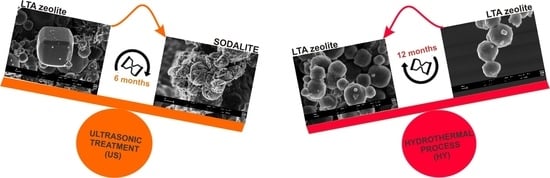
Influence of Synthesis Method on LTA Time-Dependent Stability
Abstract
:1. Introduction
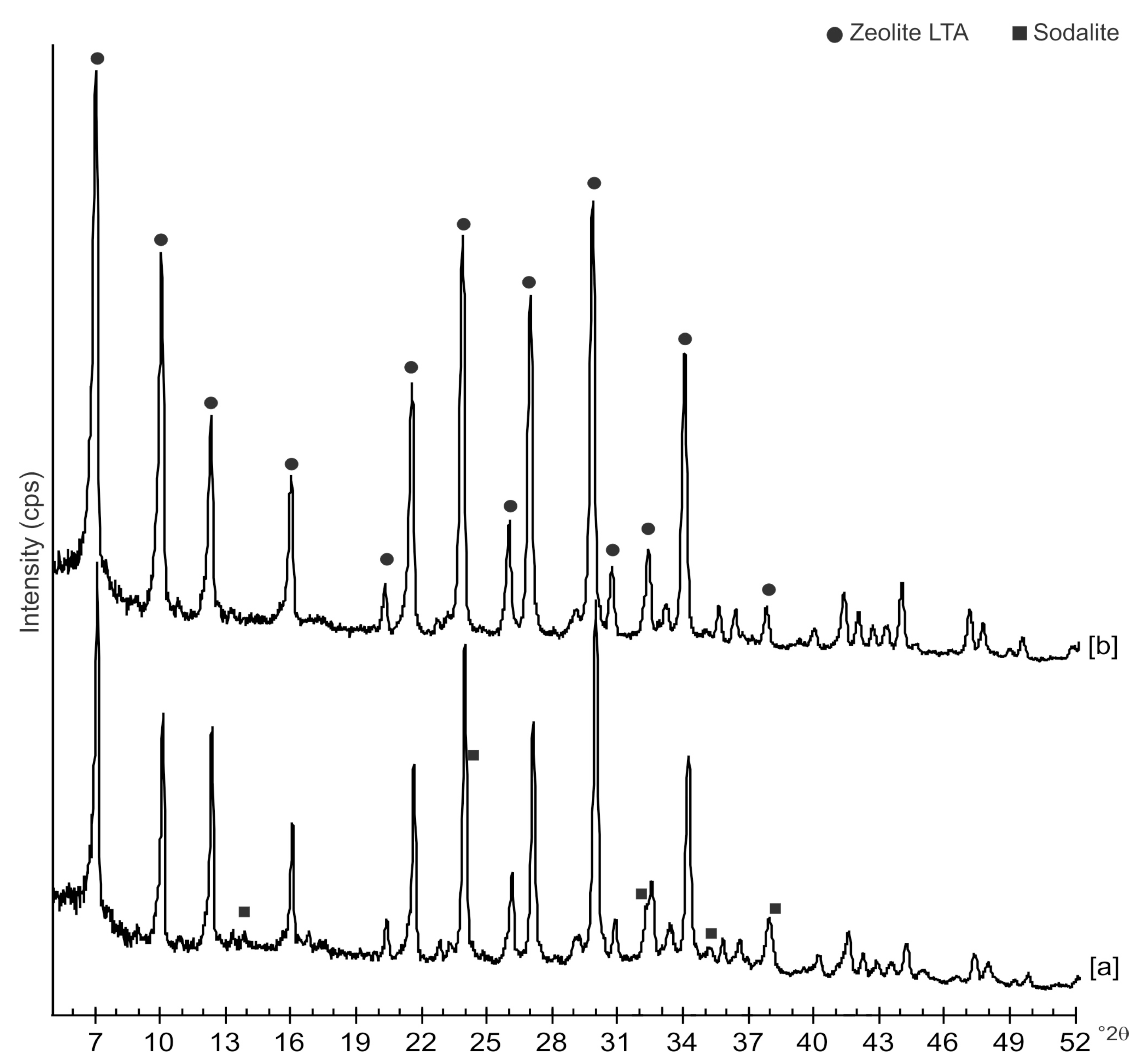
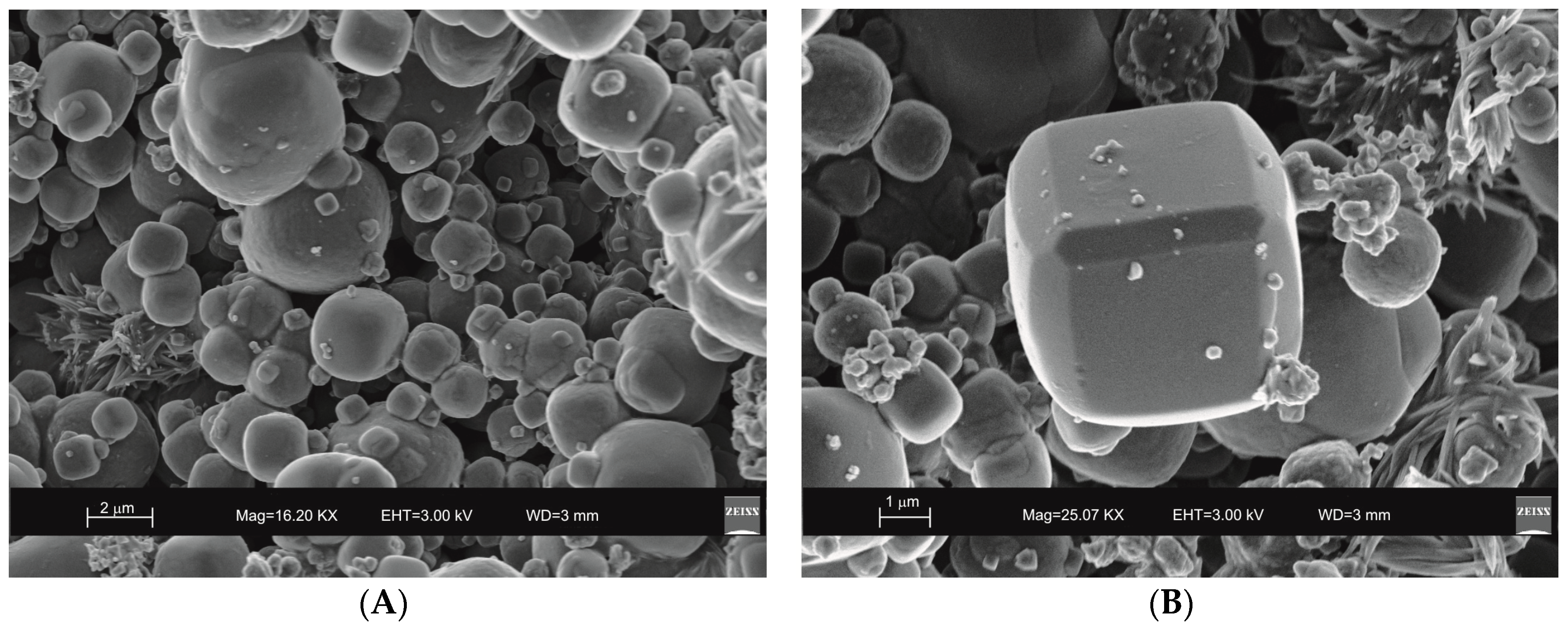
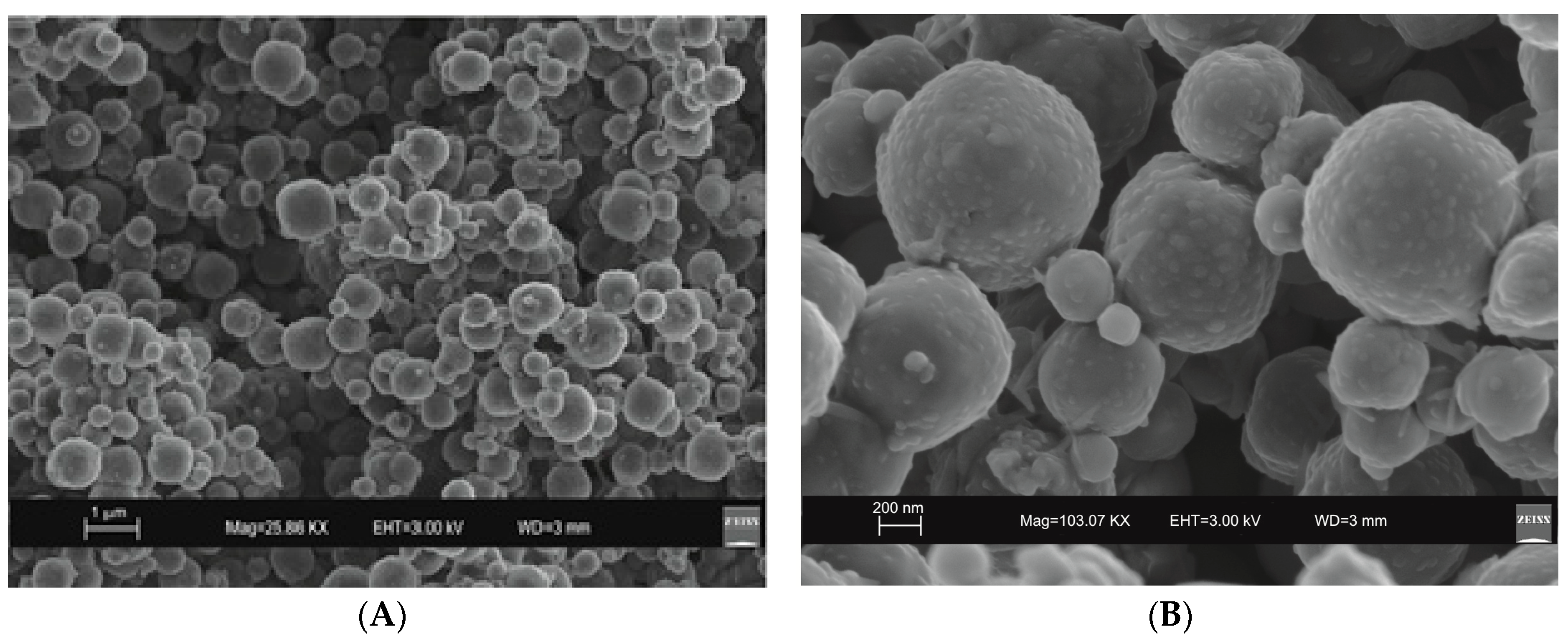
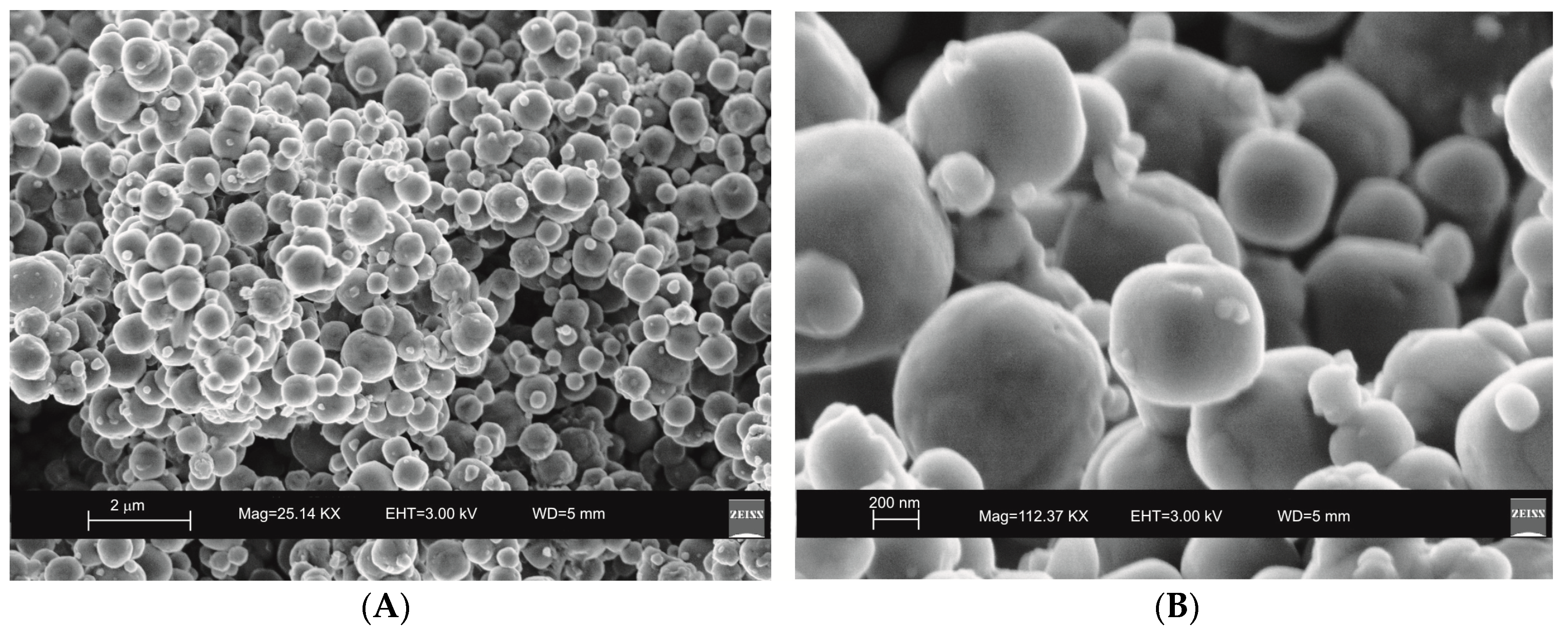
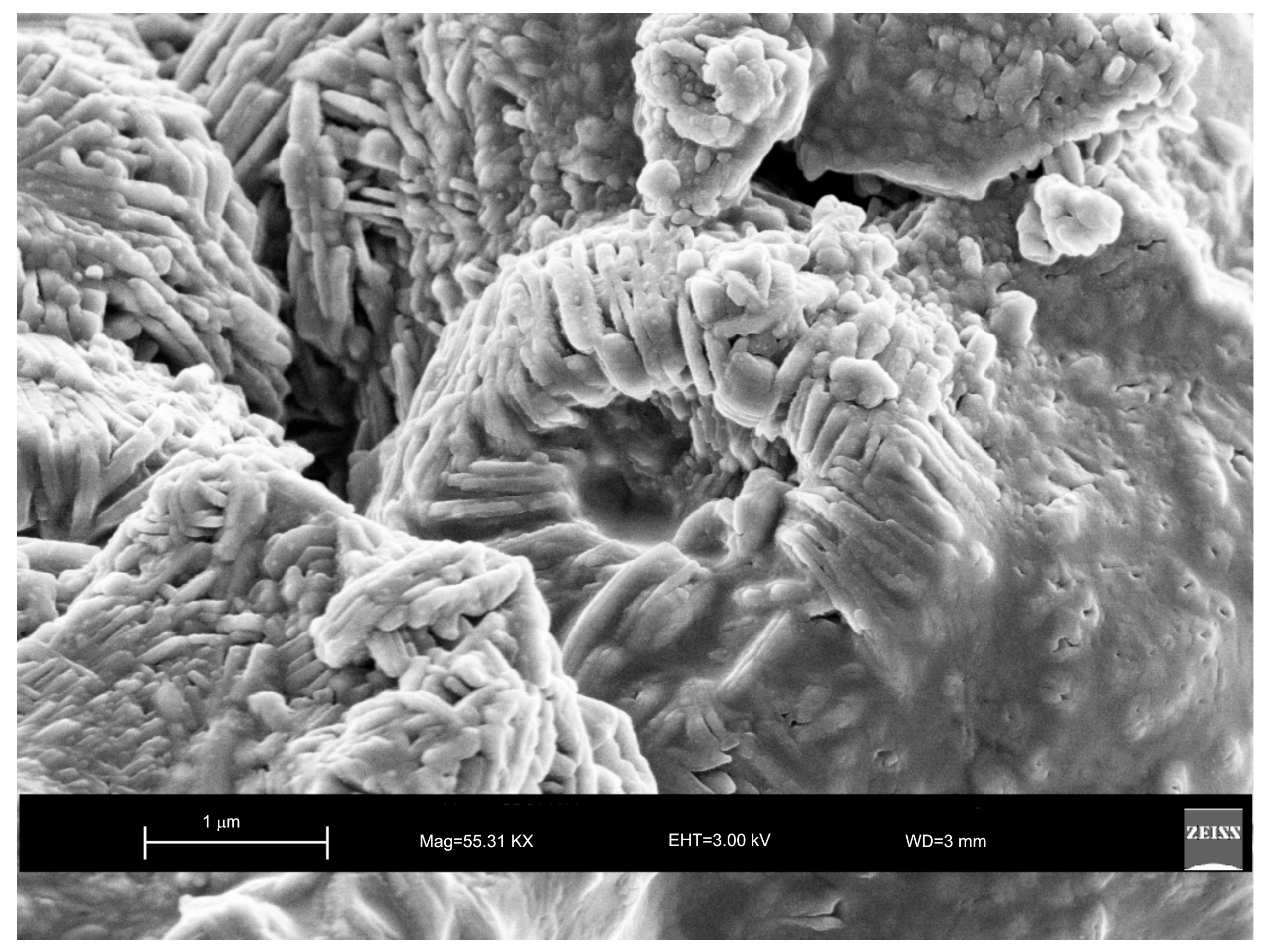
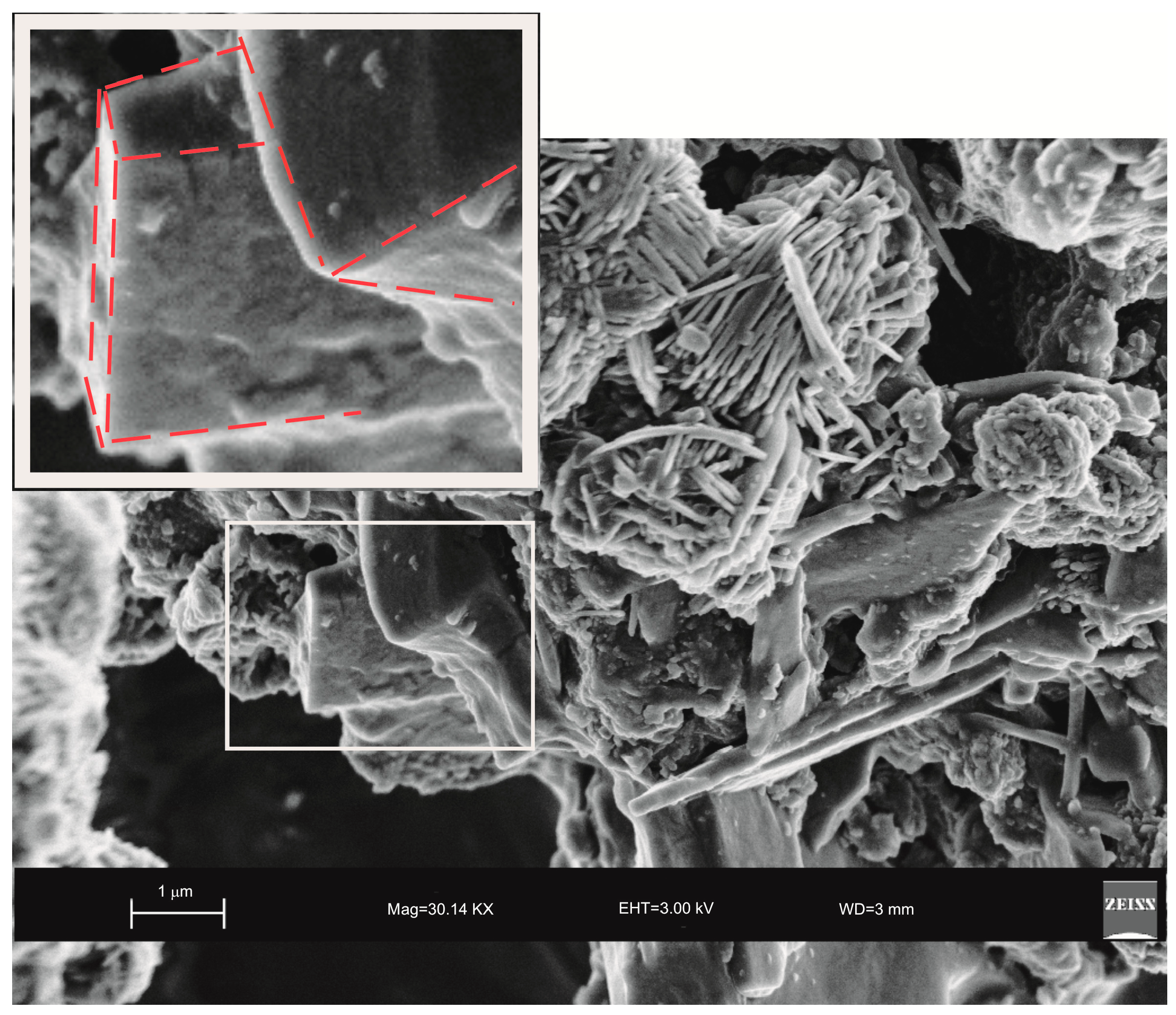
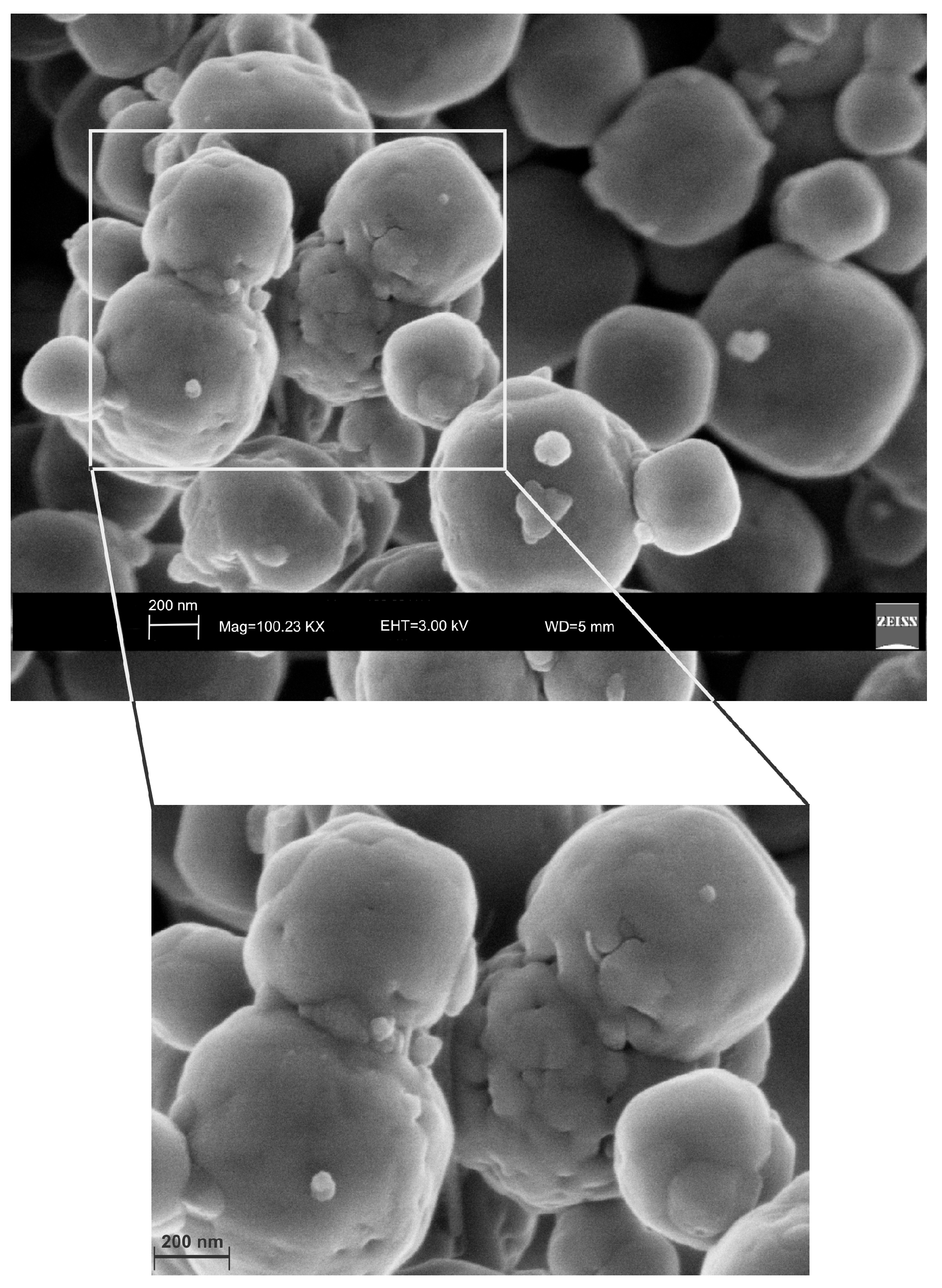
2. Results
3. Discussion
4. Experimental Section
4.1. Synthesis of LTA Zeolite
4.2. Characterization of Synthetic Products
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mintova, S.; Olson, N.H.; Bein, T. Electron Microscopy Reveals the Nucleation Mechanism of Zeolite Y from Precursor Colloids. Angew. Chem. 2009, 38, 3201–3204. [Google Scholar] [CrossRef]
- Yang, S.Y.; Navrotsky, A.; Phillips, B.L. An In Situ Calorimetric Study of the Synthesis of FAU Zeolite. Micropor. Mesopor. Mater. 2001, 46, 137–151. [Google Scholar] [CrossRef]
- Dhainaut, J.; Daou, T.J.; Chappaz, A.; Bats, N.; Harbuzaru, B.; Lapisardi, G.; Chaumeil, H.; Defoin, A.; Rouleau, L.; Patarin, J. Synthesis of FAU and EMT-type zeolites using structure-directing agents specifically designed by molecular modelling. Micropor. Mesopor. Mater. 2013, 174, 117–125. [Google Scholar] [CrossRef]
