Abstract
A novel series of pyrazolyl 1,3,4-thiadiazines 5a–c, 8a–c, 12, 15a–c, 17a–c, and 20 was prepared from the reaction of pyrazole-1-carbothiohydrazide 1a,b with 2-oxo-N′-arylpropanehydrazonoyl chloride, 2-chloro-2-(2-arylhydrazono)acetate, and 3-bromoacetylcoumarin. Moreover, the regioselective reaction of 5-pyrazolone-1-carbothiohydrazide 1a with 4-substituted diazonium salts and 4-(dimethylamino)benzaldehyde gave the corresponding hydrazones 21a–c and 22. The newly prepared compounds were characterized by spectroscopy and elemental analysis. Many new synthesized compounds showed considerable antimicrobial activity against tested microorganisms. Hydrazones 21a–c and 22 showed remarkable antibacterial and antifungal activities. 4-(2-(p-tolyl)hydrazineylidene)-pyrazole-1-carbothiohydrazide 21a displayed the highest antibacterial and antifungal activities with minimum inhibitory concentration (MIC) values lower than standard drugs chloramphenicol and clotrimazole, in the range of 62.5–125 and 2.9–7.8 µg/mL, respectively.
1. Introduction
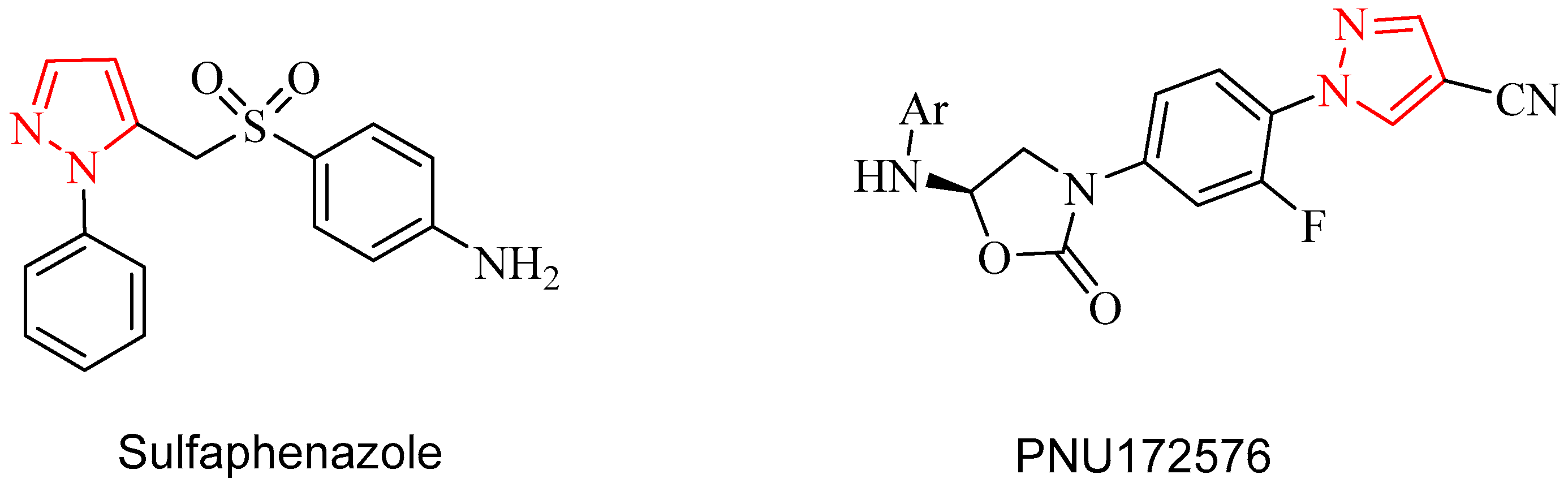
Recently, the incidence of microbial infections had been increased dramatically because of the misuse of antibiotics has caused the pathogens to become resistant to them and which has led to serious health hazards [1]. The rate of bacterial resistance to antibiotics is higher than the rate of development of new classes of antibiotics [2] so the design and synthesis of new compounds have potential antimicrobial activity are very important issue. Pyrazole derivatives have great attention due to their interesting biological and pharmaceutical activities such as antidepressant [3], antioxidant [4], anti-inflammatory [5], anticancer [6], antimicrobial [7,8,9], antiviral [10,11], anticonvulsant [12], and insecticidal activities [13]. In addition, the natural pyrazole C-glycoside, pyrazofurin (4-hydroxy-3β-d-dribofuranosyl-1H-pyrazole-5-carboxamide) has a broad spectrum of antimicrobial, antiviral, and antitumor activities [14]. It is well known that pyrazoles possess significant antibacterial activity. There are many antibiotic drugs containing pyrazole moiety such as Sulfaphenazole and PNU172576 (Figure 1).
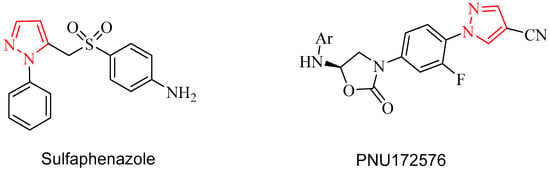
Figure 1.
Representative structures having pyrazole moieties as antimicrobial agents.
Heterocycles containing the thiadiazines moiety have biological and pharmaceutical importance [15,16,17,18]. Recently, Khidre et al. [19] reported that 1,3,4-thiadiazine derivatives have a good antimicrobial activity. Motivated by the preceding information and continuation of my research program on the synthesis of novel bioactive heterocycles [7,20,21,22,23] I designed and synthesized a novel series of pyrazole and pyrazolyl 1,3,4-thiadiazine derivatives, for antimicrobial evaluation, starting from pyrazole-1-carbothiohydrazide 1a,b.
2. Results
2.1. Chemistry
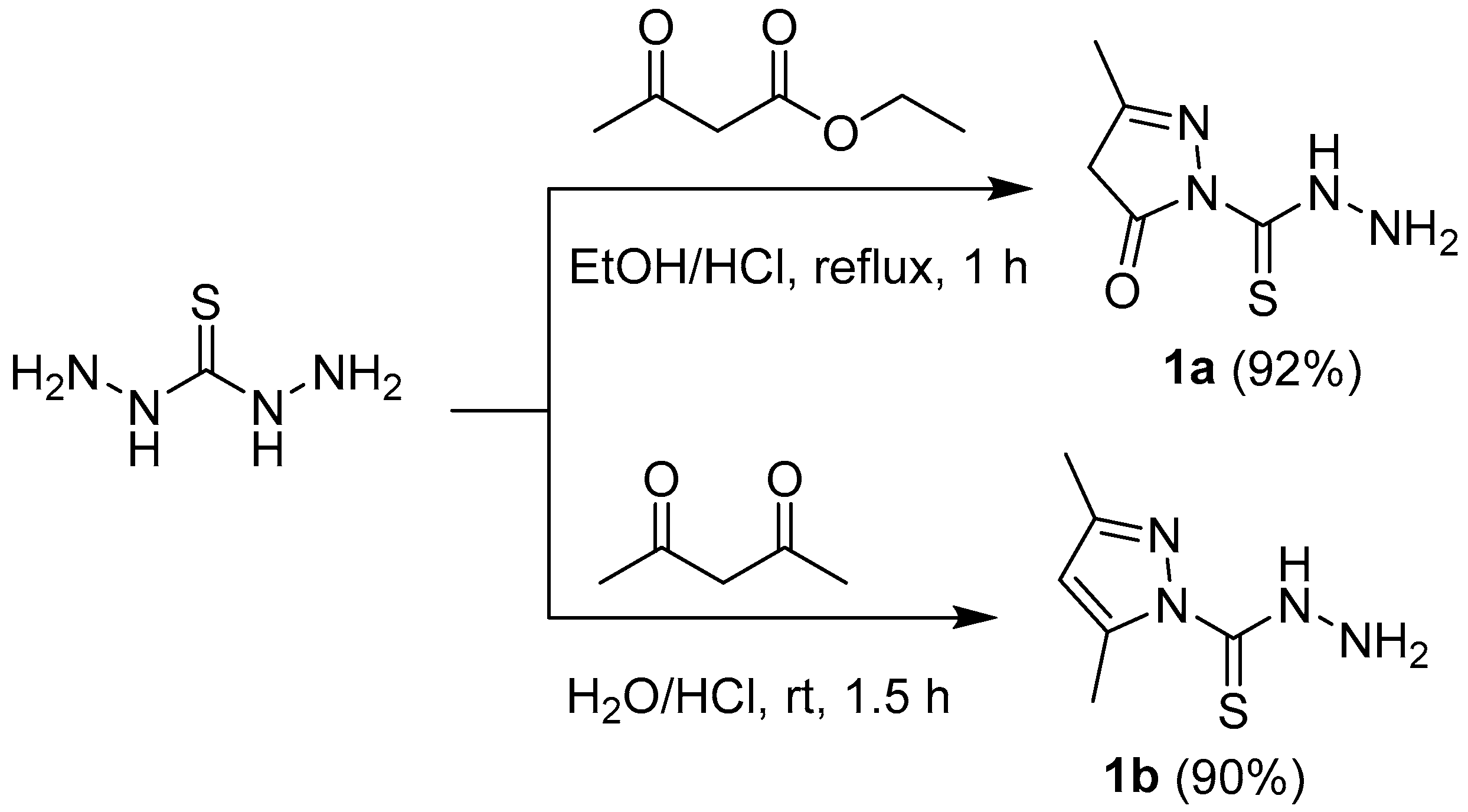
My strategy to synthesize a new heterocyclic compounds involved the use of pyrazole-1-carbothiohydrazide 1a,b that contains a number of chemically distinct functionalities, which can be reacted with different hydrazonyl chlorides, α-haloketones, diazonium salts, and aldehydes to a library of molecular diverse compounds (Scheme 1, Scheme 2, Scheme 3 and Scheme 4). As illustrated in Scheme 1, 3-methyl-5-oxo-4,5-dihydro-1H-pyrazole-1-carbothiohydrazide 1a was prepared in a very good yield by reaction of equimolar amount of ethyl acetoacetate with thiocarbohydrazide in ethanol containing a catalytic amount of HCl at reflux temperature. Treatment with a mixture of thiocarbohydrazide in (HCl, 0.05 M) with acetyl acetone at RT for 1.5 h afforded 3,5-dimethyl-1H-pyrazole-1-carbothiohydrazide 1b [24]. The molecular structure of compounds 1a was confirmed by elemental analyses and spectroscopic methods. The infrared spectrum of 1a showed a characteristic bands at 3292, 3250, 3182, and 1685 cm−1 due to NH, NH2, and C=O functions, respectively. 1H-NMR revealed two singlet peaks at 2.02 and 3.28 ppm due to the CH3 and CH2, respectively. Also, molecular weight determination (MS) of 1a showed the molecular ion peaks at m/z 172.
