Transesterification of Lactic Acid Oligomers with Ethanol, a Way to Anhydrous Ethyl Lactate: A Kinetic Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Derivation of the Kinetic Model
2.2. List of Symbols
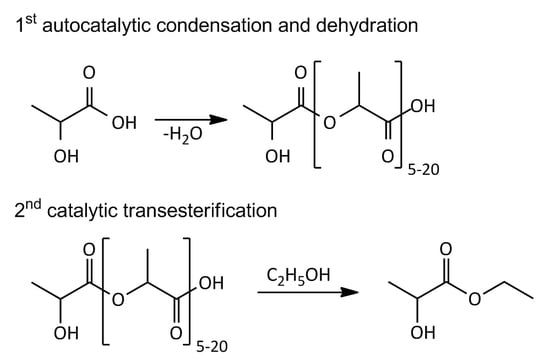
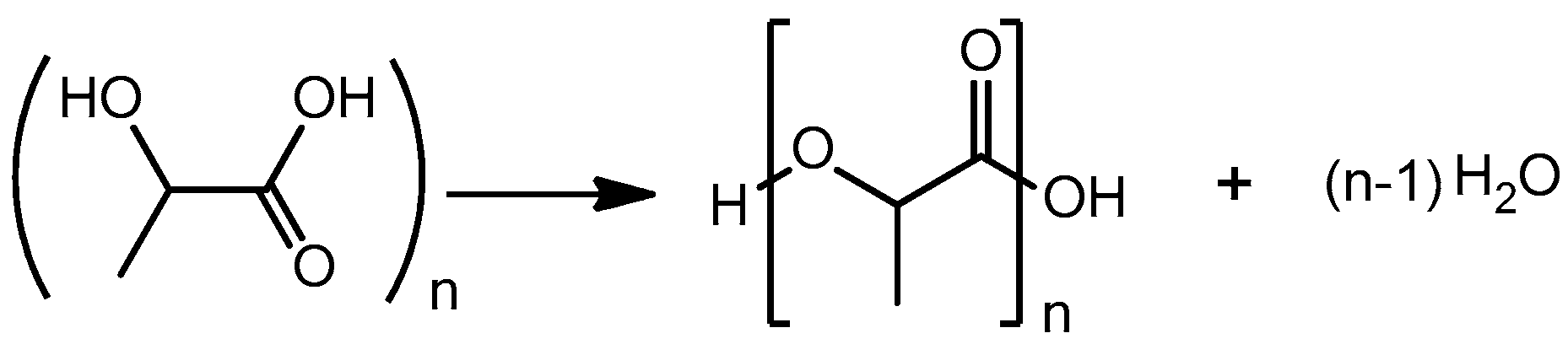
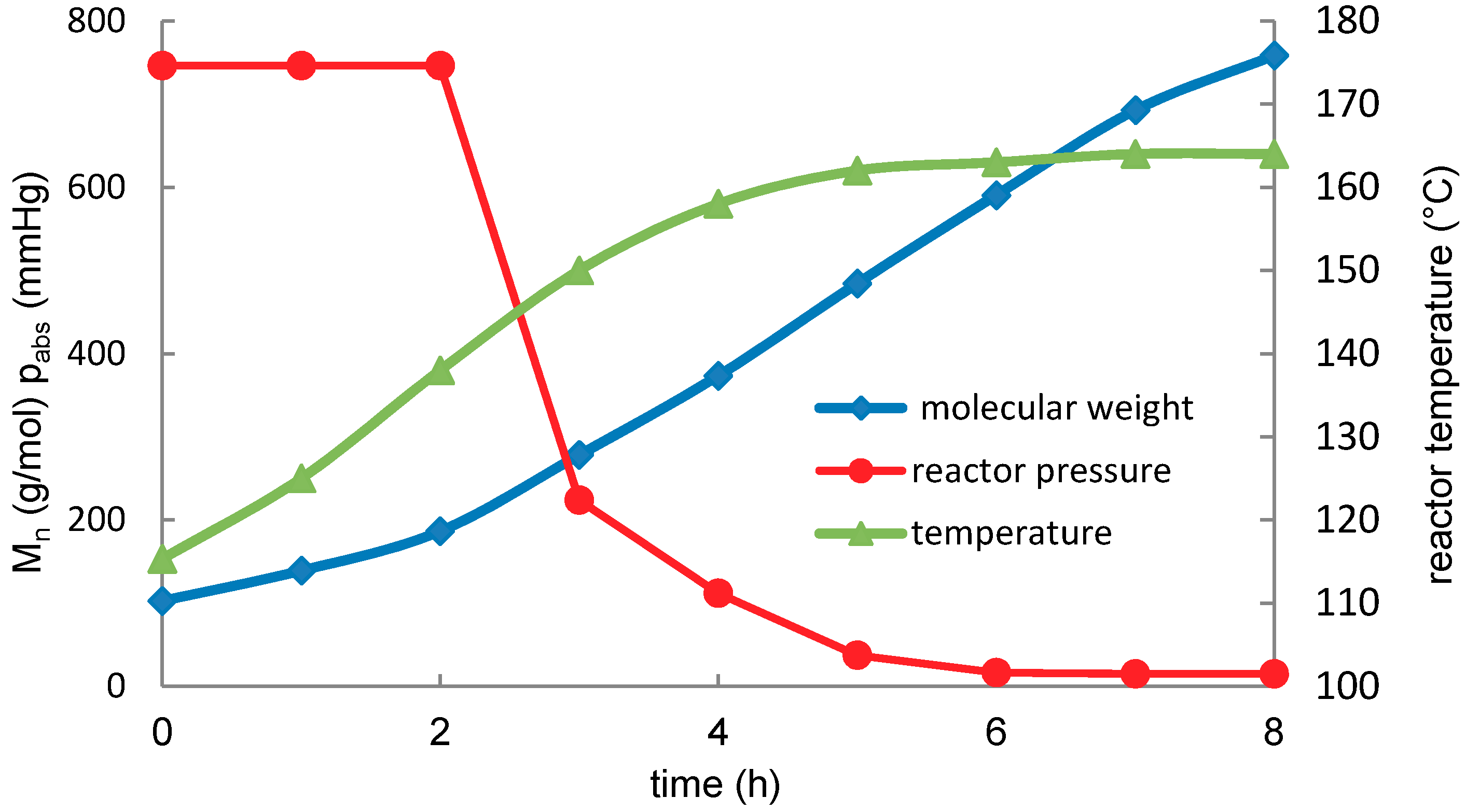
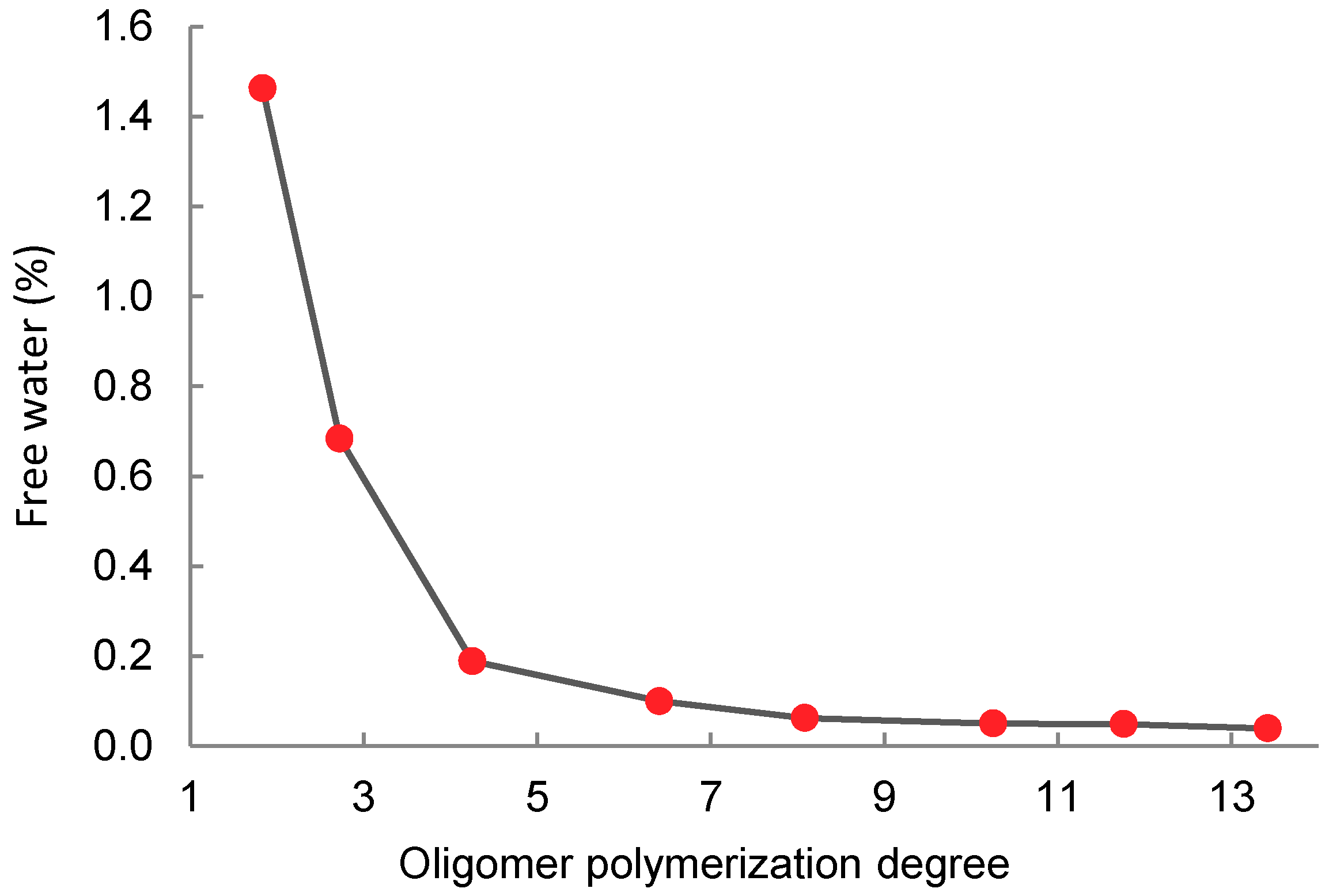
2.3. Lactic Acid Oligomerization
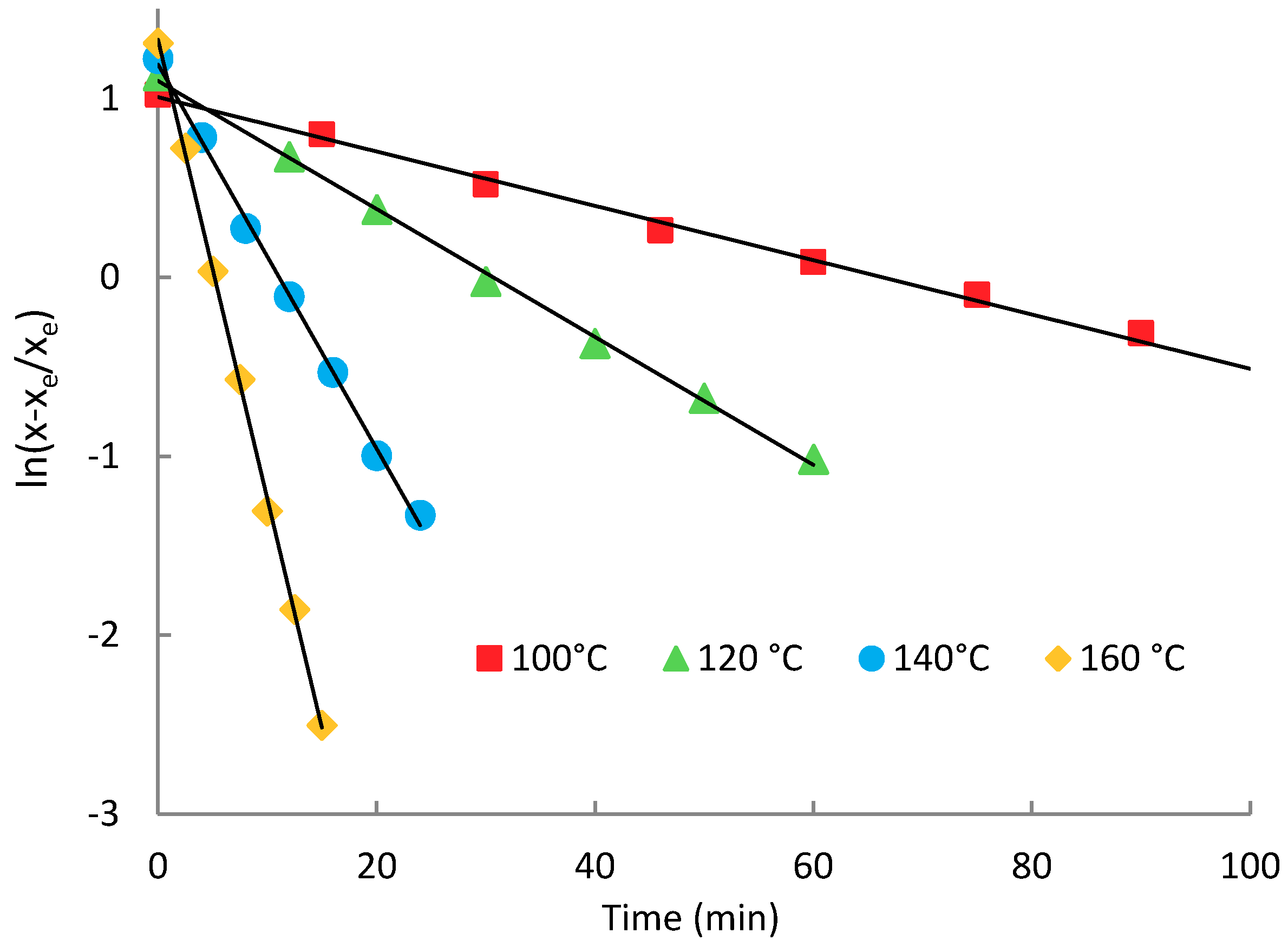
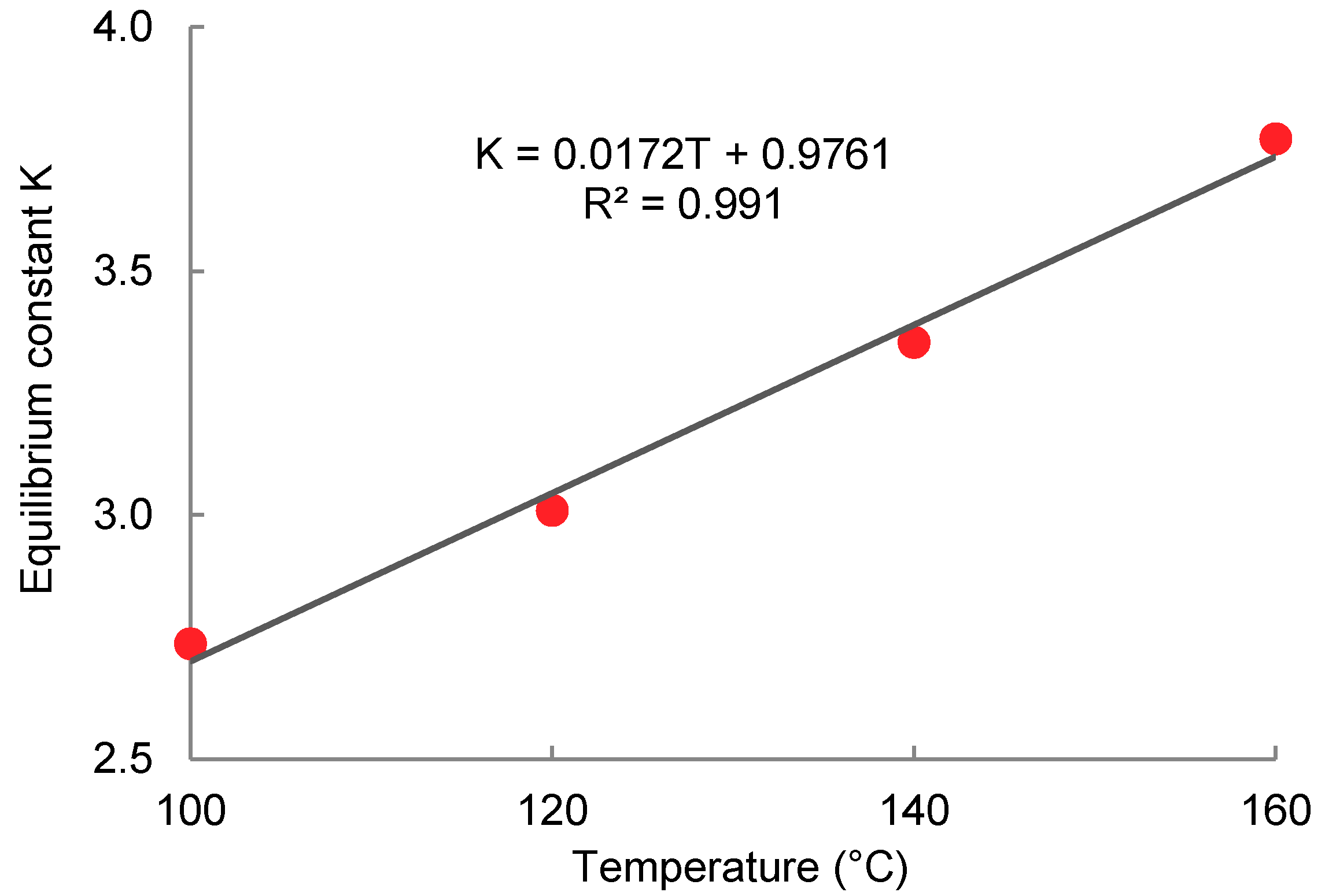
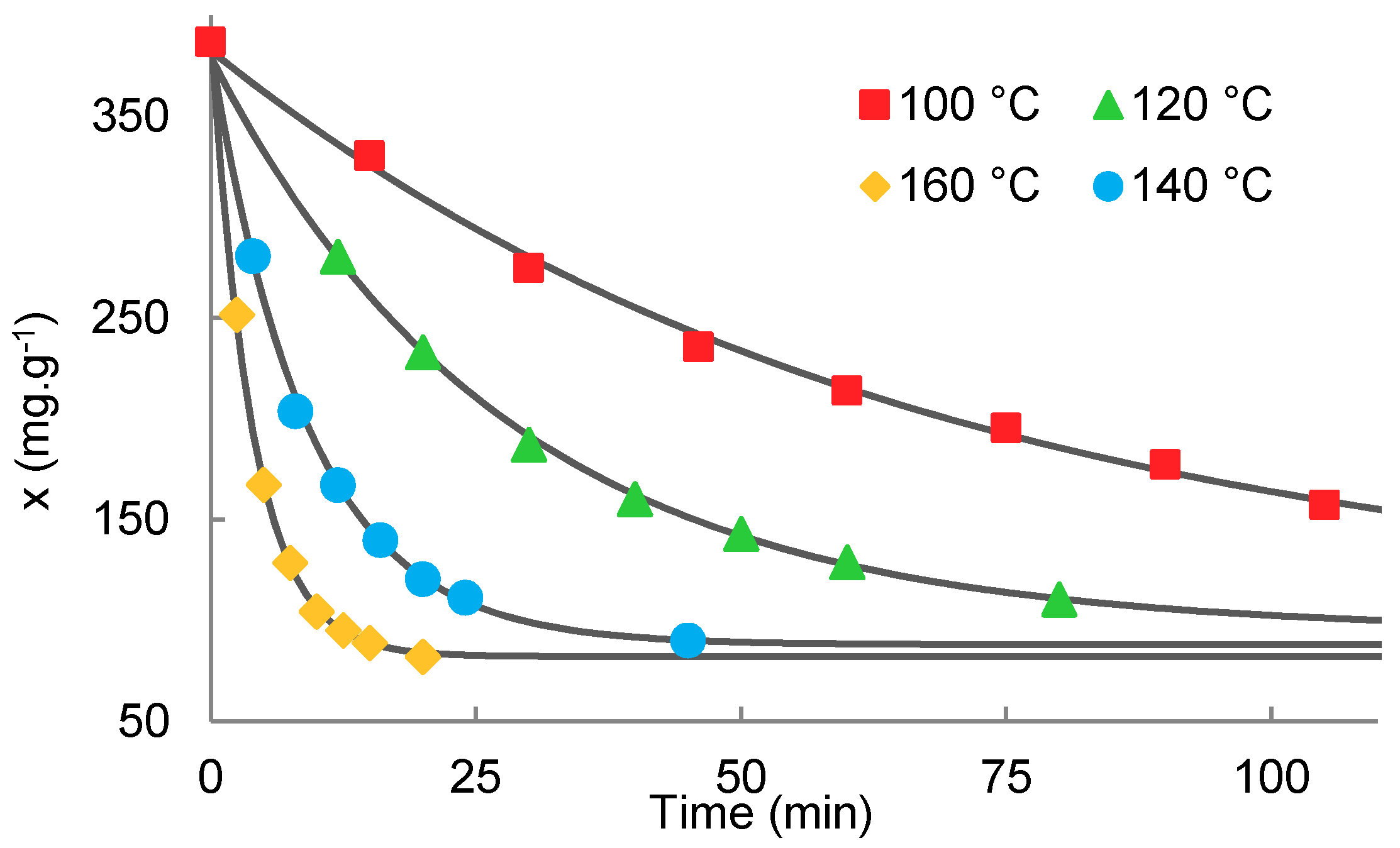
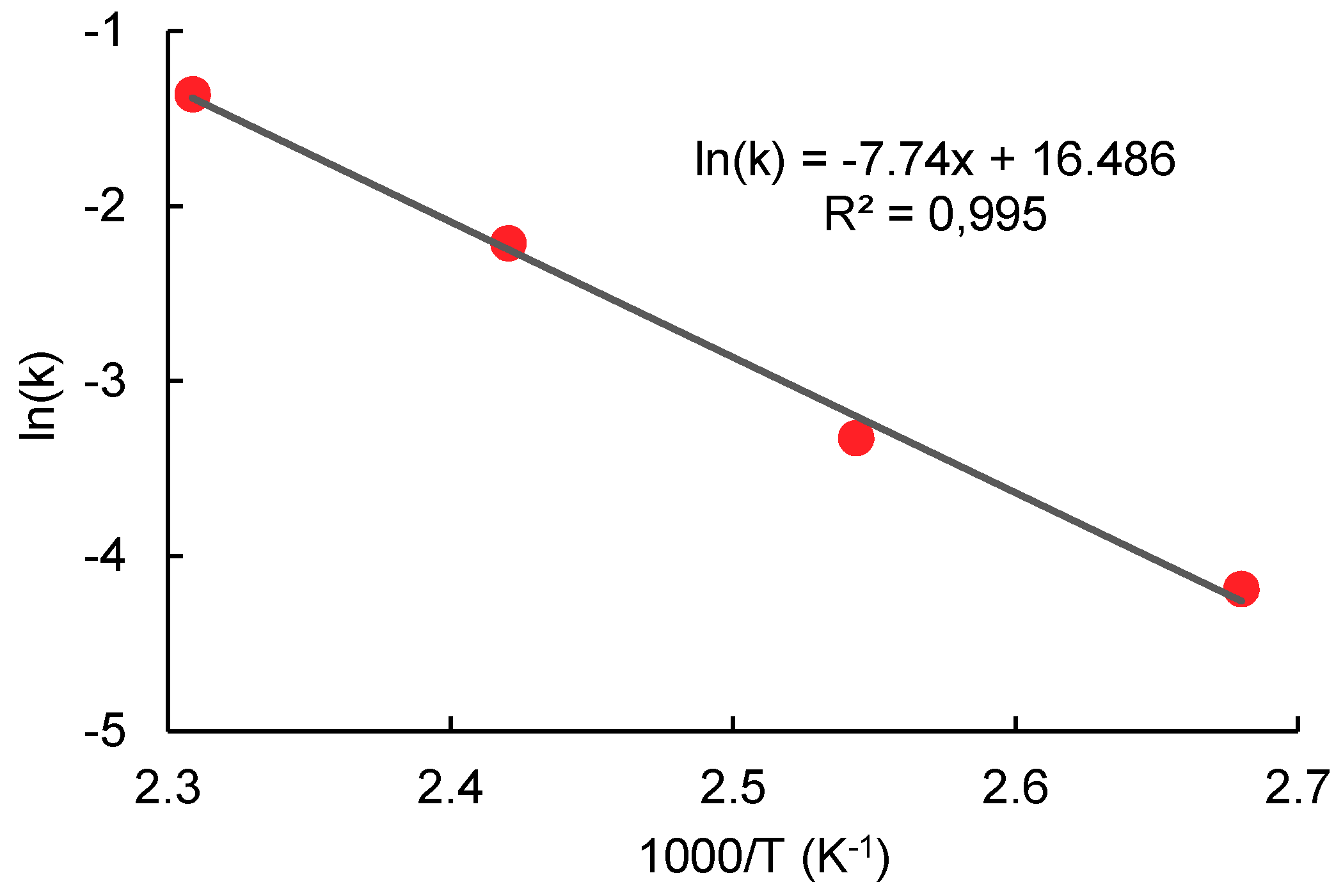
2.4. Transesterification of Oligomer with Ethanol
3. Experimental
3.1. Materials
3.2. Oligomeric LA Preparation
3.3. Oligomer Alcoholysis
4. Instruments and Analysis
4.1. Determination of Molecular Weight and Polymerization Degree of Oligomer
4.2. Ethanol and Ethyl Lactate Concentration Determination by Gas Chromatography
4.3. Melting Point of the Oligomer
4.4. Determination of Water Content
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pereira, C.S.M.; Silva, V.M.N.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes. Green Chem. 2011, 13, 2658–2671. [Google Scholar] [CrossRef]