- Li, Q.H.; Creaser, D.; Sterte, J. An Investigation of the Nucleation/Crystallization Kinetics of Nanosized Colloidal Faujasite Zeolites. Chem. Mater. 2012, 14, 1319–1324. [Google Scholar] [CrossRef]
- Holmberg, B.A.; Wang, H.; Norbeck, J.M.; Yan, Y. Controlling size and yield of zeolite Y nanocrystals using tetramethylammonium bromide. Micropor. Mesopor. Mater. 2013, 59, 13–28. [Google Scholar] [CrossRef]
- Lechert, H.; Kacirek, H. Investigations on the crystallization of X-type zeolites. Zeolites 1991, 11, 720–728. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Z.; Li, W.; Zhao, Y.; Pan, H.; Liu, Y.; Li, M.; Kong, L.; He, M. Synthesis, characterization, and catalytic performance of high-silica Y zeolites with different crystallite size. Micropor. Mesopor. Mater. 2013, 167, 102–108. [Google Scholar] [CrossRef]
- Awala, H.; Gilson, J.P.; Retoux, R.; Boullay, P.; Goupil, J.M.; Valtchev, V.; Mintova, S. Template-free nanosized faujasite-type zeolites. Nat. Mater. 2015, 14, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Huo, Z.; Xu, X.; Lu, Z.; Song, J.; He, M.; Li, Z.; Wang, Q.; Yan, L. Synthesis of zeolite NaP with controllable morphologies. Micropor. Mesopor. Mater. 2012, 158, 137–140. [Google Scholar] [CrossRef]