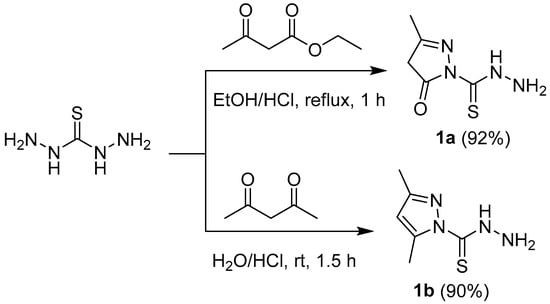
Scheme 1.
Synthesis of pyrazole-1-carbothiohydrazides 1a and 1b.
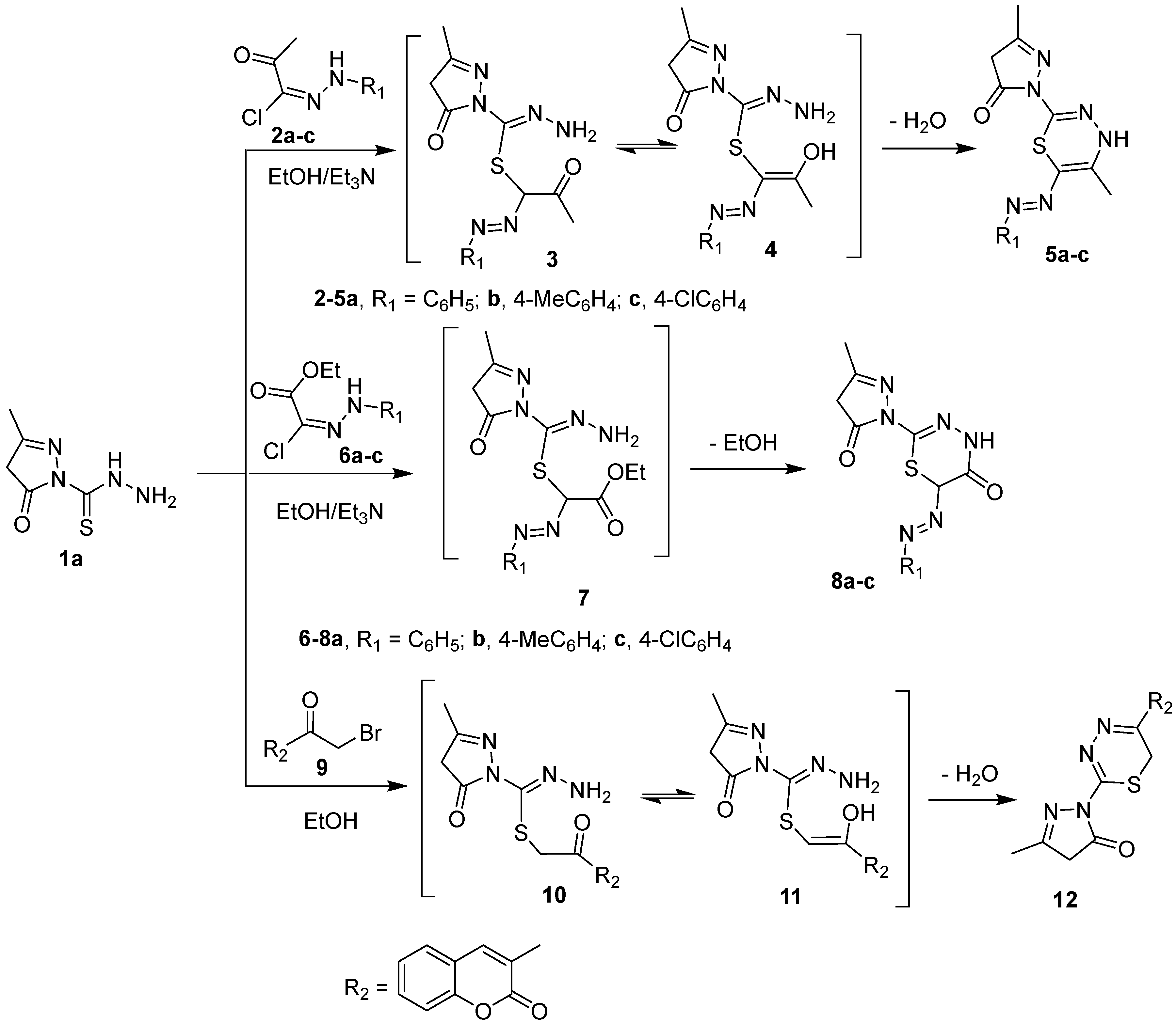
Scheme 2.
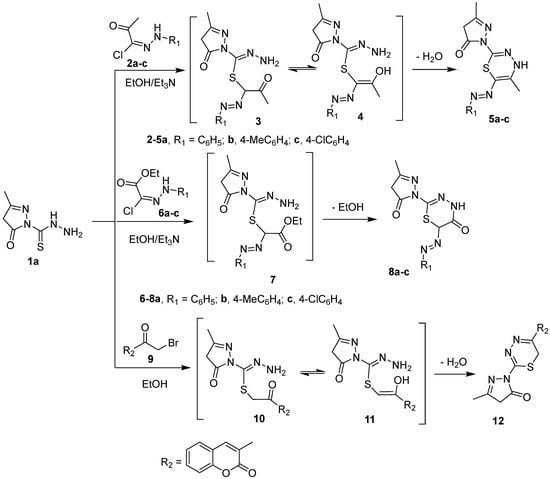
Synthesis of compounds 5a–c, 8a–c, and 12.
