Preparation and Characterization of Antioxidative and UV-Protective Larch Bark Tannin/PVA Composite Membranes
Abstract
1. Introduction
2. Results and Discussion
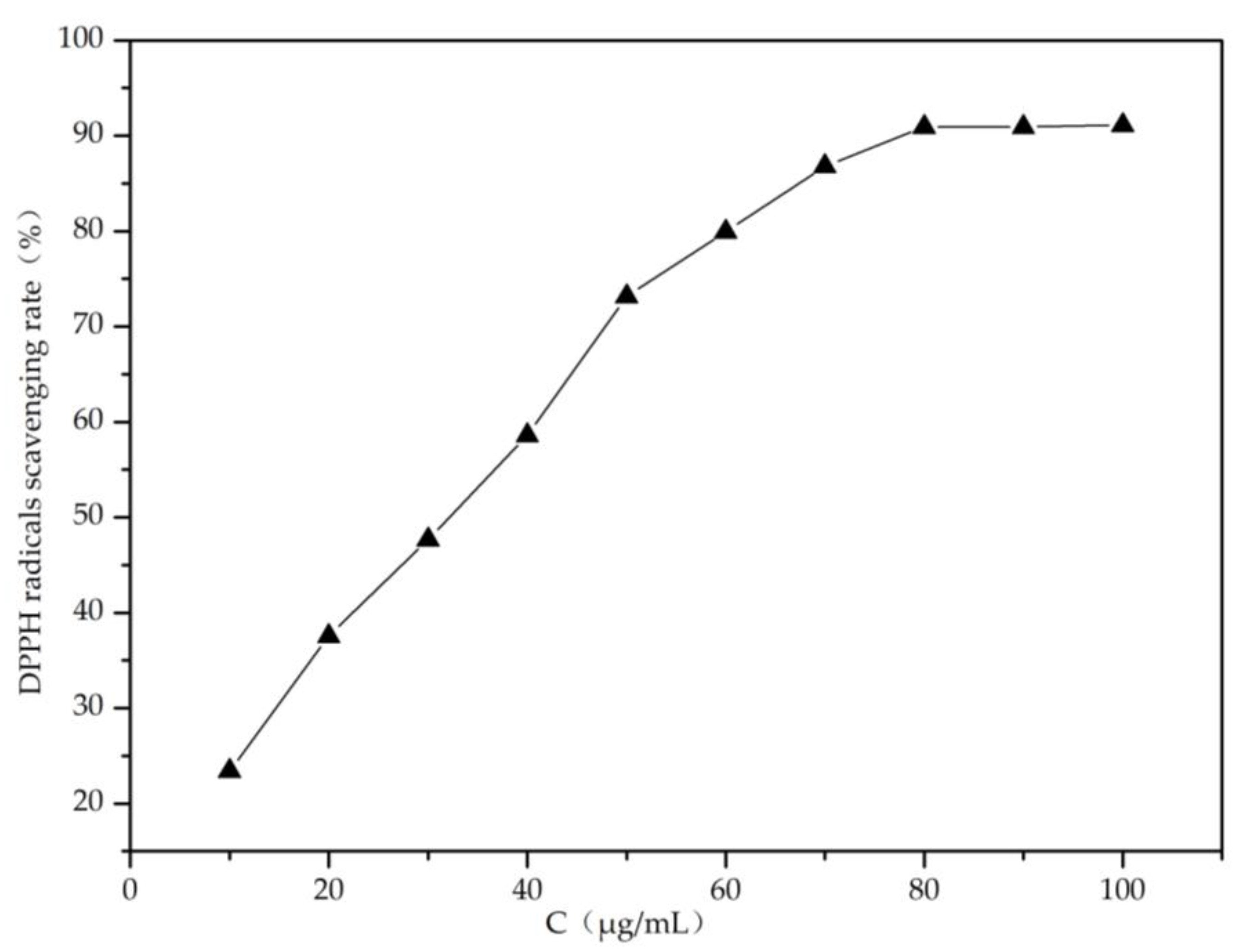
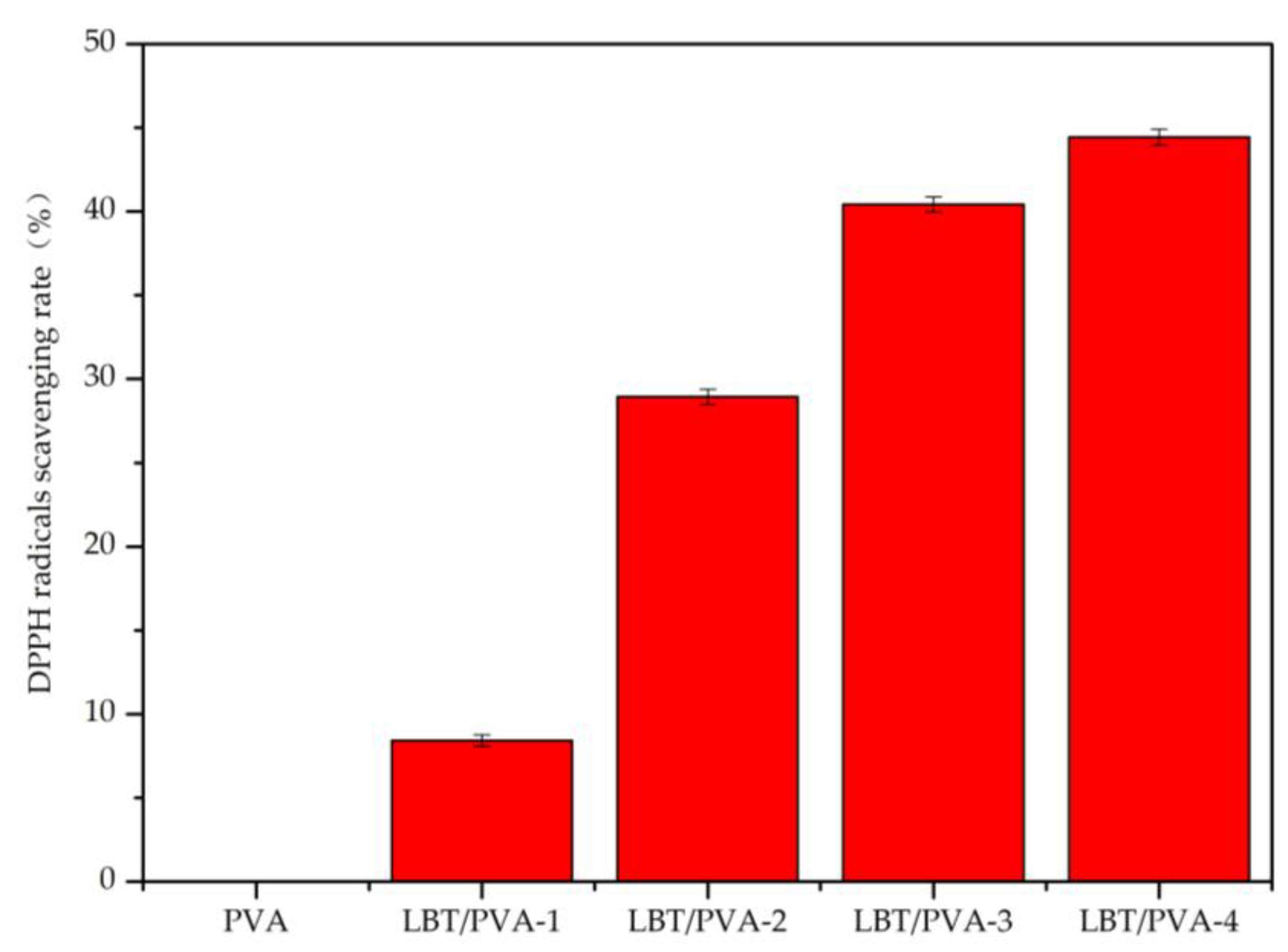
2.1. Analysis of Membrane Antioxidative Activity
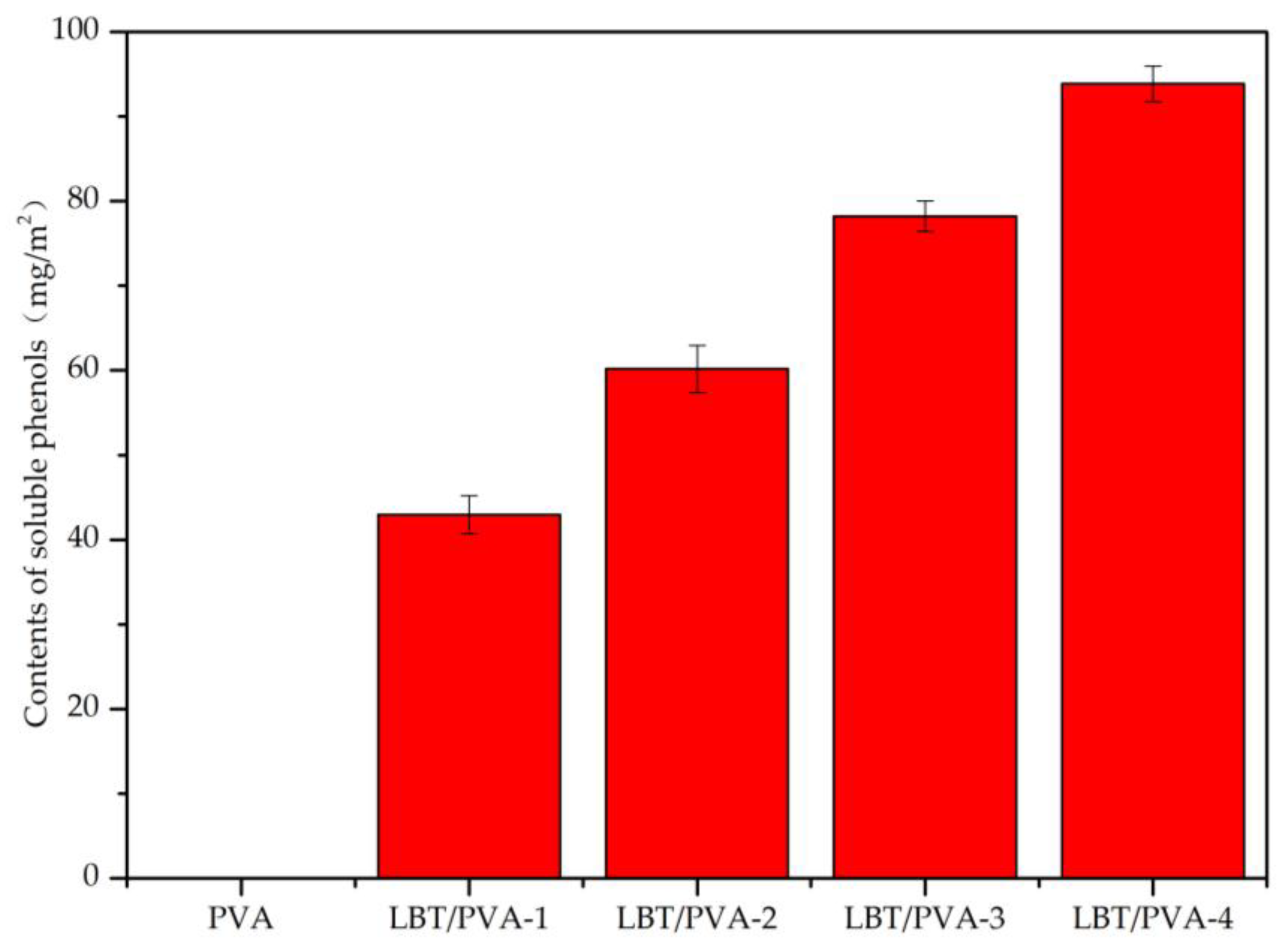
2.2. Membrane Soluble Phenols Content
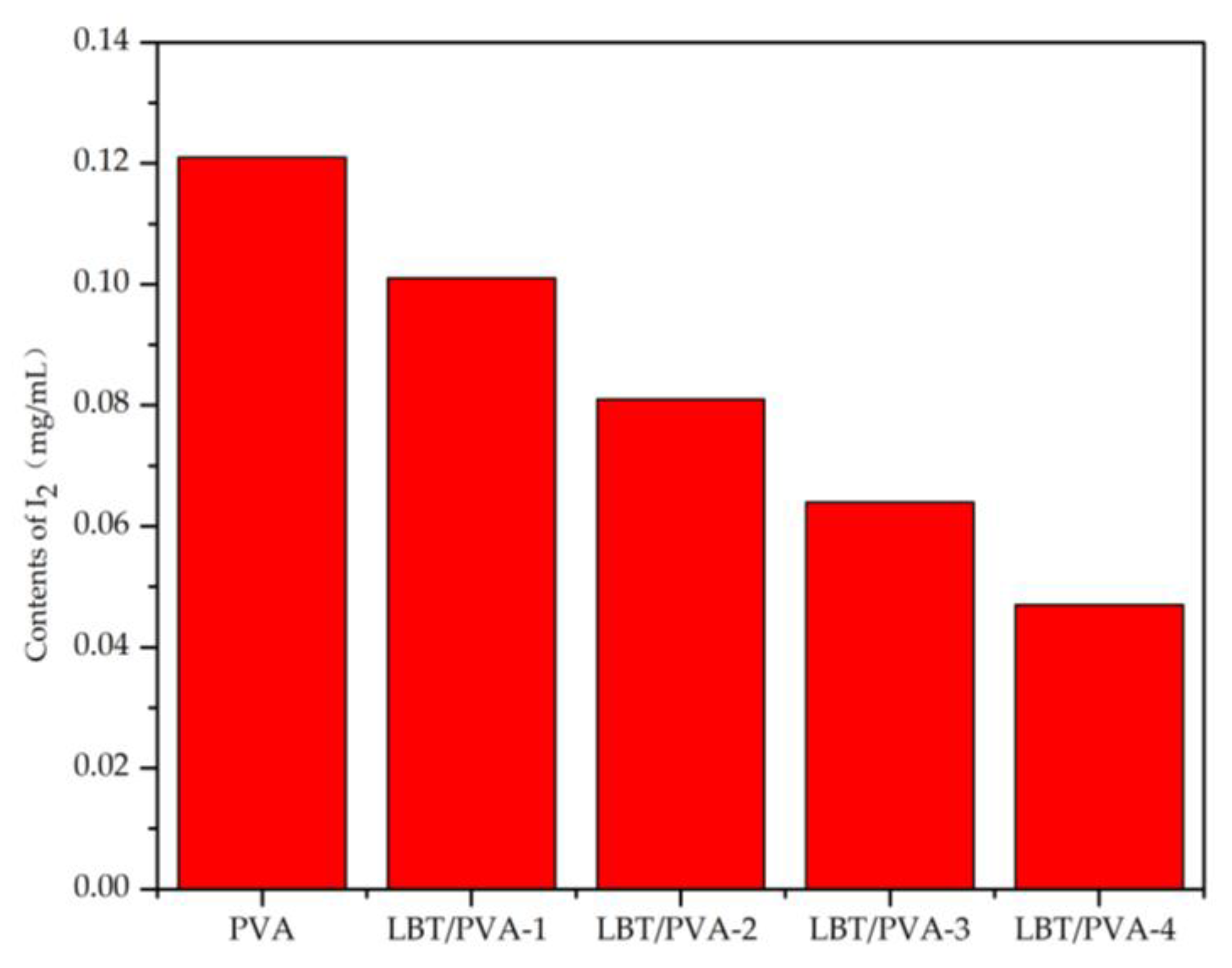
2.3. Effect of Increasing Amounts of LBT in the Membrane on Starch-KI Oxidation–Discoloration Reaction
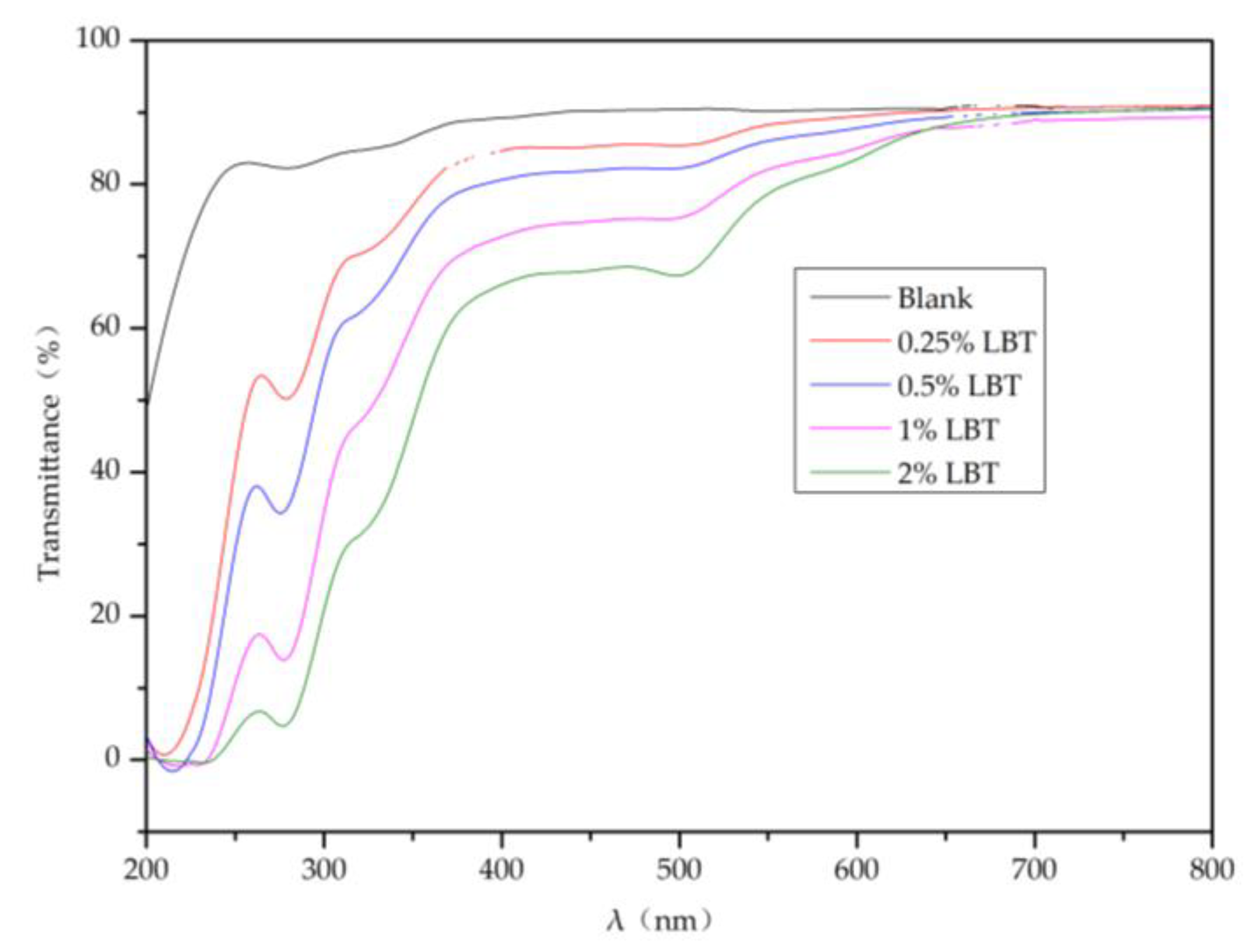
2.4. Light Transmittance of the Composite Membranes
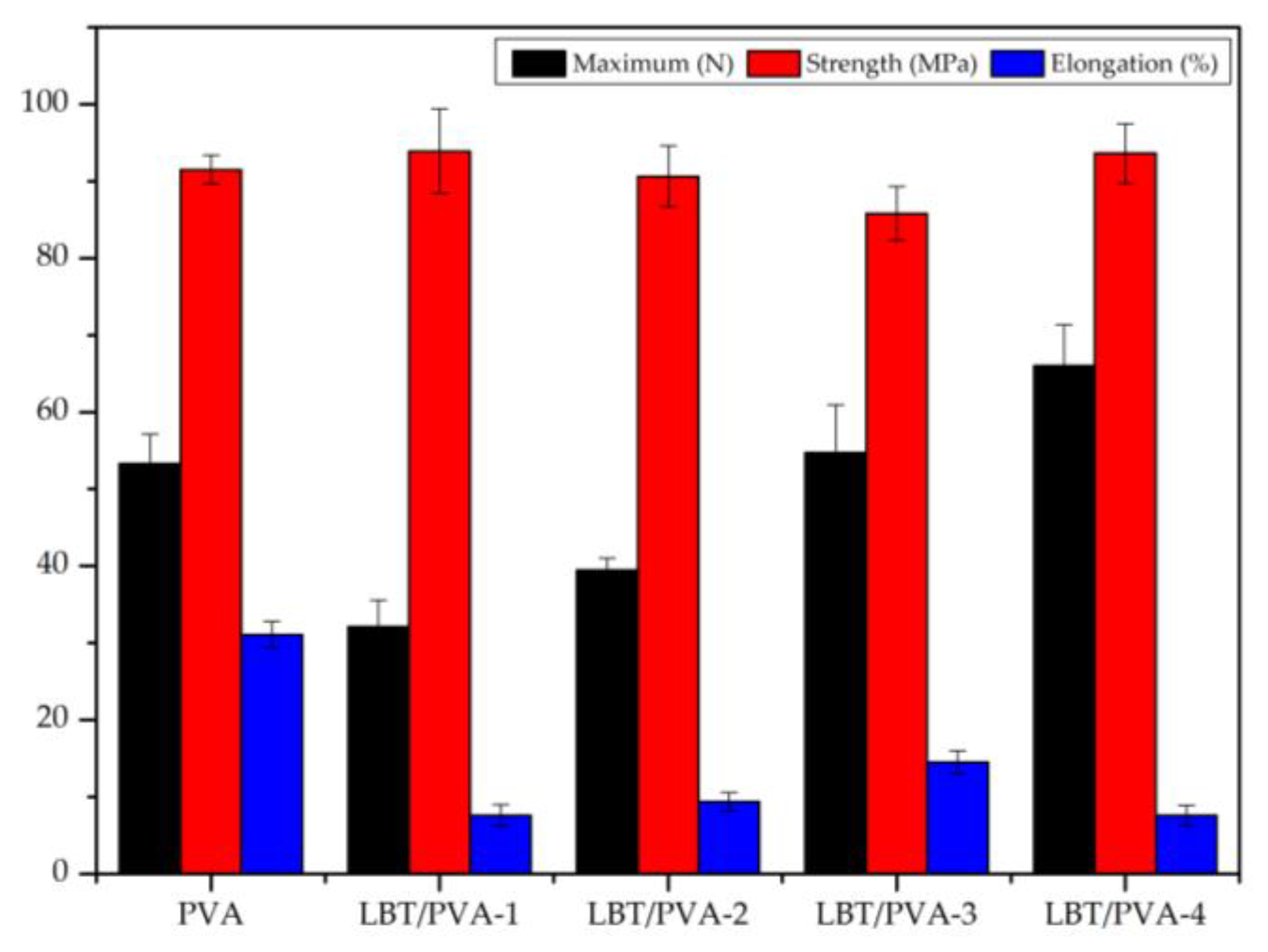
2.5. Analysis of the Mechanical Properties of Membranes
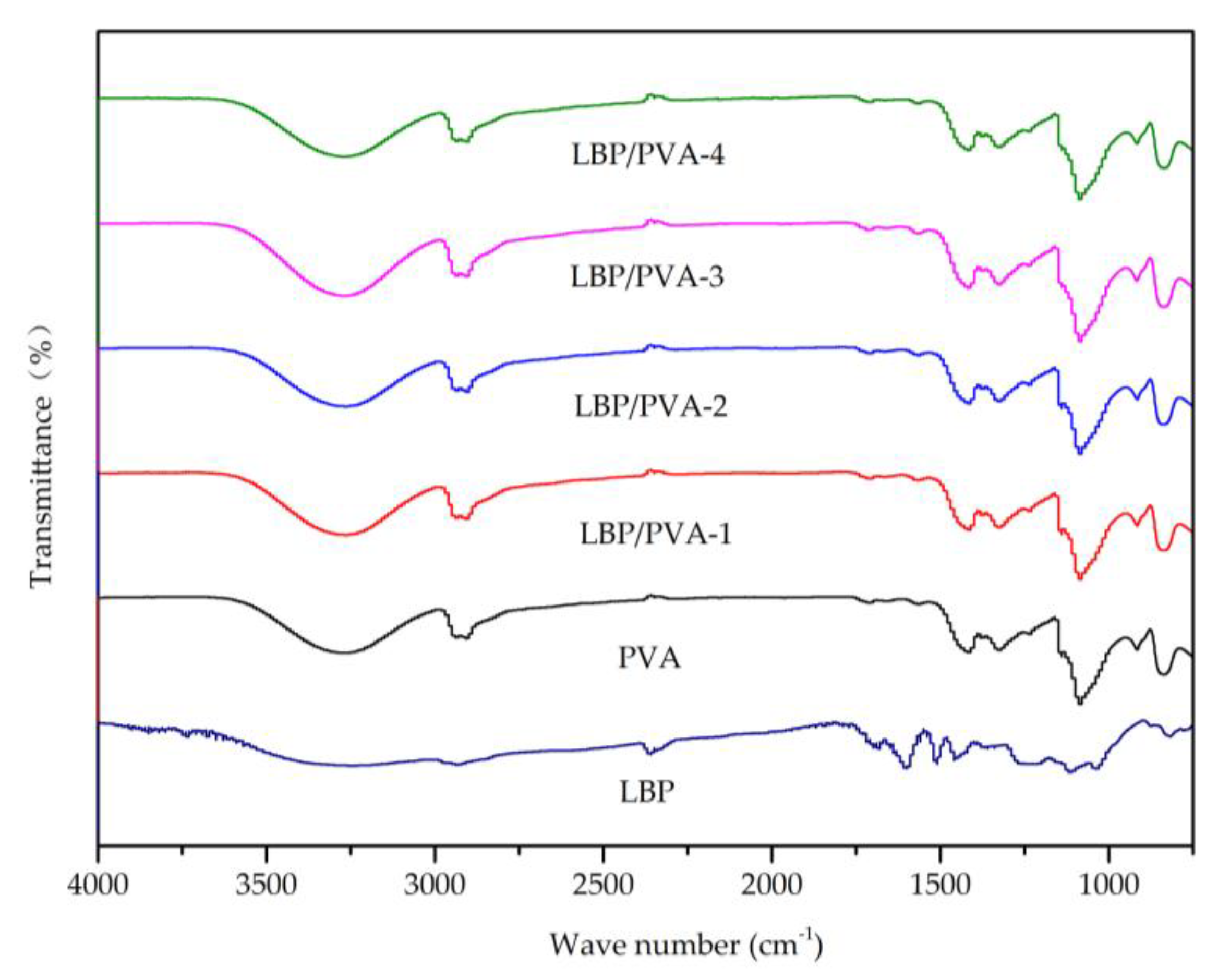
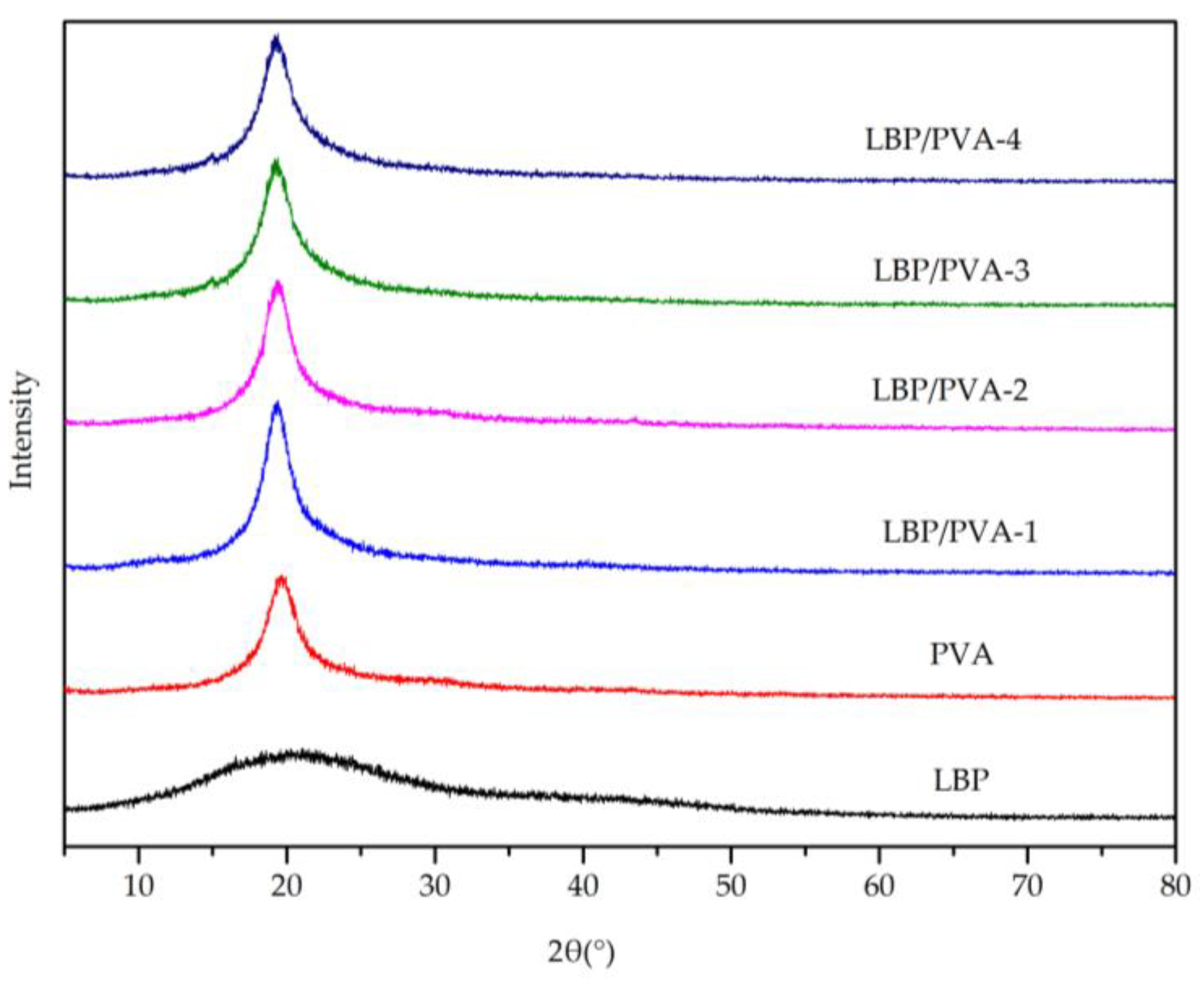
2.6. Other Analytical Characterization
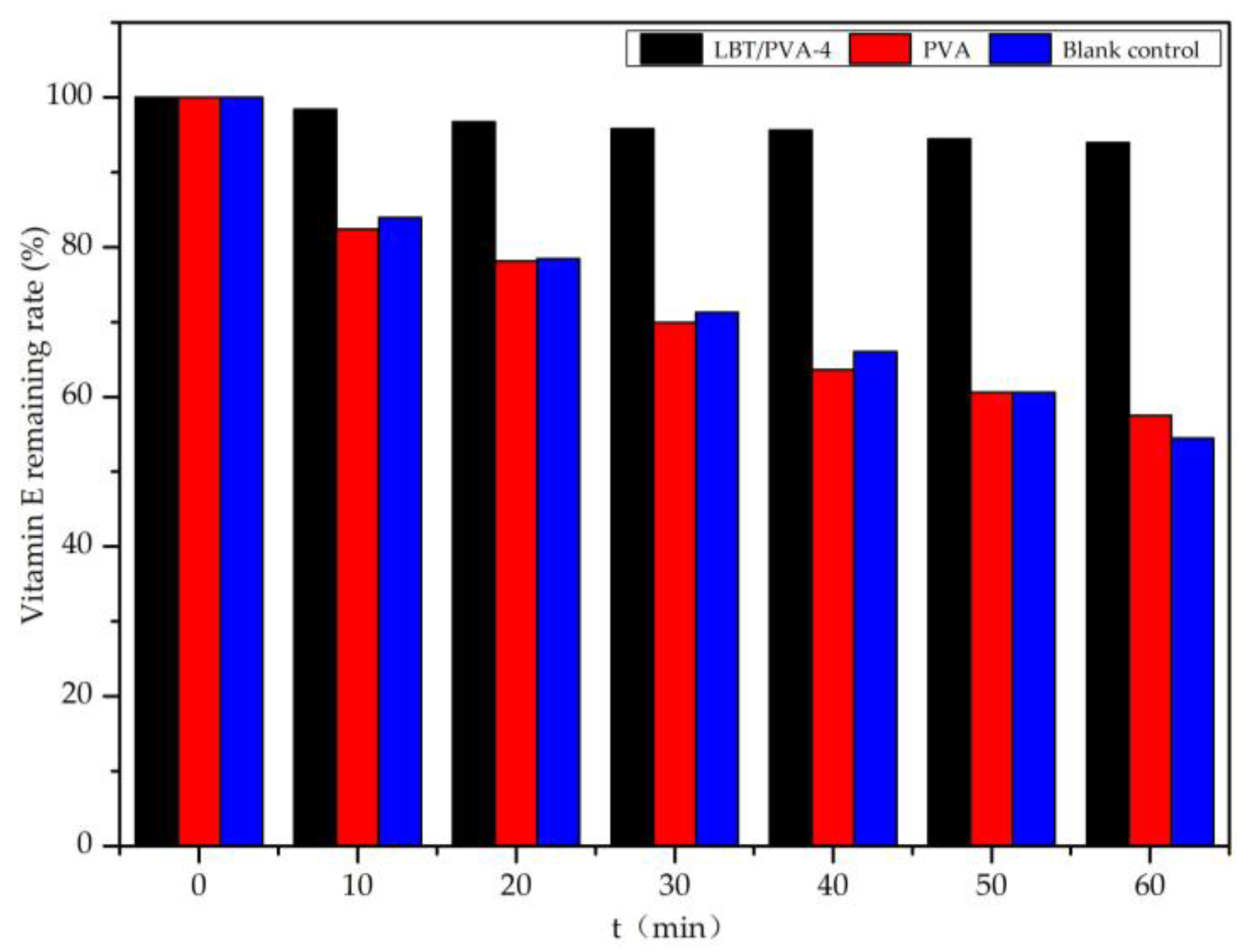
2.7. Application for Vitamin E Protection
3. Materials and Methods
3.1. Materials
3.2. Extraction of Tannin from Larch Bark
3.3. Preparation of the Composite Membrane