- Belviso, S.; Cavalcante, F.; Lettino, A.; Ragone, P.; Belviso, C. Fly ash as raw material for the synthesis of zeolite-encapsulated porphyrazine and metallo porphyrazine tetrapyrrolic macrocycles. Micropor. Mesopor. Mater. 2016, 236, 228–234. [Google Scholar] [CrossRef]
- Belviso, C.; Agostinelli, E.; Belviso, S.; Cavalcante, F.; Pascucci, S.; Peddis, D.; Varvaro, G.; Fiore, S. Synthesis of magnetic zeolite at low temperature using a waste material mixture: Fly ash and red mud. Micropor. Mesopor. Mater. 2015, 202, 208–216. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites: History and development from the earliest days to the present times. Chem. Rev. 2003, 103, 663–701. [Google Scholar] [CrossRef] [PubMed]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites: Precursors, intermediates and reaction mechanism. Micropor. Mesopor. Mater. 2005, 82, 1–78. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Fiore, S. Synthesis of zeolite from Italian coal fly ash. Differences in crystallization temperature using seawater instead of distilled water. Waste Manag. 2010, 30, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Belviso, C.; Cavalcante, F.; Huertas, F.J.; Lettino, A.; Ragone, P.; Fiore, S. The crystallisation of zeolite (X and A-type) from fly ash at 25 °C in artificial sea water. Micropor. Mesopor. Mater. 2012, 162, 115–121. [Google Scholar] [CrossRef]