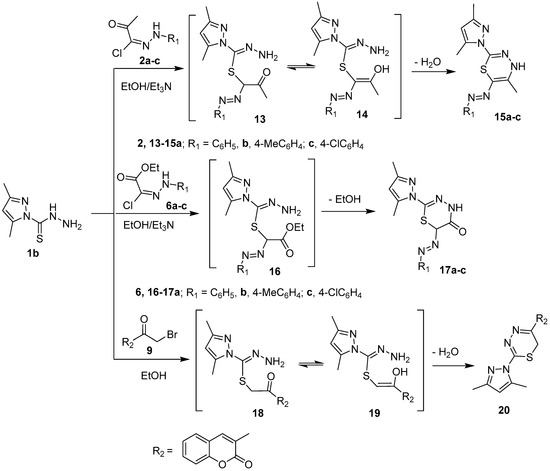
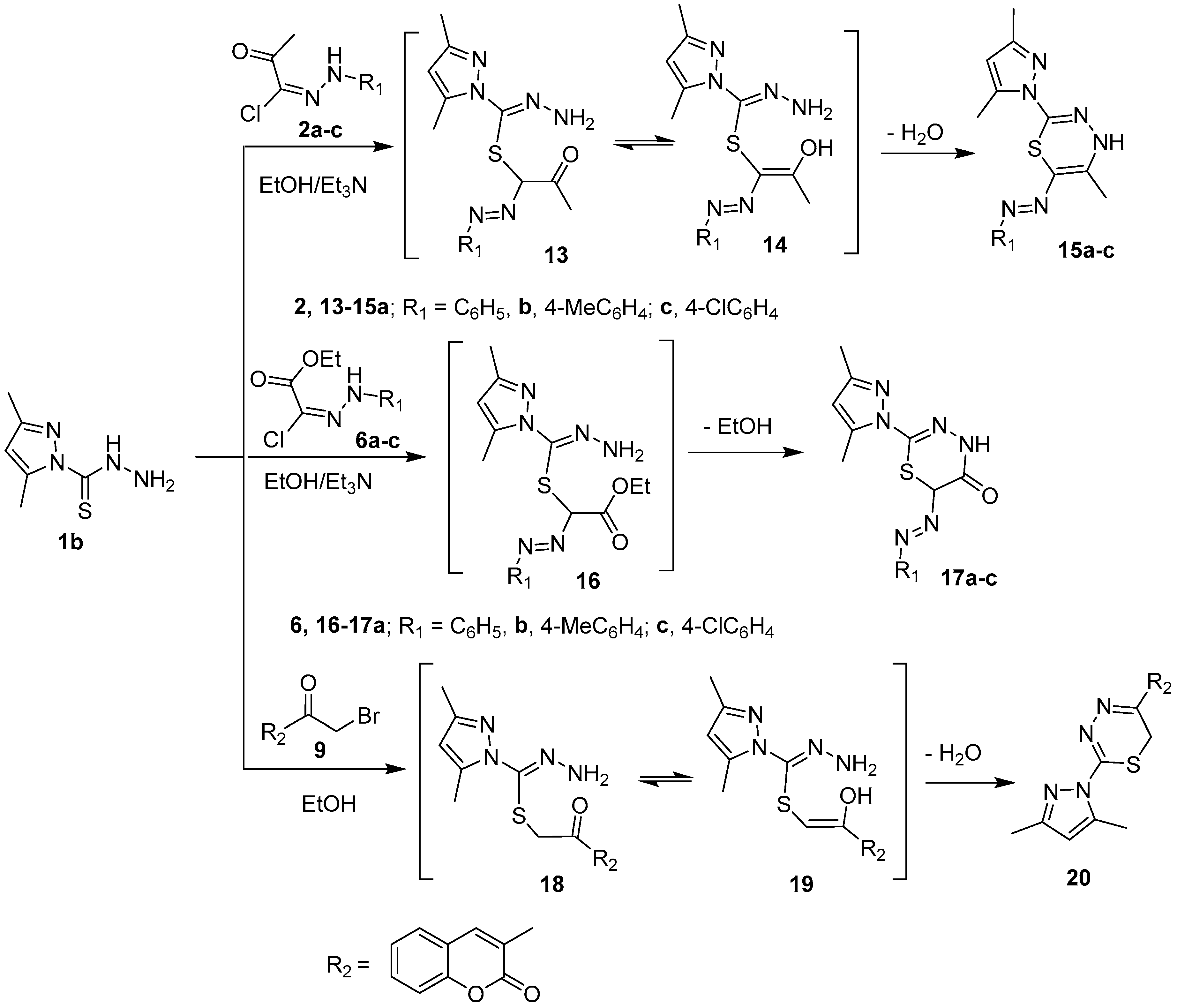
Scheme 3.
Synthesis of compounds 15a–c, 17a–c and 20.
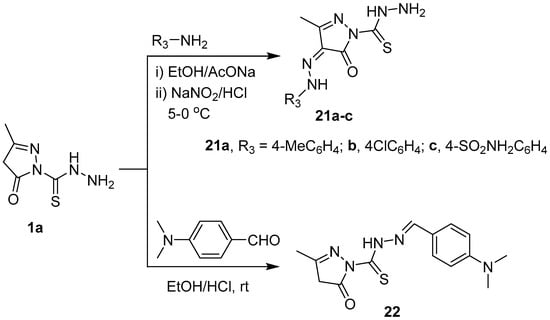
Scheme 4.
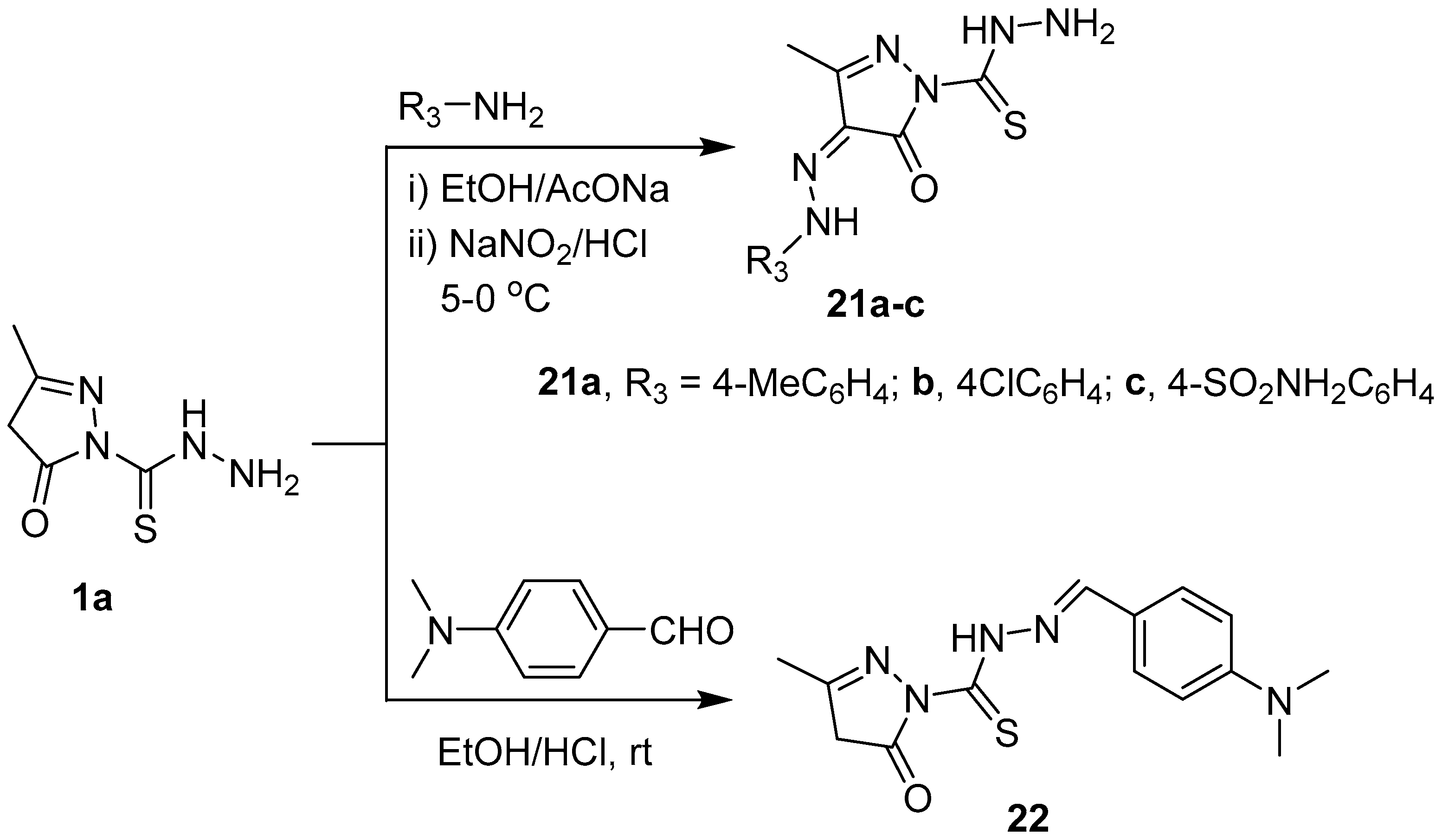
Synthesis of compounds 21a–c and 22.
The carbothiohydrazide moiety in 1a,b was reacted with selected electrophiles to prepare pyrazolyl 1,3,4-thiadiazine derivatives. Pyrazole-1 carbothiohydrazide 1a was reacted with 2-oxo-N′-arylpropanehydrazonoyl chlorides 2a–c in hot ethanol in the presence of Et3N to yield 5-methyl-2-(5-methyl-6-(aryldiazenyl)-4H-1,3,4-thiadiazin-2-yl)-2,4-dihydro-3H-pyrazol-3-ones 5a–c, in good yields, via intermediates 3 and 4 (Scheme 2). Similarly, 2-(3-methyl-5-oxo-4,5-dihydro-1H-pyrazol-1-yl)-6-(phenyldiazenyl)-4H-1,3,4-thiadiazin-5(6H)-ones 8a–c were synthesized, in high yields, from the reaction of 1a with ethyl 2-chloro-2-(2-arylhydrazono)acetate derivatives 6a–c under the same reaction conditions described for the preparation of 5a–c. 5-methyl-2-(5-(2-oxo-2H-chromen-3-yl)-4H-1,3,4-thiadiazin-2-yl)-2,4-dihydro-3H-pyrazol-3-one 12 is furnished in a good yield when 1a was refluxed with 3-bromoacetylcoumarin 9 in ethanol (Scheme 2).