3.4. Determination of the Antioxidative Activity of LBT and Composite Membranes by the DPPH Assay
3.5. Determination of Soluble Phenol Content
3.6. Effect of the Composite Membranse on the Oxidation and Discoloration of Starch-Potassium Iodide (KI)
3.7. UV–Visible Light Transmittance of the Composite Membranes
3.8. Mechanical Properties Test
3.9. Other Analysis and Characterizations
3.10. Protective Effect of the Composite Membranes on Vitamin E
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gemili, S.; Yemenicioğlu, A.; Altınkaya, S.A. Development of antioxidant food packaging materials with controlled release properties. J. Food Eng. 2010, 96, 325–332. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Lee, J.Y.; Lee, Y.S. Development of polyvinyl alcohol and apple pomace bio-composite film with antioxidant properties for active food packaging application. J. Food Sci. Technol. 2016, 53, 1608–1619. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Sobral, P.J. Investigation of the physicochemical, antimicrobial and antioxidant properties of gelatin-chitosan edible film mixed with plant ethanolic extracts. Food Biosci. 2016, 16, 17–25. [Google Scholar] [CrossRef]
- Duthie, G.G. Parsley, polyphenols and nutritional antioxidants. Br. J. Nutr. 1999, 81, 425–426. [Google Scholar] [PubMed]
- Yang, L.; Sun, X.; Yang, F.; Zhao, C.; Zhang, L.; Zu, Y. Application of ionic liquids in the microwave-assisted extraction of proanthocyanidins from Larix gmelini bark. Int. J. Mol. Sci. 2012, 13, 5163–5178. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Jin, Z.; Yang, L.; Hao, J.; Zu, Y.; Wang, W.; Liu, W. Ultrasonic-assisted extraction of procyanidins using ionic liquid solution from Larix gmelini bark. J. Chem. 2012, 2013, 541037. [Google Scholar] [CrossRef]
- Castro López, M.A.D.M.; López de Dicastillo, C.; López Vilariño, J.M.; González Rodríguez, M.a.V. Improving the capacity of polypropylene to be used in antioxidant active films: Incorporation of plasticizer and natural antioxidants. J. Agric. Food Chem. 2013, 61, 8462–8470. [Google Scholar] [CrossRef] [PubMed]