- Lores, M.; Pájaro, M.; Casas, M.A.; Domínguez, J.; Jares, C. Use of ethyl lactate to extract bioactive compounds from Cytisus scoparius: Comparison of pressurized liquid extraction and medium scale ambient temperature systems. Talanta 2015, 140, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Dandia, A.; Jain, A.K.; Laxkar, A.K. Ethyl lactate as a promising bio based green solvent for the synthesis of spiro-oxindole derivatives via 1,3-dipolar cycloaddition reaction. Tetrahedron Lett. 2013, 54, 3929–3932. [Google Scholar] [CrossRef]
- Procopio, A.; Costanzo, P.; Curini, M.; Nardi, M.; Oliverio, M.; Sindona, G. Erbium(III) Chloride in Ethyl Lactate as a Smart Ecofriendly System for Efficient and Rapid Stereoselective Synthesis of trans-4,5-Diaminocyclopent-2-enones. Sustain. Chem. Eng. 2013, 1, 541–544. [Google Scholar] [CrossRef]
- Esson, J.M.; Scott, R.; Hayes, C.J. Chemistry and Art: Removal of Graffiti Ink from Paints Grounded in a Real-Life Scenario. J. Chem. Educ. 2018, 95, 400–402. [Google Scholar] [CrossRef]