The structure of the compounds 5, 8, and 12 was confirmed by elemental analyses and spectroscopic methods. The IR spectrum of thiadiazinyl pyrazolone 5c, as a representative example, revealed the lack of an NH2 absorption peak at 3292 and 3250 cm−1 and appearance of an absorption peak at 3149 cm−1 owing to the NH group. The 1H-NMR spectrum of 5c exhibited new signals at δ 1.41, 7.33, and 7.37 ppm assigned to methyl and aromatic protons, in addition, the D2O exchangeable signal at δ 11.57 ppm due to cyclic NH. Its 13C-NMR spectrum of 5c revealed the lack of C=S signal at 180 ppm and appearance 12 carbon signals. Moreover, the mass spectra of compounds 5a–c gave molecular ion peaks at m/z 314, 328, and 348, respectively. This clearly indicates the carbothiohydrazide moiety was involved in cyclization reaction with hydrazonyl chlorides 2a–c to give thiadiazine.
In a similar way, 6-(aryldiazenyl)-4H-1,3,4-thiadiazines 15a–c, 6-(aryldiazenyl)-4H-1,3,4-thiadiazin-5(6H)-ones 17a–c, and 5-aryl-4H-1,3,4-thiadiazines 20 were synthesized in very good yields from the reaction of pyrazole-1-carbothiohydrazide 1b with hydrazonyl cholrides 2a–c, 6a–c, and α-haloketone 9, respectively, under similar reaction condition as described before (Scheme 3). Compound 20 was previously synthesized from a one pot reaction of 3-(2-bromoacetyl)-2H-chromen-2-ones, thiocarbohydrazide, and pentane-2,4-dione [25]. The IR spectrum of 17b revealed the lack of NH2 band present in the IR spectra of starting pyrazole 1b and the appearance of new absorption bands at 3176 and 1680 cm−1 corresponding to NH and CO functional groups, respectively. Likewise, the 1H-NMR spectra showed a new singlet signal at δ 3.11 ppm due to H-6 of thiadiazine, two doublet signals at δ 7.13, 7.25 ppm integrated for four protons of 4-disubstitued benzene ring, and D2O-exchangeable signals at 11.23 ppm due to NH. Its 13C-NMR spectrum did not exhibit the C=S signal at 180 ppm which observed in the starting material, but instead displayed 13 carbon signals. The mass spectra of 17a–c showed molecular ion peaks at m/z 314, 328, and 348, respectively, which were in an accord with the calculated masses (c.f. experimental section).
The coupling reaction of 1a with 4-substituted arenediazonium chloride was performed in ethanol containing sodium acetate at 0–5 °C to give the corresponding hydrazones 21a–c. Also, the reaction of 1a with 4-(dimethylamino)benzaldehyde in ethanol containing few drops of HCl gave N′-(4-(dimethylamino)benzylidene)-3-methyl-5-oxo-4,5-dihydro-1H-pyrazole-1-carbothiohydrazide 22 (Scheme 4). The 1H-NMR spectra of 21a–c showed lack the singlet signal due to CH2 (C-4 of pyrazolone) and showed an aromatic multiplets in the region 7.22–7.84 ppm. In addition, new D2O-exchangeable signals appeared in the region 11.74–12.24 ppm. These data support the successful coupling C-4 of pyrazolone with 4-substituted arenediazonium chloride. The IR spectrum of hydrazone 22 did not show an NH2 band. The 1H-NMR spectrum of 22 showed a new singlet signal at 9.65 ppm due to the azamethine proton (N=CH) and an aromatic multiplets at 6.80 and 7.91 ppm. Also, the mass spectra of 21a–c and 22 are in an agreement with the calculated masses.
2.2. Antimicrobial Activity
In vitro antimicrobial screening of the newly synthesized compounds was carried out by the agar diffusion method using cultures of two fungal strains (Candida albicans (ATCC 10231) and Aspergillus niger (ATCC 16404), as well as four bacteria strains, two Gram positive bacteria (Staphylococcus aureus (ATCC 29213), Bacillus subtilus (ATCC 6051), and two Gram negative bacteria (Klebsiella pneumoniae (ATCC 700603) and Escherichia coli (ATCC 25922). The standard antibiotic Chloramphenicol and Antifungal Clotrimazole was used as controls to evaluate the potency of the compounds being studied under the same conditions.
As shown in Table 1 compounds 5b, 8a, 12, and 17a were found to be inactive against all microorganisms while compounds 5a, 5c, 8c, 15a, 15b, 15c, 17b, 17c, and 20 exhibited low activity against some microorganisms only and inactive against others. Compounds 8b showed good activities against fungi and Gram positive bacteria. Compound 22 displayed good activities against all microorganisms except Candida albicans did not show any activity. Compounds 21a–c displayed a broad spectrum activity against all microorganisms. Compound 21c showed the highest activity against Candida albicans with inhibition zones of 25 mm while compound 21a showed the highest activity against other strains, e.g., Aspergillus niger, Staphylococcus aureus, Bacillus subtilus, Klebsiella pneumoniae, and Escherichia coli with inhibition zones 35, 22, 30, 20, and 27 mm, respectively. The variation in the effectiveness of different compounds against microorganism depends on either the impermeability of the cells of the microbes or on differences in the ribosomes of microbial cells [26]. It may be concluded that the antimicrobial activity of the compounds is related to the cell wall structure of the bacterium as well as the structure of the pyrazole derivatives itself. It is possible because the cell wall is essential to the survival of bacteria and some antibiotics are able to kill bacteria by inhibiting a step in the synthesis of peptidoglycan. Gram-positive bacteria possess a thick cell wall containing many layers of peptidoglycan and teichoic acids, but in contrast, Gram negative bacteria have a relatively thin cell wall consisting of a few layers of peptidoglycan surround by a second lipid membrane containing lipopolysaccharides and lipoproteins. These differences in cell wall structure can produce differences in antibacterial susceptibility and some antibiotics can kill only Gram-positive bacteria and are ineffective against Gram-negative pathogens [27].

Table 1.
In vitro antimicrobial activity of the synthesized compounds a,b.
On the other hand, it is obvious that compounds 21a > 21b > 21c exhibited potent inhibition activity owing to its characteristic skeleton that containing free carbothiohydrazide moiety that confer its softness, six donating N atoms, and planar 4-substituted phenyl group compared with the other pyrazolyl 1,3,4-thiadiazine derivatives.
The compounds which showed greater antibacterial and antifungal activities were further assayed for minimum inhibitory concentration (MIC), and the values are listed in Table 2. MIC is the lowest concentration of an antimicrobial that will inhibit the visible growth of a microorganism. Compounds 21a displayed low MIC value on Aspergillus niger, Staphylococcus aureus, B. subtilis, and Klebsiella pneumoniae than standard drug Clotrimazole and Chloramphenicol and showed MIC value on Candida albicans and Escherichia coli equal to standard drugs. The MIC value of compound 21b against Aspergillus niger, B. subtilis, and Klebsiella pneumoniae was equal to standard drugs Clotrimazole and Chloramphenicol. Moreover, Compound 21c showed a MIC value on Aspergillus niger Staphylococcus aureus, B. subtilis, Klebsiella pneumonia, and Escherichia coli equal to the standard drugs. The structure–activity relationship revealed that compounds with pyrazole-1-carbothiohydrazide unit 21a, 21b, 21c, and 22 showed higher activity than compounds have pyrazolyl thiadiazine unit. Further, the presence of free carbothiohydrazide moiety increases the activity of 21a–c and the presence of electron donating substituents at the aromatic ring increased the activity of 21a.

Table 2.
Minimum inhibitory concentration (MIC) in (µg/mL) for compounds 8b, 21a–c, and 22.
3. Experimental Section
3.1. General Information
All melting points were determined on a digital Gallen-Kamp MFB-595 instrument (Gallenkamp, London, UK) using open capillary tubes and are uncorrected. IR spectra were recorded on a Schimadzu FTIR 440 spectrometer (Shimadzu, Tokyo, Japan) using KBr pellets. Mass spectra were performed at 70 eV on an MS-50 Kratos (A.E.I.) spectrometer (Shimadzu, Tokyo, Japan) provided with a data system. 1H-NMR and 13C-NMR spectra were recorded on a Bruker model (500 MHz) Ultra Shield NMR spectrometer (Bruker, Coventry, UK) in CDCl3 or DMSO-d6 using tetramethylsilane (TMS) as an internal standard; chemical shifts are reported as δ ppm units. Solvents were dried by standard techniques. The monitoring of the progress of all reactions and homogeneity of the synthesized compounds was carried out and was run using thin layer chromatography (TLC) aluminum sheets silica gel 60 F254 (Merck, Darmstadt, Germany). Compound 1b was prepared previously by Alekseev et al. [24].