- Pohl, F.; Goua, M.; Bermano, G.; Russell, W.; Maciel, P.; Lin, P.K.T. Study into the polyphenol content and antioxidant activity of rapeseed pomace extracts. Proc. Nutr. Soc. 2016, 75. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Rao, M.; Chawla, S.; Sharma, A. Active chitosan–polyvinyl alcohol films with natural extracts. Food Hydrocoll. 2012, 29, 290–297. [Google Scholar] [CrossRef]
- Ma, Q.; Du, L.; Wang, L. Tara gum/polyvinyl alcohol-based colorimetric nh3 indicator films incorporating curcumin for intelligent packaging. Sens. Actuators B Chem. 2017, 244, 759–766. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.A., Jr.; de Arruda, I.N.Q.; Stefani, R. Active chitosan/pva films with anthocyanins from brassica oleraceae (red cabbage) as time–temperature indicators for application in intelligent food packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar] [CrossRef]
- Cano, A.I.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical and microstructural properties of biodegradable films based on pea starch and pva. J. Food Eng. 2015, 167, 59–64. [Google Scholar] [CrossRef]
- Pourjavaher, S.; Almasi, H.; Meshkini, S.; Pirsa, S.; Parandi, E. Development of a colorimetric ph indicator based on bacterial cellulose nanofibers and red cabbage (brassica oleraceae) extract. Carbohydr. Polym. 2017, 156, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A. Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Schaich, K. Re-evaluation of the 2, 2-diphenyl-1-picrylhydrazyl free radical (dpph) assay for antioxidant activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Maranesi, B.; Chailan, J.-F.; Khadhraoui, M.; Polini, R.; Di Vona, M.L.; Knauth, P. Crosslinked speek membranes: Mechanical, thermal, and hydrothermal properties. J. Mater. Res. 2012, 27, 1950–1957. [Google Scholar] [CrossRef]
