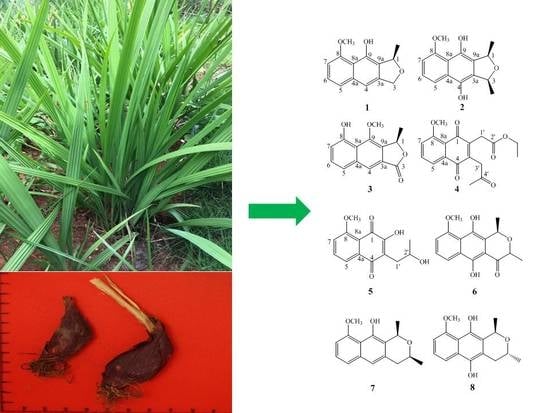
New Naphthalene Derivatives from the Bulbs of Eleutherine americana with Their Protective Effect on the Injury of HUVECs
Abstract
:1. Introduction
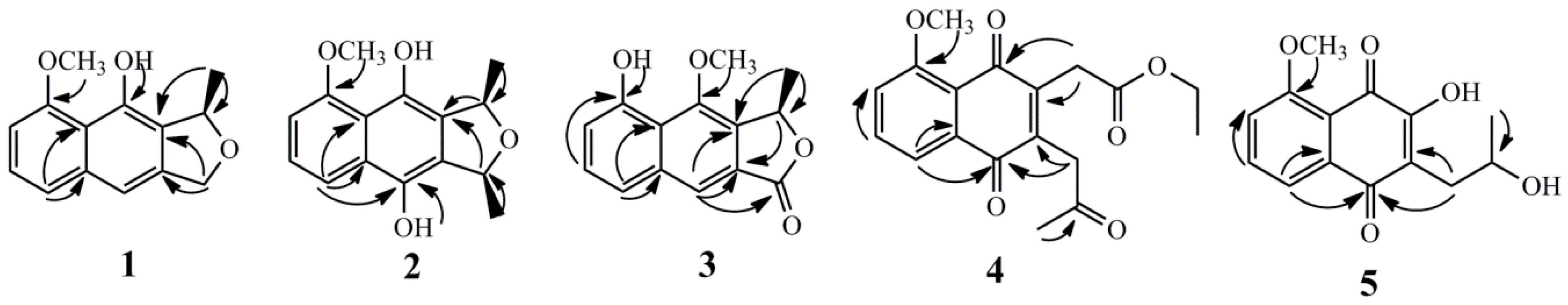
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
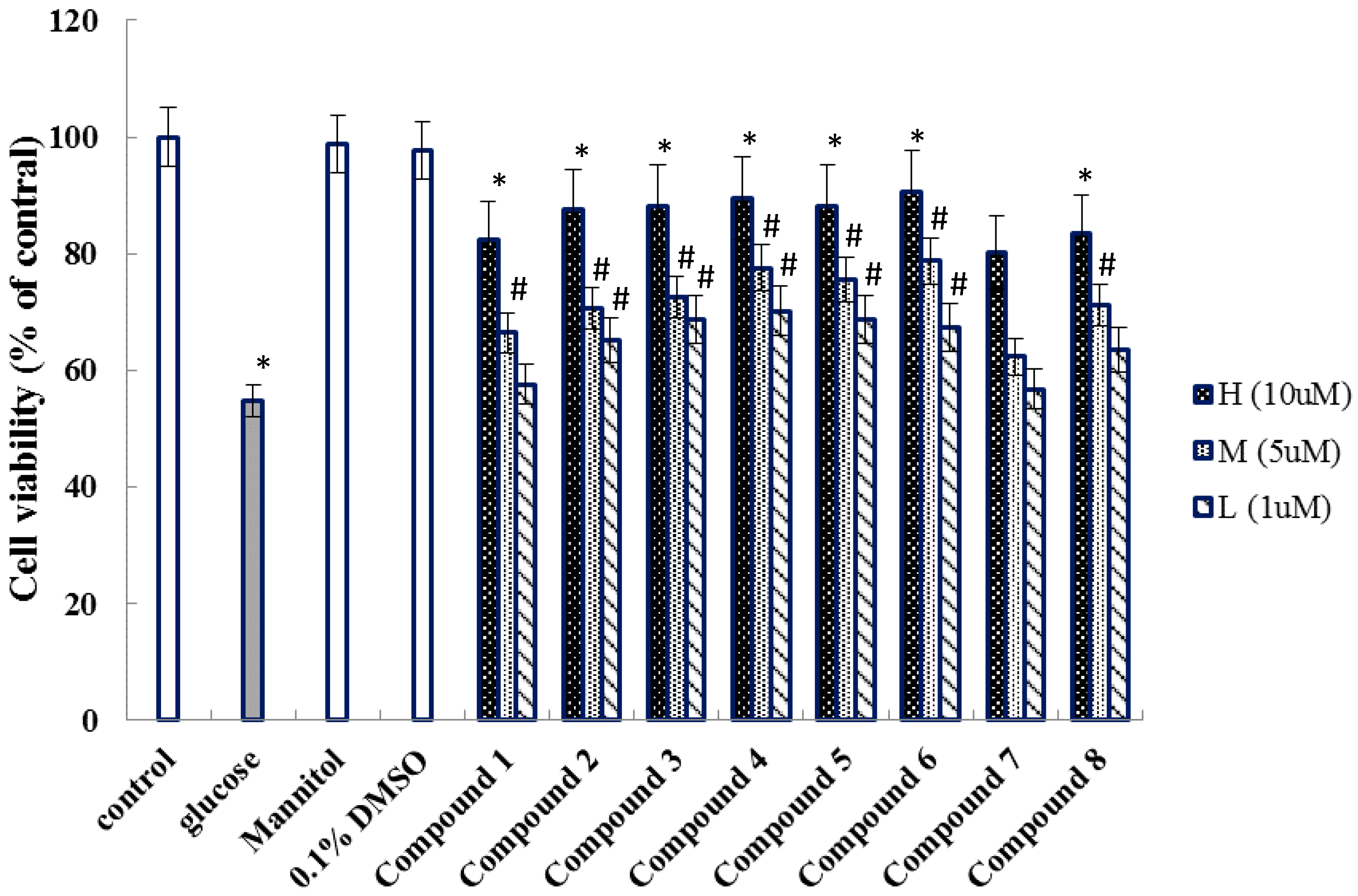
4.4. Activity Assay
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| UV | Ultra violet |
| IR | Infrared |
| NMR | Nuclear magnetic resonance |
| HR-ESIMS | High resolution electrospray ionization mass spectroscopy |
| HMBC | Heteronuclear multiple bond correlation |
| HSQC | Heteronuclear single quantum correlation |
| COSY | Homonuclear chemical shift Correlation Spectroscopy |
| NOESY | Nuclear overhauser effect spectroscopy |
| ODS | Octadecylsilane |
| HPLC | High performance liquid chromatography |
| MSO | Petroleum ether |
| CH2Cl2 | Dichloromethane |
| EtOAc | Ethyl acetate |
| nBuoH | n-Butanol |
| MeOH | Methanol |
| HUVECs | human umbilical vein endothelial cells |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| DMEM | DuIbecco’s modified eagIe’s medium |
References
- Kusuma, I.W.; Arung, E.T.; Rosamah, E.; Purwatiningsih, S.; Kuspradini, H.; JuliAstuti, J.S.; Kim, Y.U.; Shimizu, K. Antidermatophyte and antimelanogenesis compound from Eleutherine americana grown in Indonesia. J. Nat. Med. 2010, 64, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Insanu, M.; Kusmardiyani, S.; Hartati, R. Recent Studies on Phytochemicals and pharmacological effects of Eleutherine Americana Merr. Procedia Chem. 2014, 13, 221–228. [Google Scholar] [CrossRef]