3.2. Synthesis
3-Methyl-5-oxo-4,5-dihydro-1H-pyrazole-1-carbothiohydrazide (1a). Thiocarbohydrazide (10.6 g, 0.1 mol) was dissolved in a mixture of ethanol (20 mL) and HCl (1 mL) and ethyl acetoacetate was added (13 mL, 0.1 mol). The mixture was refluxed for 1 h. After cooling, the white precipitate was filtered off, washed with ethanol, and dried under reduced pressure. White crystals, yield (92%), m.p. 135–136 °C. IR (KBr) ν (cm−1): 3292, 3250, 3182 (NH2 & NH), 1685 (C=O), 1647 (C=N), 1H-NMR (500 MHz, CDCl3) δ (ppm): 2.02 (s, 3H, CH3), 3.28 (s, 2H, pyrazole-H4), 8.7 (d, D2O exchangeable, 2H, NH2), 10.18 (s, D2O exchangeable, 1H, NH); 13C-NMR (125 MHz, CDCl3) δC (ppm): 15.5 (CH3), 43.0 (CH2, pyrazole-C4), 160.5 (pyrazole-C3), 166 (C=O), 179 (C=S); MS m/z (%): 172 [M]+ (11%), 130 (100); Anal. Calcd. for C5H8N4OS (172.21): C, 34.87; H, 4.68; N, 32.54, Found: C, 34.57; H, 4.50; N, 32.41%.
3.2.1. General procedure for synthesis compounds 5, 8, 12, 15, 17, and 20
Equimolar amounts of 1a or 1b (1 mmol) and 2-oxo-N-arylpropanehydrazonoyl chloride 2a–c; ethyl 2-chloro-2-(2-arylhydrazineylidene)acetate 6a–c or 3-(2-bromoacetyl)-2H-chromen-2-one 8 (1 mmol) in absolute ethanol (30 mL) (few drops of triethylamine was added in case of 2a–c and 6a–c) was heated under reflux for 3–6 h (TLC), then left to cool. The solid was isolated by filtration, washed with ethanol, dried, and recrystallized from (EtOH).
5-Methyl-2-(5-methyl-6-(phenyldiazenyl)-4H-1,3,4-thiadiazin-2-yl)-2,4-dihydro-3H-pyrazol-3-one (5a). Red crystals, yield (85%), m.p. 193–194 °C (EtOH); IR (νmax, cm−1): 3242 (NH), 1690 (C=O), 1600 (C=N), 1600–1440 (C=C); 1H-NMR (500 MHz, CDCl3) δH (ppm): 1.25 (s, 3H, CH3), 1.44 (s, 3H, CH3), 3.10 (s, 2H, pyrazole-H4), 7.33 (t, 2H, Ar-H), 7.44 (t, 2H, Ar-H), 8.04 (d, 1H, J = 8.5 Hz, Ar-H), 11.52 (s, D2O exchangeable, 1H, NH); 13C-NMR (125 MHz, CDCl3) δC (ppm): 12.2 (CH3), 16.0 (CH3), 44.0 (CH2, pyrazole-C4), 95.4, 121.7, 129.7, 148.2, 151.8, 154.6, 160.5, 166.3 (C=O); MS m/z (%): 314 [M]+ (21%), 245(100); Anal. Calcd. for C14H14N6OS (314.37): C, 53.49; H, 4.49; N, 26.73, Found: C, 53.69; H, 4.19; N, 26.67%.
5-Methyl-2-(5-methyl-6-(p-tolyldiazenyl)-4H-1,3,4-thiadiazin-2-yl)-2,4-dihydro-3H-pyrazol-3-one (5b). Brown powder, yield (86%), m.p. 219–220 °C (EtOH); IR (νmax, cm−1):, 3244 (NH), 1687 (C=O), 1591 (C=N), 1558–1440 (C=C); 1H-NMR (500 MHz, CDCl3) δH (ppm): 1.27 (s, 3H, CH3), 1.48 (s, 3H, CH3), 1.54 (s, 3H, CH3), 3.11 (s, 2H, pyrazole-H4), 7.37 (dd, 2H, J = 8.5, 2.5 Hz, Ar-H), 7.82 (dd, 2H, J = 7.6, 2.5 Hz, Ar-H), 11.54 (s, D2O exchangeable, 1H, NH); 13C-NMR (125 MHz, CDCl3) δC (ppm): 12.9 (CH3), 16.5 (CH3), 21.5 (CH3), 42.9 (CH2, pyrazole-C4), 94.9, 128.7, 129.9, 138.6, 146.1, 152.4, 155.4, 160.7, 165.7 (C=O); MS m/z (%): 328 [M]+ (31%), 245 (100); Anal. Calcd. for C15H16N6OS (328.39): C, 54.86; H, 4.91; N, 25.59, Found: C, 55.01; H, 4.67; N, 25.51%.
2-(6-((4-Chlorophenyl)diazenyl)-5-methyl-4H-1,3,4-thiadiazin-2-yl)-5-methyl-2,4-dihydro-3H-pyrazol-3-one (5c). Brown powder, yield (87%), m.p. 230–231 °C (EtOH); IR (νmax, cm−1): 3149 (NH), 1692 (C=O), 1654 (C=N), 1593–1462 (C=C); 1H-NMR (500 MHz, CDCl3) δH (ppm): 1.22 (s, 3H, CH3), 1.41 (s, 3H, CH3), 2.53 (s, 2H, pyrazole-H4), 7.33 (dd, 2H, J = 9, 2.5 Hz, Ar-H), 7.37 (dd, 2H, J = 7, 2.5 Hz, Ar-H), 11.57 (s, D2O exchangeable, 1H, NH); 13C-NMR (125 MHz, CDCl3) δC (ppm): 12.2 (CH3), 15.8 (CH3), 43.4 (CH2, pyrazole-C4), 96.0, 129.7, 131.7, 134.8, 147.0, 151.6, 154.0, 161.4, 165.6 (C=O); MS m/z (%): 348 [M]+ (35%), 350 [M + 2]+ (10), 245(100); Anal. Calcd. for C14H13ClN6OS (348.81): C, 48.21; H, 3.76; N, 24.09, Found: C, 48.62; H, 3.46; N, 24.14%.
2-(3-Methyl-5-oxo-4,5-dihydro-1H-pyrazol-1-yl)-6-(phenyldiazenyl)-4H-1,3,4-thiadiazin-5(6H)-one (8a). Yellow crystals, yield (87%), m.p. 220–221 °C (EtOH); IR (νmax, cm−1): 3180 (NH), 1695 (C=O), 1681 (C=O), 1633 (C=N), 1598–1496 (C=C); 1H-NMR (500 MHz, CDCl3) δH (ppm): 2.14 (s, 3H, CH3), 2.27 (s, 2H, pyrazole-H4), 3.52 (s, 1H, thiadiazine-H6), 7.31 (t, 2H, Ar-H), 7.45 (t, 2H, Ar-H), 7.64 (d, 1H, J = 8.5 Hz, Ar-H), 10.6 (s, D2O exchangeable, 1H, NH); 13C-NMR (125 MHz, CDCl3) δC (ppm): 14.9 (CH3), 42, 81.5, 121.4, 126.5, 129.5, 151.4, 152.9, 159.7, 165 (C=O), 169 (C=O); MS m/z (%): 316 [M]+ (20%), 350 (8), 98 (100); Anal. Calcd. for C13H12N6O2S (316.34): C, 49.36; H, 3.82; N, 26.57, Found: C, 49.06; H, 3.79; N, 26.35%.
2-(3-Methyl-5-oxo-4,5-dihydro-1H-pyrazol-1-yl)-6-(p-tolyldiazenyl)-4H-1,3,4-thiadiazin-5(6H)-one (8b). Yellow crystals, yield (84%), m.p. 238–239 °C (EtOH); IR (νmax, cm−1): 3176 (NH), 1699 (C=O), 1695 (C=O), 1621 (C=N), 1593–1506 (C=C); 1H-NMR (500 MHz, CDCl3) δH (ppm): 2.16 (s, 3H, CH3), 2.22 (s, 2H, pyrazole-H4), 3.42 (s, 1H, thiadiazine-H6), 3.45 (s, 3H, CH3), 7.2 (d, 2H, J = 8 Hz, Ar-H), 7.3 (dd, 2H, J = 6.5, 2 Hz, Ar-H), 10.9 (s, D2O exchangeable, 1H, NH); 13C-NMR (125 MHz, CDCl3) δC (ppm): 14.5 (CH3), 21 (CH3), 42 (CH2), 79.5, 123.4, 129.5, 138.4, 148, 151.4, 159.7, 165 (C=O), 169 (C=O); MS m/z (%): 330 [M]+ (25%), 350 (8), 98 (100); Anal. Calcd. for C14H14N6O2S (330.37): C, 50.90; H, 4.27; N, 25.44, Found: C, 50.43; H, 4.15; N, 25.27%.
6-((4-Chlorophenyl)diazenyl)-2-(3-methyl-5-oxo-4,5-dihydro-1H-pyrazol-1-yl)-4H-1,3,4-thiadiazin-5(6H)-one (8c). Yellow powder, yield (81%), m.p. 260–261 °C (EtOH); IR (νmax, cm−1): 3184 (NH), 1693, 1689 (C=O), 1625 (C=N), 1583–1529 (C=C); 1H-NMR (500 MHz, CDMSO-d6) δH (ppm): 2.08 (s, 3H, CH3), 2.13 (s, 2H, pyrazole-H4), 3.74 (s, 1H, thiadiazinone-H6), 7.24 (dd, 2H, J = 7, 2 Hz, Ar-H), 7.33 (dd, 2H, J = 9, 2 Hz, Ar-H), 10.58 (s, D2O exchangeable, 1H, NH); 13C-NMR (125 MHz, CDCl3) δC (ppm): 14.7 (CH3), 42 (CH2), 78, 123.9, 129.5, 134.4, 148, 153, 159.5, 164 (C=O), 168 (C=O); MS m/z (%): 349.87 [M]+ (2%), 293.77 (100); Anal. Calcd. for C13H11ClN6O2S (350.78): C, 44.51; H, 3.16; N, 23.96, Found: C, 44.19; H, 3.02; N, 23.62%.