- Chen, H.; Wydra, J.; Zhang, X.; Lee, P.-S.; Wang, Z.; Fan, W.; Tsapatsis, M. Hydrothermal Synthesis of Zeolites with Three-Dimensionally Ordered Mesoporous-Imprinted Structure. J. Am. Chem. Soc. 2011, 133, 12390–12393. [Google Scholar] [CrossRef] [PubMed]
- Belviso, C.; Cavalcante, F.; Niceforo, G.; Lettino, A. Sodalite, faujasite and A-type zeolite from 2:1dioctahedral and 2:1:1 trioctahedral clay minerals. A singular review of synthesis methods through laboratory trials at a low incubation temperature. Powder Technol. 2017, 320, 483–497. [Google Scholar] [CrossRef]
- Belviso, C. EMT-type zeolite synthesized from obsidian. Micropor. Mesopor. Mater. 2016, 226, 325–330. [Google Scholar] [CrossRef]
- Belviso, C.; Giannossa, L.G.; Huertas, F.J.; Lettino, A.; Mangone, A.; Fiore, S. Synthesis of zeolites at low temperatures in fly ash-kaolinite misture. Micropor. Mesopor. Mater. 2015, 212, 35–47. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Lettino, A.; Fiore, S. A and X type zeolite synthesized from kaolinite at low temperature. Appl. Clay Sci. 2013, 80, 162–168. [Google Scholar] [CrossRef]
- Kunecki, P.; Panek, R.; Koteja, A.; Franus, W. Influence of the reaction time on the crystal structure of Na-P1 zeolite obtained from coal fly ash microspheres. Micropor. Mesopor. Mater. 2018, 266, 102–108. [Google Scholar] [CrossRef]