- Ieyama, T.; Gunawan-Puteri, M.D.P.T.; Kawabata, J. α-Glucosidase inhibitors from the bulb of Eleutherine americana. Food Chem. 2011, 128, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.X.; Huang, H.Z.; Wang, C.R. Study on effective constituents isolated from Eleutherine americana. Zhong Cao Yao 1981, 12, 84. [Google Scholar]
- Mahabusarakam, W.; Hemtasin, C.; Chakthong, S.; Voravuthikunchai, S.P.; Olawumi, I.B. Naphthoquinones, anthraquinones and naphthalene derivatives from the bulbs of Eleutherine americana. Planta Med. 2010, 76, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Han, A.R.; Min, H.Y.; Nam, J.W.; Lee, N.Y.; Wiryawan, A.; Suprapto, W.; Lee, S.K.; Lee, K.R.; Seo, E.K. Identification of a new naphthalene and its derivatives from the bulb of Eleutherine americana with inhibitory activity on lipopolysaccharide induced nitric oxide production. Chem. Pharm. Bull. 2008, 56, 1314–1316. [Google Scholar] [CrossRef] [PubMed]
- Alves, T.M.A.; Kloos, H.; Zani, C.L. Eleutherinone, a novel fungitoxicnaphthoquinone from Eleutherinebulbosa (Iridaceae). Mem. Inst. Oswaldo Cruz. 2003, 98, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, H.; Fukushima, T.; Ohashi, K.; Nakamura, A.; Riswan, S.; Kitagawa, I. Indonesian medicinal plants. XX. Chemical structures of Eleuthosides A, B, and C, three new aromatic glucosides from the bulbs of Eleutherine palmifolia (Iridaceae). Chem. Pharm. Bull. 1997, 45, 1130–1134. [Google Scholar] [CrossRef]
- Francesca, R.G.; Palazzino, G.; Federici, E.; Iurilli, R.; Galeffi, C.; Chifundera, K.; Nicoletti, M. Polyketides from Eleutherine bulbosa. Nat. Prod. Res. 2010, 24, 1578–1586. [Google Scholar]
- Chen, Z.X.; Huang, H.Z.; Wang, C.R.; Li, Y.H.; Ding, J.M.; Ushio, S.; Hiroshi, N.C.; Yoichi, I. Hongconin, a new naphthalene derivative from Hong-Cong, the rhizome of Eleutherine americana Merr. et Heyne. Chem. Pharm. Bull. 1986, 34, 2743–2746. [Google Scholar]