- Hill, E.A.; Carter, J. An Alternative to Chlorinated Solvents for Cleaning Melat Parts. In Proceedings of the 1993 International CFC and Halon Alternatives Conference-Statospheric Ozone Protection for the 90’s, Washington, DC, USA, October 1993; pp. 465–471. [Google Scholar]
- Ahmadkalaei, S.P.J.; Gan, S.; Ng, H.K.; Talib, S.A. Evaluation of ethyl lactate as solvent in Fenton oxidation for the remediation of total petroleum hydrocarbon (TPH)-contaminated soil. Environ. Sci. Pollut. Res. 2017, 24, 17779–17789. [Google Scholar] [CrossRef] [PubMed]
- Ahmadkalaei, S.P.J.; Gan, S.; Ng, H.K.; Talib, S.A. Investigation of ethyl lactate as a green solvent for desorption of total petroleum hydrocarbons (TPH) from contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 22008–22018. [Google Scholar] [CrossRef] [PubMed]
- Steinborn-Rogulska, I.; Parzuchowski, P.; Rokicki, G. Melt/solid-state polytransesterification supported by an inert gas flow—An alternative route for the synthesis of high molar mass poly(l-lactic acid). Polym. Chem. 2014, 5, 5412–5423. [Google Scholar] [CrossRef]