- Wdowin, M.; Franus, M.; Panek, R.; Badura, L.; Franus, W. The conversion technology of fly ash into zeolites. Clean Technol. Environ. Policy 2014, 16, 1217–1223. [Google Scholar] [CrossRef] [Green Version]
- Gordon, J.; Kazemian, H.; Rohani, S. Rapid and efficient crystallization of MIL-53(Fe) by ultrasound and microwave irradiation. Micropor. Mesopor. Mater. 2012, 162, 36–43. [Google Scholar] [CrossRef]
- Park, J.; Kim, B.C.; Park, A.S.; Park, H.C. Conventional versus ultrasonic synthesis of zeolite 4A from kaolinite. J. Mater. Sci. Lett. 2001, 20, 531–533. [Google Scholar] [CrossRef]
- Wu, J.B.; Wang, B.Y.; Li, N.; Xiang, S.H. Effect of aging with ultrasound on the synthesis of MCM-49 zeolite. Chin. J. Catal. 2006, 27, 375–377. [Google Scholar] [CrossRef]
- Wang, B.; Wu, J.; Yuan, Z.-Y.; Li, N.; Xiang, S. Synthesis of MCM-22 zeolite by an ultrasonic-assisted aging procedure. Ultrason. Sonochem. 2008, 15, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.S.; Behin, J.; Kazemian, H.; Rohani, S. Conversion of coal fly ash to zeolite utilizing microwave and ultrasound energies: A review. Fuel 2015, 140, 250–266. [Google Scholar] [CrossRef]