- Qiu, F.; Xu, J.Z.; Duan, W.J.; Qu, G.X.; Wang, N.L.; Yao, X.S. New constituents from Eleutherine americana. Chem. J. Chin. Univ. 2005, 26, 2057–2060. [Google Scholar]
- Xu, J.Z.; Qiu, F.; Qu, G.X.; Wang, N.L.; Yao, X.S. Studies on the antifungal constituents isolated from Eleutherine americana. Chin. J. Med. Chem. 2005, 15, 157–161. [Google Scholar]
- Hara, H.; Maruyama, N.; Yamashita, S.; Hayashi, Y.; Lee, K.H.; Bastow, K.F.; Marumoto, C.R.; Imakura, Y. Elecanacin, a novel new naphthoquinone from the bulb of Eleutherine americana. Chem. Pharm. Bull. 1997, 45, 1714–1716. [Google Scholar] [CrossRef]
- Le, M.H.; Do, T.T.H.; Phan, V.K.; Chau, V.M.; Nguyen, T.H.V.; Nguyen, X.N.; Bui, H.T.; Pham, Q.L.; Bui, K.A.; Seung, H.K.; et al. Chemical constituents of the rhizome of Eleutherine bulbosa and their inhibitory effect on the pro-Inflammatory cytokines production in lipopolysaccharide-Stimulated Bone marrow-derived dendritic cells. Bull. Korean Chem. Soc. 2013, 34, 633–636. [Google Scholar] [CrossRef]
- Mitscher, L.A.; Gollapudi, S.R.; Oburn, D.S.; Drake, S. Antimicrobial agents from higher plants: Two dimethylbenzisochromans from Karwinskia humboldtiana. Phytochemistry 1985, 24, 1681–1683. [Google Scholar] [CrossRef]
- Schmid, H.; Ebnöther, A. Über die Konfiguration der Eleutherine-Chinone (Inhaltsstoffeaus Eleutherinebulbosa (Mill.) Urb. V.). Helv. Chim. Acta 1951, 34, 1041–1049. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, L.; Liu, S.; Peng, Z.; Zhang, S. Total flavonoids from Plumulanelumbinis suppress angiotensin II-induced fractalkine product by inhibiting the ROS/NF-κB pathway in human umbilical vein endothelial cells. Exp. Ther. Med. 2014, 7, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Vásquez, N.; Paez, A.; Zapata, E.; Alcázar-Leyva, S.; Zenteno, E. HUVECs from newborns with a strong family history of diabetes show diminished ROS synthesis in the presence of high glucose concentrations. Diabetes Metab. Res. Rev. 2007, 23, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Long, H.P.; Zou, H.; Li, F.S.; Li, J.; Luo, P.; Zou, Z.X.; Hu, C.P.; Xu, K.P.; Tan, G.S. Involvenflavones A–F, six new flavonoids with 3’-aryl substituent from Selaginella involven. Fitoterapia 2015, 105, 254–259. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–8 are available from the authors. |
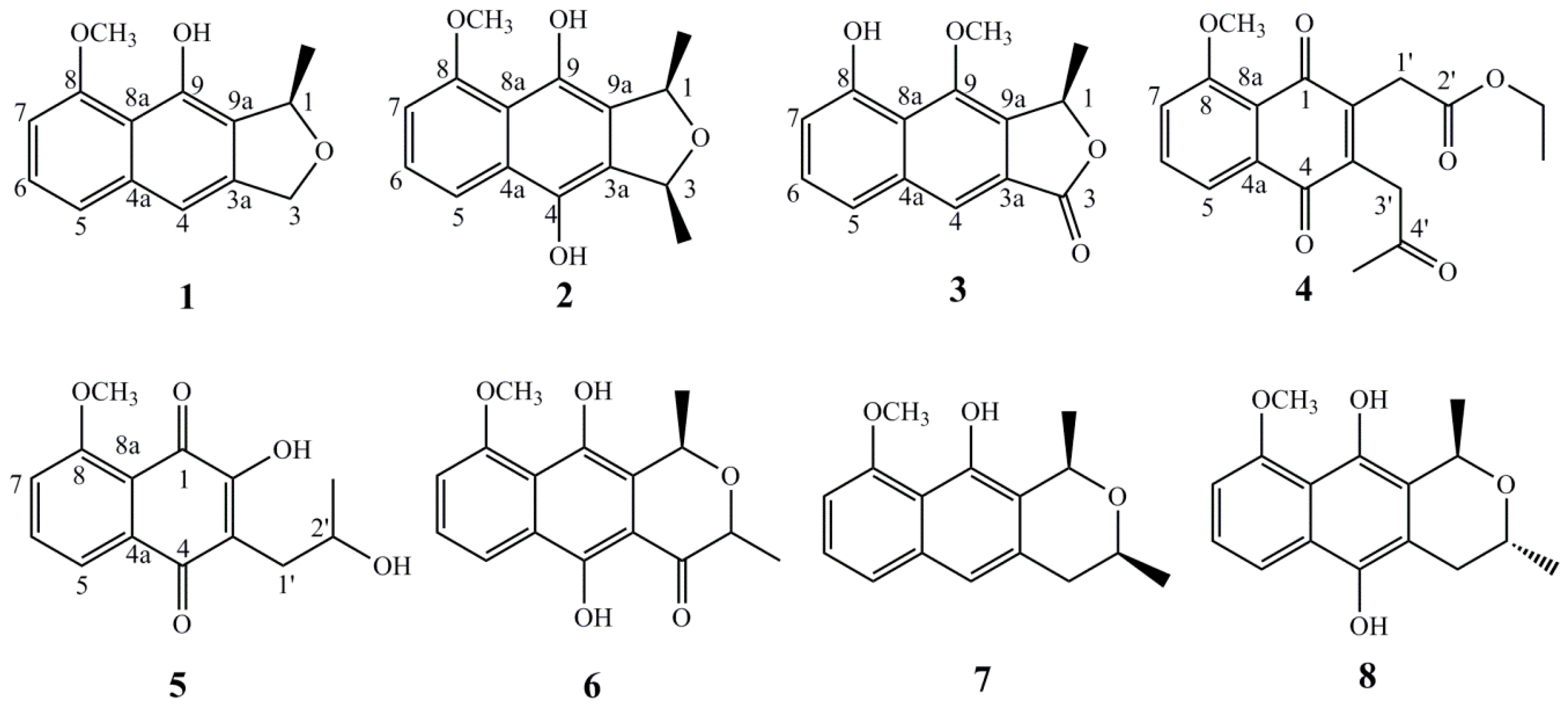
| No. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 79.5 | 5.53, q, 6.0 | 69.6 | 4.71, q, 6.6 | 78.1 | 5.74, q, 6.0 |
| 3 | 72.0 | 5.10, d, 12.6 5.23, d, 12.6 | 67.3 | 5.50, q, 6.6 | 170.6 | |
| 3a | 141.0 | 120.9 | 127.9 | |||
| 4 | 110.1 | 7.12, s | 154.4 | 116.6 | 7.91, s | |
| 4a | 137.5 | 126.0 | 137.2 | |||
| 5 | 122.0 | 7.36, d, 7.8 | 118.1 | 8.04, d, 8.4 | 123.7 | 7.60, d, 7.8 |
| 6 | 125.6 | 7.28, t, 7.8 | 125.3 | 7.39, t, 8.4 | 126.6 | 7.41, t, 7.8 |
| 7 | 103.7 | 6.75, d, 7.8 | 109.1 | 7.02, d, 8.4 | 106.3 | 6.94, d, 7.8 |
| 8 | 156.8 | 155.7 | 156.6 | |||
| 8a | 114.7 | 107.7 | 117.5 | |||
| 9 | 148.4 | 139.4 | 149.2 | |||
| 9a | 125.2 | 119.6 | 125.9 | |||
| CH3-1 | 20.6 | 1.61, d, 6.6 | 16.1 | 1.53, d, 6.6 | 19.2 | 1.74, d, 6.6 |
| CH3-3 | 17.4 | 1.64, d, 6.6 | ||||
| OCH3-8 | 56.3 | 4.06, s | 56.2 | 4.07, s | ||
| OCH3-9 | 56.4 | 4.12, s | ||||
| 4-OH | 8.99, s | |||||
| 8-OH | 9.67, s | |||||
| 9-OH | 9.44, s | 12.83, s | ||||
| No. | 4 | 5 | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 182.8 | 179.6 | ||
| 2 | 143.8 | 154.8 | ||
| 3 | 140.4 | 118.7 | ||