5-Methyl-2-(5-(2-oxo-2H-chromen-3-yl)-6H-1,3,4-thiadiazin-2-yl)-2,4-dihydro-3H-pyrazol-3-one (12). Brown powder, yield (87%), m.p. 220–221 °C (EtOH); IR (νmax, cm−1): 1692, 1681 (2C=O), 1606 (C=N), 1558–1452 (C=C); 1H-NMR (500 MHz, CDCl3) δH (ppm): 2.17 (s, 3H, CH3), 3.42 (s, 2H, pyrazole-H4), 4.97 (s, 2H, thiadiazine-H6), 7.45 (dd, 1H, J = 7.5, 3.5Hz, coumarin-H8), 7.66 (dd, 1H, J = 6, 1.5 Hz, coumarin-H6), 7.81 (dd, 1H, J = 7.5, 1.5 Hz, coumarin-H7), 8.1 (dd, 1H, J = 7.8, 1, coumarin-H5), 8.94 (s, 1H, coumarin-H4); 13C-NMR (125 MHz, CDCl3) δC (ppm): 13.7 (CH3), 41.5 (CH2), 43.4 (CH2), K 116.4, 122.7, 128.6, 130.8, 135.2, 141.9, 144.5, 148.7, 152.7, 154.7, 158.5, 163.6, 190.3; MS m/z (%): 340 [M]+ (13%), 105 (100); Anal. Calcd. for C16H12N4O3S (340.36): C, 56.46; H, 3.55; N, 16.46, Found: C, 56.19; H, 3.29; N, 16.28.
2-(3,5-Dimethyl-1H-pyrazol-1-yl)-5-methyl-6-(phenyldiazenyl)-4H-1,3,4-thiadiazine (15a). Brown powder, yield (85%), m.p. 180–181 °C (EtOH); IR (νmax, cm−1): 3151 (NH), 1657 (C=N), 1598–1489 (C=C); 1H-NMR (500 MHz, CDCl3) δH (ppm): 2.25 (s, 3H, CH3), 2.29 (s, 3H, CH3), 2.57 (s, 3H, CH3), 6.05 (s, 1H, H4 pyrazole), 7.27 (t, 2H, Ar-H), 7.42 (t, 2H, Ar-H), 8.04 (d, 1H, J = 8.5 Hz, Ar-H), 11.54 (s, D2O exchangeable, 1H, NH); 13C-NMR (125 MHz, CDCl3) δC (ppm): 8.85 (CH3), 14.3 (CH3), 14.9 (CH3), 110, 117.5, 128, 129, 142.0, 148.5, 150.2, 155.0, 160, 189.5; MS m/z (%): 312 [M]+ (10%), 239.9 (100); Anal. Calcd. for C15H16N6S (312.40): C, 57.67; H, 5.16; N, 26.90, Found: C, 57.39; H, 5.04; N, 26.55%.
2-(3,5-Dimethyl-1H-pyrazol-1-yl)-5-methyl-6-(p-tolyldiazenyl)-4H-1,3,4-thiadiazine (15b). Brown powder, yield (86%), m.p. 205–206 °C (EtOH); IR (νmax, cm−1): 3156 (NH), 1683 (C=N), 1591–1456 (C=C); 1H-NMR (500 MHz, CDCl3) δH (ppm): 2.26 (s, 3H, CH3), 2.32 (s, 3H, CH3), 2.51 (s, 3H, CH3), 2.57 (s, 3H, CH3), 6.04 (s, 1H, pyrazole-H4), 7.32 (dd, 2H, J = 6 Hz, Ar-H), 7.86 (dd, 2H, J = 8.5 Hz, Ar-H), 11.52 (s, D2O exchangeable, 1H, NH); 13C-NMR (125 MHz, CDCl3) δC (ppm): 8.54 (CH3), 13.2 (CH3), 18.6 (CH3), 18.6 (CH3), 103.3, 114.9, 121.0, 129.1, 129.3, 129.6, 148.9, 150.6, 159.2, 189.5; MS m/z (%): 326 [M]+ (30%), 299 (100); Anal. Calcd. for C16H18N6S (326.42): C, 58.87; H, 5.56; N, 25.75, Found: C, 58.49; H, 5.27; N, 25.41%.
6-((4-Chlorophenyl)diazenyl)-2-(3,5-dimethyl-1H-pyrazol-1-yl)-5-methyl-4H-1,3,4-thiadiazine (15c). Brown crystals, yield (84%), m.p. 187–188 °C (EtOH); IR (νmax, cm−1): 3153 (NH), 1654 (C=N), 1595–1487 (C=C); 1H-NMR (500 MHz, CDCl3) δH (ppm): 2.25 (s, 3H, CH3), 2.29 (s, 3H, CH3), 2.57 (s, 3H, CH3), 6.05 (s, 1H, pyrazole-H4), 7.33 (dd, 2H, J = 9, Ar-H), 7.98 (dd, 2H, J = 9, Ar-H), 11.56 (s, D2O exchangeable, 1H, NH); 13C-NMR (125 MHz, CDCl3) δC (ppm): 8.58 (CH3), 13.3 (CH3), 14.4 (CH3), 111, 116.4, 126.8, 128.9, 141.0, 149.6, 151.2, 154.0, 159.2, 189.5; MS m/z (%): 348.97 [M + 3]+ (20%), 345.94 [M]+ (10%), 317 (100); Anal. Calcd. for C15H15ClN6S (346.84): C, 51.95; H, 4.36; N, 24.23, Found: C, 51.60; H, 4.15; N, 24.07%.
2-(3,5-dimethyl-1H-pyrazol-1-yl)-6-(phenyldiazenyl)-4H-1,3,4-thiadiazin-5(6H)-one (17a). Green powder, yield (85%), m.p. 126–127 °C (EtOH); IR (νmax, cm−1): 3178 (NH), 1681 (C=O), 1602 (C=N), 1575–1473 (C=C); 1H-NMR (500 MHz, CDCl3) δH (ppm): 2.12 (s, 3H, CH3), 2.24 (s, 3H, CH3), 3.2 (s, 1H, H6 thiadiazinone), 6 (s, 1H, pyrazole-H4), 7.33 (t, 2H, Ar-H), 7.44 (t, 2H, Ar-H), 7.65 (d, 1H, J = 8.5 Hz, Ar-H), 11 (s, D2O exchangeable, 1H, NH); 13C-NMR (125 MHz, CDCl3) δC (ppm): 12.7 (CH3), 14.9 (CH3), 85.2, 114, 121.4, 129.5, 135.4, 142.9, 145.7, 148.6, 152.0, 169.1; MS m/z (%):314 [M]+ (45), 350 (8), 86 (100); Anal. Calcd. for C14H14N6OS (314.37): C, 53.49; H, 4.49; N, 26.73, Found: C, 53.17; H, 4.19; N, 26.43%.
2-(3,5-Dimethyl-1H-pyrazol-1-yl)-6-(p-tolyldiazenyl)-4H-1,3,4-thiadiazin-5(6H)-one (17b). Green powder, yield (80%), m.p. 164–165 °C (EtOH); IR (νmax, cm−1): 3176 (NH), 1680 (C=O), 1612 (C=N), 1599–1465 (C=C); 1H-NMR (500 MHz, CDCl3) δH (ppm): 2.13 (s, 3H, CH3), 2.23 (s, 3H, CH3), 2.46 (s, 3H, CH3), 3.11 (s, 1H, thiadiazine-H6), 6.04 (s, 1H, pyrazole-H4), 7.13 (d, 2H, J = 8 Hz, Ar-H), 7.25 (dd, 2H, J = 6.5, 2 Hz, Ar-H), 11.23 (s, D2O exchangeable, 1H, NH); 13C-NMR (125 MHz, CDCl3) δC (ppm): 12.4 (CH3), 14.8 (CH3), 21.7 (CH3), 86.0, 113, 125.7, 130.7, 134.8, 144.0, 147.6, 149, 154.0, 169.6; MS m/z (%): 328 [M]+ (3%), 321(20), 148.8 (90), 85.9 (100); Anal. Calcd. for C15H16N6OS (328.39): C, 54.86; H, 4.91; N, 25.59, Found: C, 54.39; H, 4.69; N, 25.41%.