- Askari, S.; Alipour, S.M.; Halladj, R.; Hossein, M.; Abadi Farahani, D. Effects of ultrasound on the synthesis of zeolites: A review. J. Porous Mater. 2013, 20, 285–302. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Fiore, S. Ultrasonic waves induce rapid zeolite synthesis in a seawater solution. Effects of ultrasonic treatment on zeolite synthesized from coal fly ash. Ultrason. Sonochem. 2013, 20, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Belviso, C.; Cavalcante, F.; Lettino, A.; Fiore, S. Effects of ultrasonic treatment on zeolite synthesized from coal fly ash. Ultrason. Sonochem. 2011, 18, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Oleksiak, M.D.; Soltis, J.A.; Conato, M.T.; Penn, R.L.; Rimer, J.D. Nucleation of FAU and LTA Zeolites from Heterogeneous Aluminosilicate Precursors. Chem. Mater. 2016, 28, 4906–4916. [Google Scholar] [CrossRef]
- Ren, L.; Wu, Q.; Yang, C.; Zhu, L.; Li, C.; Zhang, P.; Zhang, H.; Meng, X.; Xiao, F.S. Solvent-Free Synthesis of Zeolites from Solid Raw Materials. J. Am. Chem. Soc. 2012, 134, 15173–15176. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Sun, Q.; Qi, G.; Yang, C.; Xu, J.; Chen, F.; Meng, X.; Deng, F.; Xiao, F.S. Solvent-Free Synthesis of Silicoaluminophosphate Zeolites. Angew. Chem. Int. Ed. 2013, 52, 9172–9175. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, X.; Qi, G.; Guo, Q.; Pan, S.; Meng, X.; Xu, J.; Deng, F.; Fan, F.; Feng, Z.; et al. Sustainable synthesis of zeolites without addition of both organotemplates and solvents. J. Am. Chem. Soc. 2014, 136, 4019–4025. [Google Scholar] [CrossRef] [PubMed]