| 4 | 184.4 | 185.7 | ||
| 4a | 140.0 | 135.0 | ||
| 5 | 119.4 | 7.74, dd, 8.4, 1.2 | 119.6 | 7.80, d, 8.4 |
| 6 | 135.0 | 7.67, t, 8.4 | 136.2 | 7.72, t, 8.4 |
| 7 | 117.9 | 7.29, d, 8.4 | 117.0 | 7.27, d, 8.4 |
| 8 | 159.8 | 160.2 | ||
| 8a | 119.8 | 116.8 | ||
| 1′ | 33.2 | 3.62, s | 33.0 | 2.81, dd, 13.2, 3.6 2.76, dd, 13.2, 7.8 |
| 2′ | 169.5 | 67.6 | 4.12, m | |
| 3′ | 41.8 | 3.78, s | ||
| 4′ | 203.1 | |||
| 2′-OCH2CH3 | 61.4 | 4.14, m | ||
| 2′-OCH2CH3 | 14.1 | 1.26, t, 6.0 | ||
| 2′-CH3 | 23.6 | 1.26, d, 6.6 | ||
| 4′-CH3 | 30.1 | 2.30, s | ||
| 8-OCH3 | 56.5 | 4.00, s | 56.7 | 4.04, s |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.-L.; Hu, M.-G.; Liu, Y.-Y.; Li, R.-T.; Yu, M.; Xu, X.-D.; Ma, G.-X. New Naphthalene Derivatives from the Bulbs of Eleutherine americana with Their Protective Effect on the Injury of HUVECs. Molecules 2018, 23, 2111. https://doi.org/10.3390/molecules23092111
Chen D-L, Hu M-G, Liu Y-Y, Li R-T, Yu M, Xu X-D, Ma G-X. New Naphthalene Derivatives from the Bulbs of Eleutherine americana with Their Protective Effect on the Injury of HUVECs. Molecules. 2018; 23(9):2111. https://doi.org/10.3390/molecules23092111
Chicago/Turabian StyleChen, De-Li, Mei-Geng Hu, Yang-Yang Liu, Rong-Tao Li, Meng Yu, Xu-Dong Xu, and Guo-Xu Ma. 2018. "New Naphthalene Derivatives from the Bulbs of Eleutherine americana with Their Protective Effect on the Injury of HUVECs" Molecules 23, no. 9: 2111. https://doi.org/10.3390/molecules23092111
APA StyleChen, D.-L., Hu, M.-G., Liu, Y.-Y., Li, R.-T., Yu, M., Xu, X.-D., & Ma, G.-X. (2018). New Naphthalene Derivatives from the Bulbs of Eleutherine americana with Their Protective Effect on the Injury of HUVECs. Molecules, 23(9), 2111. https://doi.org/10.3390/molecules23092111