6-((4-Chlorophenyl)diazenyl)-2-(3,5-dimethyl-1H-pyrazol-1-yl)-4H-1,3,4-thiadiazin-5(6H)-one (17c). Brown powder, yield 83%, m.p. 140–141 °C (EtOH); IR (νmax, cm−1): 3169 (NH), 1681 (C=O), 1614 (C=N), 1599–1541 (C=C); 1H-NMR (500 MHz, CDCl3) δH (ppm): 2.12 (s, 3H, CH3), 2.24 (s, 3H, CH3), 3.2 (s, 1H, H6 thiadiazinone), 6.05 (s, 1H, H4 pyrazole), 7.17 (d, 2H, J = 8 Hz, Ar-H), 7.30 (dd, 2H, J = 6.5, 2 Hz, Ar-H), 11.17 (s, D2O exchangeable, 1H, NH); 13C-NMR (125 MHz, CDCl3) δC (ppm): 12.4 (CH3), 14.8 (CH3), 81.0, 112, 124.2, 131.5, 135.6, 143.0, 146.7, 148.9, 153.0, 168.6; MS m/z (%): 348 [M]+ (25%), 350 (8), 86 (100); Anal. Calcd. for C14H13ClN6OS (348.81): C, 48.21; H, 3.76; N, 24.09, Found: C, 47.99; H, 3.62; N, 23.89%.
3.2.2. General procedure for synthesis 21a–c
To a stirred solution of compound 1a (0.516 g, 3 mmol) in ethanol (30 mL) sodium acetate trihydrate (0.39 g, 3 mmol) was added. After stirring for 15 min, the mixture was chilled at 0 °C and treated with a cold solution of the respective aniline (4-chloroaniline (0.381 g, 3 mmol), p-toluidine (0.310 g, 3 mmol), or 4-aminobenzenesulfonamide (0.516 g, 3 mmol)) in 6 M hydrochloric acid (1.5 mL) with a sodium nitrite solution (0.21 g, 3 mmol, in 3 mL water). The addition of the diazonium salt was stirred for an additional 2 h at 0–5 °C and then left for 8 h in a refrigerator (4 °C). The resulting solid was collected by filtration, washed thoroughly with water, and dried. The crude product was crystallized from ethanol to give hydrazones 21a–c.
3-Methyl-5-oxo-4-(2-(p-tolyl)hydrazineylidene)-4,5-dihydro-1H-pyrazole-1-carbothiohydrazide (21a). Brown powder, yield (80%), m.p. 120–121 °C (EtOH); IR (νmax, cm−1): 3254–3142 (NH2 & 2NH), 1698 (C=O), 1595 (C=N), 1556–1485 (C=C); 1H-NMR (500 MHz, CDMSO-d6) δH (ppm): 2.31 (s, 3H, CH3), 2.35 (s, 3H, CH3), 6.85 (d, D2O exchangeable, 2H, NH2), 7.46 (m, 4H, Ar-H), 11.31 (s, D2O exchangeable, 1H, NH), 11.74 (s, D2O exchangeable, 1H, NH); 13C-NMR (125 MHz, DMSO-d6) δC (ppm): 11.9 (CH3), 21.4 (CH3), 116.4, 127.4, 128.4, 130, 142.1, 147.5, 163.8, 194.0; MS m/z (%): 290 [M]+ (5%), 95 (100); Anal. Calcd. for C12H14N6OS (290.09): C, 49.64; H, 4.86; N, 28.95, Found: C, 49.41; H, 4.64; N, 28.77%.
4-(2-(4-Chlorophenyl)hydrazineylidene)-3-methyl-5-oxo-4,5-dihydro-1H-pyrazole-1-carbothiohydrazide (21b). Red crystals, yield (85%), m.p. 117–118 °C (EtOH); IR (νmax, cm−1): 3383–3172 (NH2 & 2NH), 1674 (C=O), 1593 (C=N), 1544–1481 (C=C); 1H-NMR (500 MHz, CDMSO-d6) δH (ppm): 2.31 (s, 3H, CH3), 6.99 (s, D2O exchangeable, 2H, NH2), 7.1 (s, D2O exchangeable, 1H, NH), 7.48 (m, 4H, Ar-H), 11.76 (s, D2O exchangeable, 1H, NH); 13C-NMR (125 MHz, DMSO-d6) δC (ppm): 11.9 (CH3), 116.4, 117.4, 118.4, 128, 129.5, 147.1, 163.8 (C=O), 194 (C=S); MS m/z (%): 310 [M]+ (5%), 285.78 (30), 267.95 (90); Anal. Calcd. for C11H11ClN6OS (310.76): C, 42.52; H, 3.57; N, 27.04, Found: C, 42.36; H, 3.29; N, 26.88%.
4-(2-(1-(Hydrazinecarbonothioyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazineyl)benzenesulfonamide (21c). Brown powder, yield (80%), m.p. 140–141 °C (EtOH); IR (νmax, cm−1): 3296–3221 (2NH2 & 2NH), 1693 (C=O), 1595 (C=N), 1543–1489 (C=C); 1H-NMR (500 MHz, CDMSO-d6) δH (ppm): 2.28 (s, 3H, CH3), 6.98 (s, D2O exchangeable, 2H, NH2), 7.08 (s, D2O exchangeable, 2H, NH2), 7.18 (s, D2O exchangeable, 2H, NH2), 7.72 (d, 2H, J = 7.5 Hz, Ar-H), 7.84 (d, 2H, J = 8.5 Hz, Ar-H), 11.31 (s, D2O exchangeable, 1H, NH), 12.24 (s, D2O exchangeable, 1H, NH); 13C-NMR (125 MHz, DMSO-d6) δC (ppm): 11.8 (CH3), 116.6, 126.8, 140.9, 144, 153.8, 160.7, 169 (C =O), 180 (C=S); MS m/z (%): 354.93 [M]+ (4%), 255.68 (100); Anal. Calcd. for C11H13N7O3S2 (355.39): C, 37.18; H, 3.69; N, 27.59, Found: C, 36.98; H, 3.56; N, 27.47%.
Synthesis of N′-(4-(Dimethylamino)benzylidene)-3-methyl-5-oxo-4,5-dihydro-1H-pyrazole-1-carbothiohydrazide (22). A few drops of HCl were added to a mixture of 1a (0.516 g, 3 mmol), and 4-(dimethylamino)benzaldehyde (0.447 g, 3 mmol) in EtOH (20 mL), and the reaction mixture was stirred 6 h. The precipitate formed was collected by filtration, dried, washed with EtOH, and recrystallized from EtOH. Red powder, yield (89%), m.p. 261–262 °C (EtOH); IR (νmax, cm−1): 3269 (NH), 1689 (C=O), 1510 (C=N), 1504–1456 (C=C); 1H-NMR (500 MHz, CDMSO-d6) δH (ppm): 2.68 (s, 3H, CH3), 3 (s, 6H, N(CH3)2), 3.04 (s, 2H, CH2, pyrazole-4), 6.80 (m, 2H, Ar-H), 7.91 (m, 2H, Ar-H), 9.65 (s, 1H, CH=N), 11.97 (s, D2O exchangeable, 1H, NH); 13C-NMR (125 MHz, DMSO-d6) δC (ppm): 16.6 (CH3), 40 (CH3), 41 (CH2),111.6, 124, 129.5, 131.3, 154.4, 158, 163 (C=O), 190.5 (C=S); MS m/z (%): 290 [M]+ (25%), Anal. Calcd. for C14H17N5OS (303.38): C, 55.43; H, 5.65; N, 23.08, Found: C, 55.13; H, 5.30; N, 22.97%.
3.3. Antimicrobial Evaluation
The antibacterial activity of the synthesized compounds was tested against a panel of two gram positive bacteria (Staphylococcus aureus and Bacillus subtilis) and two Gram-negative bacteria (Klebsiella pneumoniae and Escherichia coli). The antifungal activities of the compounds were tested against two fungi (Candida albicans and Aspergillus flavus). A solution of each compounds in DMSO with concentration 1 mg/mL was prepared separately, paper discs of Whatman filter paper were prepared with standard size (5 cm) were cut and sterilized in an autoclave. The paper discs soaked in the desired concentration of the compound solution were placed aseptically in the petri dishes containing nutrient agar media (agar 20 g + beef extract 3 g + peptone 5 g) seeded with Staphylococcus aureus, Bacillus subtilis, E. coli, Pseudomonas aeuroginosa, Candida albicans, and Aspergillus flavus. The petri dishes were incubated at 36 °C and the inhibition zones were recorded after 24 h of incubation. Each treatment was replicated three times. The antibacterial activity of a common standard antibiotic chloramphenicol and antifungal clotrimazole were also recorded using the same procedure as above at the same concentration and solvents [28].
The MIC was determined using the disc diffusion technique by preparing discs containing 1.9–1000 µg/mL of each compound against gram positive, gram negative, and fungi. Twofold dilutions of the solution were prepared. The microorganism suspensions at 10 CFU/mL (colony forming unit/mL) concentrations were inoculated to the corresponding wells. The plates were incubated at 36 °C for 24 h for the bacteria. The standard antibiotic chloramphenicol and antifungal clotrimazole was also recorded using the same procedure as above at the same concentration and solvents. At the end of the incubation period, the minimum inhibitory concentrations (MIC) values were recorded as the lowest concentration of the substance that had no visible turbidity. Control experiments with DMSO and uninoculated media were run parallel to the test compounds under the same condition [28].
