2016, 10, 1–10. [Google Scholar] [CrossRef]
- Arvand, M.; Erfanifar, Z.; Ardaki, M.S. A new core@shell silica-coated magnetic molecular imprinted nanoparticles for selective detection of sunset yellow in food samples. Food Anal. Method 2017, 10, 1–14. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Modified Electrodes | Method | Metrological Parameters | Linear Range (μmol/L) | LOD (μmol/L) | Reference |
|---|---|---|---|---|---|
| PLPA/GCE | DPV | 0.1 M phosphate-citrate buffer solution (pH 7.0); accumulation for 60 s | 0.04–14 | 0.040 | [42] |
| MIP/f-MWCNTs/GCE | DPV | 0.1 M CBS solution (pH 5.0); accumulation for 30 min; scanned at 10 mV/s | 0.05–100 | 0.005 | [43] |
| Au-Pd-RGO/GCE | DPV | 0.1 M PBS (pH 4.0); scanned at 50 mV/s | 0.69–332 | 0.0015 | [17] |
| CTAB-Gr-Pt/GCE | DPV | 0.1 M PBS (pH 3.0); accumulation for 3 min | 0.0085–1.0; 1.0–30 | 0.0042 | [44] |
| GO/AgNPs-MIPs/GCE | LSV | 0.1 M PBS (pH 5.5); accumulation for 7 min; scanned at 50 mV/s | 0.1–0.6; 0.6–12 | 0.02 | [45] |
| ILRGO-Au/GCE | SWV | 0.1 M BR buffer solution (pH 7.0); accumulation for 300 s | 0.004–1.0 | 0.00052 | [13] |
| Au NPs/CPE | DPV | 0.1 M PBS (pH 4.0); accumulation for 1 min; modulation amplitude = 60 mV and scan rate = 60 mV/s | 0.1–2.0 | 0.03 | [7] |
| MWCNT/GCE | DPV | 0.1 M PBS (pH 7.0); accumulation at open circuit potential for 2 min; potential increment of 0.004 V, pulse amplitude of 0.05 V, and pulse period of 0.2 s | 0.55–7.0 | 0.12 | [46] |
| PDDA-Gr-Pd/GCE | DPV | 0.1 M PBS (pH 3.0); accumulation for 5 min | 0.01–10 | 0.002 | [47] |
| Fe3O4@SiO2-NPs@MIP/Gr/GCE | DPV | 0.1 M PBS (pH 8.0); pulse amplitude = 0.05 V; pulse interval time = 0.05 s, and scan rate = 0.02 V/s for differential pulse voltammetry | 0.02–20 | 0.0055 | [48] |
| Cu2O-ErGO/GCE | SDLSV | 0.1 M PBS (pH 3.8); accumulation at 0.4 V for 180 s; scanned at 100 mV/s | 0.02–20; 20–100 | 0.006 | This work |
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| ipa (μA) | 21.07 | 22.34 | 21.36 | 22.04 | 22.29 | 22.85 | 22.24 |
| Average value (μA) | 22.02 | ||||||
| RSD (%) | 2.78 | ||||||
| Samples | Original (μmol/L) | Added (μmol/L) | RSD (%) | Found (μmol/L) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|---|
| Sodas | ND 1 | 4 | 1.28 | 4.06 | 102.0 | 1.50 |
| Orange juice | 0.085 | 0.080 | 2.46 | 0.082 | 102.5 | 2.31 |
| Candies | 0.162 | 0.160 | 3.25 | 0.158 | 98.75 | 2.85 |
| Protocols | Advantages | Drawbacks |
|---|---|---|
| Liquid chromatography | Reliable; good repeatability; high sensitivity; low LOD | Limited separation ability; Time-consuming; expensive equipment |
| Thin layer chromatography | Low cost apparatus | Organic solvents are often used; they have intensive disagreeable smell and cancerogenic activity |
| Spectrophotometry | Simultaneous identification and quantification; simple technique | Low sensitivity; extraction separation is needed for detection of dyes in complex product composition |
| Capillary electrophoresis | High column efficiency, short analysis time and minimal amounts of samples | Limited sensitivity & selectivity; severe matrix interferences |
| Electrochemical analysis (This work) | Low cost, rapid response, facile operate, high sensitivity and good selectivity | Portability needs to be improved; not disposable |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Liu, J.; Liu, X.; Xia, Y.; Li, G.; Deng, P.; Chen, D. Novel Electrochemical Sensors Based on Cuprous Oxide-Electrochemically Reduced Graphene Oxide Nanocomposites Modified Electrode toward Sensitive Detection of Sunset Yellow. Molecules 2018, 23, 2130. https://doi.org/10.3390/molecules23092130
He Q, Liu J, Liu X, Xia Y, Li G, Deng P, Chen D. Novel Electrochemical Sensors Based on Cuprous Oxide-Electrochemically Reduced Graphene Oxide Nanocomposites Modified Electrode toward Sensitive Detection of Sunset Yellow. Molecules. 2018; 23(9):2130. https://doi.org/10.3390/molecules23092130
Chicago/Turabian StyleHe, Quanguo, Jun Liu, Xiaopeng Liu, Yonghui Xia, Guangli Li, Peihong Deng, and Dongchu Chen. 2018. "Novel Electrochemical Sensors Based on Cuprous Oxide-Electrochemically Reduced Graphene Oxide Nanocomposites Modified Electrode toward Sensitive Detection of Sunset Yellow" Molecules 23, no. 9: 2130. https://doi.org/10.3390/molecules23092130
APA StyleHe, Q., Liu, J., Liu, X., Xia, Y., Li, G., Deng, P., & Chen, D. (2018). Novel Electrochemical Sensors Based on Cuprous Oxide-Electrochemically Reduced Graphene Oxide Nanocomposites Modified Electrode toward Sensitive Detection of Sunset Yellow. Molecules, 23(9), 2130. https://doi.org/10.3390/molecules23092130