- Adams, T.A.; Seider, W.D. Semicontinuous Distillation for Ethyl Lactate Production. Semicontinuous distillation for ethyl lactate production. AIChE J. 2008, 54, 2539–2552. [Google Scholar] [CrossRef]
- Lira, D.T.; Aspi, N.S.A.; Kolah, K.; Miller, D.J. Vapor–Liquid Equilibria in the Systems Ethyl Lactate + Ethanol and Ethyl Lactate + Water. J. Chem. Eng. Data 2006, 51, 1220–1225. [Google Scholar]
- Komesu, A.; Jaimes, F.J.; Rios, L.M.; Lunelli, O.J.B.; Maciel, R.F.; Wolf, M.M.R. Evaluation of Operational Parameters for Ethyl Lactate Production Using Reactive Distillation Process. Chem. Eng. Trans. 2015, 43, 1141–1146. [Google Scholar]
- Lira, D.T.; Aspi, N.S.A.; Kolah, K.; Miller, D.J.; Vu, D.T. A Continuous Reactive Separation Process for Ethyl Lactate Formation. Org. Process Res. Dev. 2005, 9, 599–607. [Google Scholar]
- Arellano-Garcia, H.; Kraus, R.; Wozny, G. A novel Process Concept for the Production of Ethyl Lactate. In Proceedings of the Distillation Absorption Conference 2010, Eindhoven, The Netherlands, 12–15 September 2010; pp. 240–246. [Google Scholar]
- Troupe, R.A.; DiMilla, E. Kinetics of the Ethyl Alcohol—Lactic Acid Reaction. Ind. Eng. Chem. 1957, 49, 847–855. [Google Scholar] [CrossRef]