4. Conclusions
A novel series of pyrazole and pyrazolone derivatives was synthesized, in good yields, starting from pyrazole-1-carbothiohydrazide 1a,b. A number of prepared compounds showed moderate to good antimicrobial activities. Hydrazones 21a–c showed significant antimicrobial activities with MIC values equal to or lower than standard drugs chloramphenicol and clotrimazole. It is clearly that the presence of free carbothiohydrazide moiety increases antimicrobial activity.
Funding
This research was funded by the Deanship of Scientific Research, Jazan University, Saudi Arabia for the financial support via project No: JUP7//00089 (2014–2015).
Acknowledgments
The author is thankful to Yasser Mokhtar A. Ibrahim, Vacsera, Cairo, Egypt; for handling the antimicrobial properties.
Conflicts of Interest
The author declares no conflict of interest.
References
- Kardas, P.; Devine, S.; Golembesky, A.; Roberts, C. A systematic review and meta analysis of misuse of antibiotic therapies in the community. Int. J. Antimicrob. Agents 2005, 26, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.G. The history of antimicrobial drug development and the current situation. Infect. Chemother. 2012, 44, 263–268. [Google Scholar] [CrossRef]
- Abdel Aziz, M.; Gamal El-Din, A.; Abuo-Rahma, A.H.A. Synthesis of novel pyrazole derivatives and evaluation of their antidepressant and anticonvulsant activities. Eur. J. Med. Chem. 2005, 44, 3480–3487. [Google Scholar] [CrossRef] [PubMed]
- Dinesha, V.S.; Priya, B.K.; Pai, K.S.R.; Naveen, S.; Lokanath, N.K.; Nagaraja, G.K. Synthesis and pharmacological evaluation of some new fluorine containing hydroxypyrazolines as potential anticancer and antioxidant agents. Eur. J. Med. Chem. 2015, 104, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Bandgar, B.P.; Gawande, S.S.; Bodade, R.G. Synthesis and biological evaluation of a novel series of pyrazole chalcones as anti-inflammatory, antioxidant and antimicrobial agents. Bioorg. Med. Chem. 2009, 17, 8168–8173. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.-C.; Li, H.-Q.; Sun, J.; Zhou, Y.; Zhu, H.-L. Synthesis and biological evaluation of pyrazole derivatives containing thiourea skeleton as anticancer agents. Bioorg. Med. Chem. 2010, 18, 4606–4614. [Google Scholar] [CrossRef] [PubMed]
- Radini, I.A.M.; Khidre, R.E.; El-Telbani, E.M. Synthesis and antimicrobial evaluation of new pyrazoline and pyrazolinyl thiazole derivatives bearing tetrazolo[1,5-a]quinoline moiety. Lett. Drug Des. Discov. 2016, 13, 921–931. [Google Scholar] [CrossRef]
- Altıntop, M.D.; Abu Mohsen, U.; Karaca, H.; Canturk, Z.; Ozdemir, A. Synthesis and Evaluation of Bis-pyrazoline Derivatives as Potential Antimicrobial Agents. Lett. Drug Des. Discov. 2014, 11, 1199–1203. [Google Scholar] [CrossRef]
- Bai, L.S.; Wang, Y.; Liu, X.H.; Zhu, H.L.; Song, B.A. Novel dihydropyrazole derivatives linked with multi(hetero)aromatic ring: Synthesis and antibacterial activity. Chin. Chem. Lett. 2009, 20, 427–430. [Google Scholar] [CrossRef]
- Ouyang, G.P.; Cai, X.J.; Chen, Z.; Song, B.-A.; Bhadury, P.S.; Yang, S.; Jin, L.-H.; Xue, W.; Hu, D.-Y.; Zeng, S. Synthesis and antiviral activities of pyrazole derivatives containing an oxime moiety. J. Agric. Food Chem. 2008, 56, 10160–10167. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.P.; Chen, Z.; Cai, X.J.; Song, B.A.; Bhadury, P.S.; Yang, S.; Jin, L.H.; Xue, W.; Hu, D.Y.; Zeng, S. Synthesis and antiviral activity of novel pyrazole derivatives containing oxime esters group. Bioorg. Med. Chem. 2008, 16, 9699–9707. [Google Scholar] [CrossRef] [PubMed]
- Alagarsamy, V.; Saravanan, G. Synthesis and anticonvulsant activity of novel quinazolin-4(3H)-one derived pyrazole analogs. Med. Chem. Res. 2013, 22, 1711. [Google Scholar] [CrossRef]
- Song, H.J.; Liu, Y.X.; Xiong, L.X.; Li, Y.; Yang, N.; Wang, Q. Design, synthesis, and insecticidal evaluation of new pyrazole derivatives containing imine, oxime ether, and dihydroisoxazoline groups based on the inhibitor binding pocket of respiratory complex I. J. Agric. Food Chem. 2013, 61, 8730–8736. [Google Scholar] [CrossRef] [PubMed]
- Comber, R.N.; Gray, R.J.; Secrist, J.A. Acyclic analogues of pyrazofurin: Syntheses and antiviral evaluation. Carbohydr. Res. 1991, 216, 441–452. [Google Scholar] [CrossRef]
- Schroder, J.; Henke, A.; Wenzel, H.; Brandstetter, H.; Stammler, H.G.; Stammler, A.; Pfeiffer, W.D.; Tschesche, H. Structure-Based Design and Synthesis of Potent Matrix Metalloproteinase Inhibitors Derived from a 6H-1,3,4-Thiadiazine Scaffold. J. Med. Chem. 2001, 44, 3231–3243. [Google Scholar] [CrossRef] [PubMed]
- Sert-Ozgur, S.; Banu, C.T.; Somuncuoglu, E.I.; Kazkayasi, I.; Ertan, M.; Tozkoparan, B. Design and Synthesis of 1,2,4-Triazolo[3,2-b]-1,3,5-thiadiazine Derivatives as a Novel Template for Analgesic/Anti-Inflammatory Activity. Arch. Pharm. Chem. Life Sci. 2017, 350, e1700052. [Google Scholar] [CrossRef] [PubMed]
- Čačić, M.; Pavić, V.; Molnar, M.; Šarkanj, B.; Has-Schön, E. Design and Synthesis of Some New 1,3,4-Thiadiazines with Coumarin Moieties and Their Antioxidative and Antifungal Activity. Molecules 2014, 19, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Zaib, S.; Ibrar, A.; Rama, N.H.; Simpson, J.; Iqbal, J. Synthesis, crystal structure and biological evaluation of some novel 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles and 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazines. Eur. J. Med. Chem. 2014, 78, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Khidre, R.E.; Abdel-Wahab, B.F.; Awad, G.E.A. Multi-component one-pot synthesis of novel (1,3,4-thiadiazin-2-ylamino)isoindoline-1,3-diones as antimicrobial agents. Heterocycles 2017, 94, 314–325. [Google Scholar] [CrossRef]
- Radini, I.A.M.; Abdel-Wahab, B.F.; Khidre, R.E. Synthetic Routes to Imidazothiazines. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 844–856. [Google Scholar] [CrossRef]
- Khidre, R.E.; Rodini, I.M.; Ibrahim, D.A. Synthesis of a novel heterocyclic scaffold utilizing 2-cyano-N-(3-cyano-4,6-dimethyl-2-oxopyridin-1-yl)acetamide. ARKIVOC 2016, 301–317. [Google Scholar] [CrossRef]
- Khidre, R.E.; Radini, I.A.M.; Abdel-Wahab, B.F. Synthesis of new heterocycles incorporating 3-(N-phthalimidomethyl)-1,2,4-triazole as antimicrobial agents. Egypt. J. Chem. 2016, 59, 731–744. [Google Scholar]
- Radini, I.A.M.; Elsheikh, T.M.Y.; El-Telbani, E.M.; Khidre, R.E. New Potential Antimalarial Agents: Design, Synthesis and Biological Evaluation of Some Novel Quinoline Derivatives as Antimalarial Agents. Molecules 2016, 21, 909. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, V.V.; Zelenin, K.N.; Yakimovich, S.I. Synthesis of Pyrazole, 1,2,4,5-Tetrazine, and 1,2,4-Triazole Derivatives from Thiocarbonohydrazides and β-Dicarbonyl Compounds. Russ. J. Org. Chem. 1995, 31, 868–873. [Google Scholar]
- Pavurala, S.; Vaarla, K.; Vedula, R.R. One-Pot Three-Component Synthesis of Pyrazolyl-thiadiazinyl-2Hchromen-2-one Derivatives. J. Heterocycl. Chem. 2015, 52, 1503–1505. [Google Scholar] [CrossRef]
- Lawrence, P.G.; Harold, P.L.; Francis, O.G. Antibiotic and Chemotherapy; Edinburgh: London, UK, 1980; Volume 5, p. 1597. [Google Scholar]
- Koch, A.L. Bacterial wall as target for attack: Past, present, and future research. Clin. Microbiol. Rev. 2003, 16, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1a, 1b, 5a, 8a, 12, 15c, 17b, 20, 21b, and 22 are available from the author. |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).