- Rosales-Castro, M.; Honorato-Salazar, J.A.; Reyes-Navarrete, M.G.; González-Laredo, R.F. Antioxidant phenolic compounds of ethanolic and aqueous extracts from Pink Cedar (Acrocarpus fraxinifolius Whight & Arn.) bark at two tree ages. J Wood Chem. Technol. 2015, 35, 270–279. [Google Scholar]
- Yuan, G.; Lv, H.; Yang, B.; Chen, X.; Sun, H. Physical properties, antioxidant and antimicrobial activity of chitosan films containing carvacrol and pomegranate peel extract. Molecules 2015, 20, 11034–11045. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.W.; Cho, M.J.; Hwang, K.Y. Correction of hydrogen peroxide interference on standard chemical oxygen demand test. Water Res. 1999, 33, 1247–1251. [Google Scholar] [CrossRef]
- López-Mata, M.A.; Ruiz-Cruz, S.; Silva-Beltrán, N.P.; Ornelas-Paz, J.D.J.; Zamudio-Flores, P.B.; Burruel-Ibarra, S.E. Physicochemical, antimicrobial and antioxidant properties of chitosan films incorporated with carvacrol. Molecules 2013, 18, 13735–13753. [Google Scholar] [CrossRef] [PubMed]
- Anicuta, S.G.; Dobre, L.; Stroescu, M.; Jipa, I. Fourier Transform Infrared (FTIR) Spectroscopy for Characterization of Antimicrobial Films Containing Chitosan. Zootehnie şi Tehnologii de Industrie Alimentară; Analele Universită Ńii din Oradea Fascicula: Ecotoxicologie, România, 2010; pp. 1234–1240. [Google Scholar]
- Chiellini, E.; Cinelli, P.; Ilieva, V.I.; Martera, M. Biodegradable thermoplastic composites based on polyvinyl alcohol and algae. Biomacromolecules 2008, 9, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Devi, D.A.; Smitha, B.; Sridhar, S.; Aminabhavi, T. Dehydration of 1, 4-dioxane through blend membranes of poly (vinyl alcohol) and chitosan by pervaporation. J. Membr. Sci. 2006, 280, 138–147. [Google Scholar] [CrossRef]
- Lin, J.Y.; Selim, M.A.; Shea, C.R.; Grichnik, J.M.; Omar, M.M.; Monteiro-Riviere, N.A.; Pinnell, S.R. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J. Am. Acad. Dermatol. 2003, 48, 866–874. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of Larch bark tannin and the composite membranes are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Y.; Wang, J.; Wang, H.; Song, T.; Hu, W.; Li, S. Preparation and Characterization of Antioxidative and UV-Protective Larch Bark Tannin/PVA Composite Membranes. Molecules 2018, 23, 2073. https://doi.org/10.3390/molecules23082073
Zhai Y, Wang J, Wang H, Song T, Hu W, Li S. Preparation and Characterization of Antioxidative and UV-Protective Larch Bark Tannin/PVA Composite Membranes. Molecules. 2018; 23(8):2073. https://doi.org/10.3390/molecules23082073
Chicago/Turabian StyleZhai, Yingxiang, Jiangtao Wang, Hao Wang, Tao Song, Weitong Hu, and Shujun Li. 2018. "Preparation and Characterization of Antioxidative and UV-Protective Larch Bark Tannin/PVA Composite Membranes" Molecules 23, no. 8: 2073. https://doi.org/10.3390/molecules23082073
APA StyleZhai, Y., Wang, J., Wang, H., Song, T., Hu, W., & Li, S. (2018). Preparation and Characterization of Antioxidative and UV-Protective Larch Bark Tannin/PVA Composite Membranes. Molecules, 23(8), 2073. https://doi.org/10.3390/molecules23082073