- Daengpradab, B.; Rattanaphanee, P. Process Intensification for Production of Ethyl Lactate from Fermentation-Derived Magnesium Lactate: A Preliminary Design. Int. J. Chem. Reactor Eng. 2015, 13, 407–412. [Google Scholar] [CrossRef]
- Asthana, N.S.; Kolah, A.K.; Vu, D.T.; Lira, K.T. A Kinetic Model for the Esterification of Lactic Acid and Its Oligomers. Ind. Eng. Chem. Res. 2006, 45, 5251–5257. [Google Scholar] [CrossRef]
- Petrus, R.; Bykowski, D.; Sobota, P. Solvothermal Alcoholysis Routes for Recycling Polylactide Waste as Lactic Acid Esters. ACS Catal. 2016, 6, 5222–5235. [Google Scholar] [CrossRef]
- Liu, H.; Song, X.; Liu, F.; Liu, S.; Yu, S. Ferric chloride as an efficient and reusable catalyst for methanolysis of poly(lactic acid) waste. J. Polym. Res. 2015, 22, 135–142. [Google Scholar] [CrossRef]
- Nardi, M.; Sindona, G.; Costanzo, P.; Oliverio, M.; Procopio, A. Eco-friendly stereoselective reduction of α,β-unsaturated carbonyl compounds by Er(OTf)3/NaBH4 in 2-MeTHF. Tetrahedron 2015, 71, 1132–1135. [Google Scholar] [CrossRef]