- Mullin, J.W. Crystallization, 4th ed.; Butterworth Heinemann: Oxford, UK, 2001; ISBN 9780080530116. [Google Scholar]
- Ng, E.-P.; Goupil, J.M.; Vincente, A.; Fernandez, C.; Retoux, R.; Valtchev, V.; Mintova, S. Nucleation and crystal growth features of EMT-type zeolite synthesized from on organic-template-free-system. Chem. Mater. 2012, 24, 4758–4765. [Google Scholar] [CrossRef]
- Oleksiak, M.D.; Rimer, J.D. Synthesis of zeolites in the absence of organic structure-directing agents: Factors governing crystal selection and polymorphism. Rev. Chem. Eng. 2014, 30, 1–49. [Google Scholar] [CrossRef]
- Conato, M.T.; Oleksiak, M.D.; McGrail, P.B.; Motkuri, R.K.; Rimer, J.D. Framework stabilization of Si-rich LTA zeolite prepared in organic-free media. Chem. Commun. 2015, 51, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Pang, W.; Yu, J.; Huo, Q.; Chen, J. Chemistry of Zeolites and Related Porous Materials: Synthesis and Structure; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 9780470822371. [Google Scholar]
- Baerlocher, C.; McCusker, L.B. Database of Zeolite Structures. Available online: http://www.iza-structure.org/database/ (accessed on 1 July 2018).
- Mintova, S.; Olson, N.H.; Valtchev, V.; Bein, T. Nanocrystal growth from colloids at room temperature. Science 1999, 283, 958–960. [Google Scholar] [CrossRef] [PubMed]
- Nikolakis, V.; Vlacho, D.G.; Tsapatsis, M. Modeling of zeolite crystallization: The role of gel microstructure. Micropor. Mesopor. Mater. 1998, 21, 337–346. [Google Scholar] [CrossRef]
- Walton, R.I.; Millange, F.; O’Hare, D.; Davies, A.T.; Sankar, G.; Catlow, C.R.A. An in Situ Energy-Dispersive X-ray Diffraction Study of the Hydrothermal Crystallization of Zeolite A. 1. Influence of Reaction Conditions and Transformation into Sodalite. J. Phys. Chem. B 2001, 105, 83–90. [Google Scholar] [CrossRef]
- Subotic, B.; Sekovanic, L.J. Transformation of zeolite A into hydroxysodalite. J. Cryst. Growth 1986, 75, 561. [Google Scholar] [CrossRef]
- Greer, H.; Wheatley, P.S.; Ashbrook, S.E.; Morris, R.E.; Zhou, W. Early Stage Reversed Crystal Growth of Zeolite A and Its Phase Transformation to Sodalite. J. Am. Chem. Soc. 2009, 131, 17986–17992. [Google Scholar] [CrossRef] [PubMed]
- Belviso, C. Effects of ultrasonic treatment on zeolite synthesized from coal fly ash. Ultrason. Sonochem. 2018, 43, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Andaç, Ö.; Murat, T.S.; Tatlier, M.; Erdem-Senatalar, A. Effects of ultrasound on the preparation of zeolite A coatings. Micropor. Mesopor. Mater. 2005, 88, 72–76. [Google Scholar] [CrossRef]
- McCausland, L.J.; Cains, P.W.; Martin, P.D. Use the powder of sonocrystallization for improved properties. Chem. Eng. Prog. 2001, 97, 56. [Google Scholar]
- Gualtieri, A.; Norby, P.; Artioli, G.; Hanson, J. Kinetics of formation of zeolite Na-A [LTA] from natural kaolinites. Phys. Chem. Miner. 1997, 24, 191–199. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Ragone, P.; Fiore, S. Immobilization of Zn and Pb in polluted soil by in situ crystallization zeolites from fly ash. Water Air Soil Poll. 2012, 223, 5357–5364. [Google Scholar] [CrossRef]
- Misaelides, P. Application of natural zeolites in environmental remediation: A short review. Micropor. Mesopor. Mater. 2011, 144, 15–18. [Google Scholar] [CrossRef]
- Ríos, C.A.; Oviedo, J.A.; Henao, J.A.; Macías, M.A. A NaY zeolite synthesized from Colombian industrial coal by-products: Potential catalytic applications. Catal. Today 2012, 190, 61–67. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belviso, C.; Lettino, A.; Cavalcante, F. Influence of Synthesis Method on LTA Time-Dependent Stability. Molecules 2018, 23, 2122. https://doi.org/10.3390/molecules23092122
Belviso C, Lettino A, Cavalcante F. Influence of Synthesis Method on LTA Time-Dependent Stability. Molecules. 2018; 23(9):2122. https://doi.org/10.3390/molecules23092122
Chicago/Turabian StyleBelviso, Claudia, Antonio Lettino, and Francesco Cavalcante. 2018. "Influence of Synthesis Method on LTA Time-Dependent Stability" Molecules 23, no. 9: 2122. https://doi.org/10.3390/molecules23092122
APA StyleBelviso, C., Lettino, A., & Cavalcante, F. (2018). Influence of Synthesis Method on LTA Time-Dependent Stability. Molecules, 23(9), 2122. https://doi.org/10.3390/molecules23092122