- Begouin, J.M.; Niggemann, M. Calcium-Based Lewis Acid Catalysts. Chem. Eur. J. 2013, 19, 8030–8041. [Google Scholar] [CrossRef] [PubMed]
- Zuoxiang, Z.; Li, C.; Weilan, X.; Jing, C. Recent Developments on the Mechanism and Kinetics of Esterification Reaction Promoted by Various Catalysts. In Chemical Kinetics; Vivek, P., Ed.; InTech: Vienna, Austria, 2012; ISBN 978-953-51-0132-1. [Google Scholar]
- Casas, A.; Ramos, M.J.; Rodríguez, J.F.; Pérez, A. Tin compounds as Lewis acid catalysts for esterification and transesterification of acid vegetable oils. Fuel Process. Technol. 2013, 106, 321–325. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Gao, Z.; Sun, X.; Wang, W.; Niu, R. Transesterification of waste cooking oil using FeCl3-modified resin catalyst and the research of catalytic mechanism. Renew. Energy 2016, 86, 643–650. [Google Scholar] [CrossRef]
- Guan, Q.; Shang, H.; Liu, J.; Gu, J.; Li, B.; Miao, R.; Chen, Q.; Ning, P. Biodiesel from transesterification at low temperature by AlCl3 catalysis in ethanol and carbon dioxide as cosolvent: Process, mechanism and application. Appl. Energy 2016, 164, 380–386. [Google Scholar] [CrossRef]
- Cho, C.S.; Kim, D.T.; Choi, H.J.; Kim, T.J.; Shim, S.C. Catalytic Activity of Tin(II) Chloride in Esterification of Carboxylic Acids with Alcohols. Bull. Korean Chem. Soc. 2002, 23, 539–540. [Google Scholar]
- McMurry, J. Organic Chemistry, 8th ed.; Brooks/Cole: Belmont, CA, USA, 2012; pp. 771–809. ISBN 0840054440. [Google Scholar]
- Beula, C.; Sai, P.S.T. Kinetics of Esterification of Acetic Acid and Ethanol with a Homogeneous Acid. Indian Chem. Eng. 2015, 57, 177–196. [Google Scholar] [CrossRef]
Sample Availability: No samples are available. |
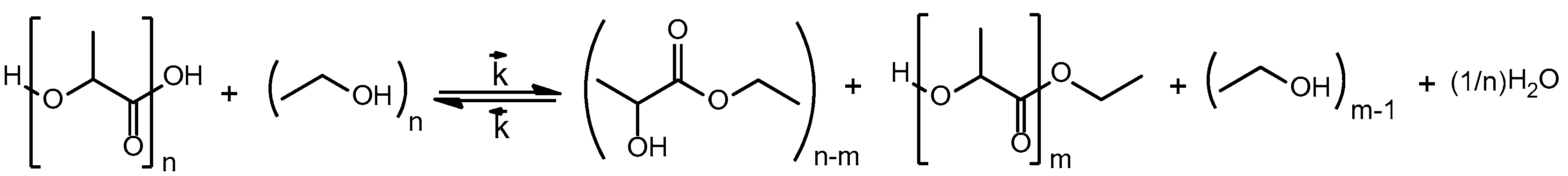

| Property of Oligomer | Value |
|---|---|
| 758.4 g·mol−1 | |
| 10.25 | |
| Melting point | 103.4 °C |
| Yield | 99.8% |
| Free water | 0.049% |
| Lactic acid in condensate | 0.17 |
| Temperature (°C) | x0 (mg·g−1) | K | xe (mg·g−1) | r2 | Half-Time (min) | Conversion (%) | |
|---|---|---|---|---|---|---|---|
| 100 (LA) * | 290 | −0.1643 | 0.921 | 148.00 | 0.995 | 4.22 | 60.8 |
| 100 | 387 | −0.0152 | 2.736 | 102.44 | 0.996 | 45.66 | 72.9 |
| 120 | 387 | −0.0359 | 3.009 | 94.67 | 0.999 | 19.28 | 75.0 |
| 140 | 387 | −0.1093 | 3.354 | 88.03 | 0.999 | 6.34 | 76.7 |
| 160 | 387 | −0.2563 | 3.771 | 82.19 | 0.999 | 2.70 | 78.3 |
| 180 | 387 | −0.3520 | 4.827 | 66.99 | 0.997 | 1.97 | 82.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figalla, S.; Petrůj, J.; Švestková, T. Transesterification of Lactic Acid Oligomers with Ethanol, a Way to Anhydrous Ethyl Lactate: A Kinetic Study. Molecules 2018, 23, 2044. https://doi.org/10.3390/molecules23082044
Figalla S, Petrůj J, Švestková T. Transesterification of Lactic Acid Oligomers with Ethanol, a Way to Anhydrous Ethyl Lactate: A Kinetic Study. Molecules. 2018; 23(8):2044. https://doi.org/10.3390/molecules23082044
Chicago/Turabian StyleFigalla, Silvestr, Jaroslav Petrůj, and Tereza Švestková. 2018. "Transesterification of Lactic Acid Oligomers with Ethanol, a Way to Anhydrous Ethyl Lactate: A Kinetic Study" Molecules 23, no. 8: 2044. https://doi.org/10.3390/molecules23082044
APA StyleFigalla, S., Petrůj, J., & Švestková, T. (2018). Transesterification of Lactic Acid Oligomers with Ethanol, a Way to Anhydrous Ethyl Lactate: A Kinetic Study. Molecules, 23(8), 2044. https://doi.org/10.3390/molecules23082044