Protomer-Dependent Electronic Spectroscopy and Photochemistry of the Model Flavin Chromophore Alloxazine
Abstract
1. Introduction
2. Results
2.1. Gas-Phase and Solution-Phase Absorption Spectroscopy of AL∙H+
2.2. Fragmentation of AL∙H+
2.2.1. Higher-Energy Collisional Dissociation
2.2.2. Photofragmentation Mass Spectroscopy
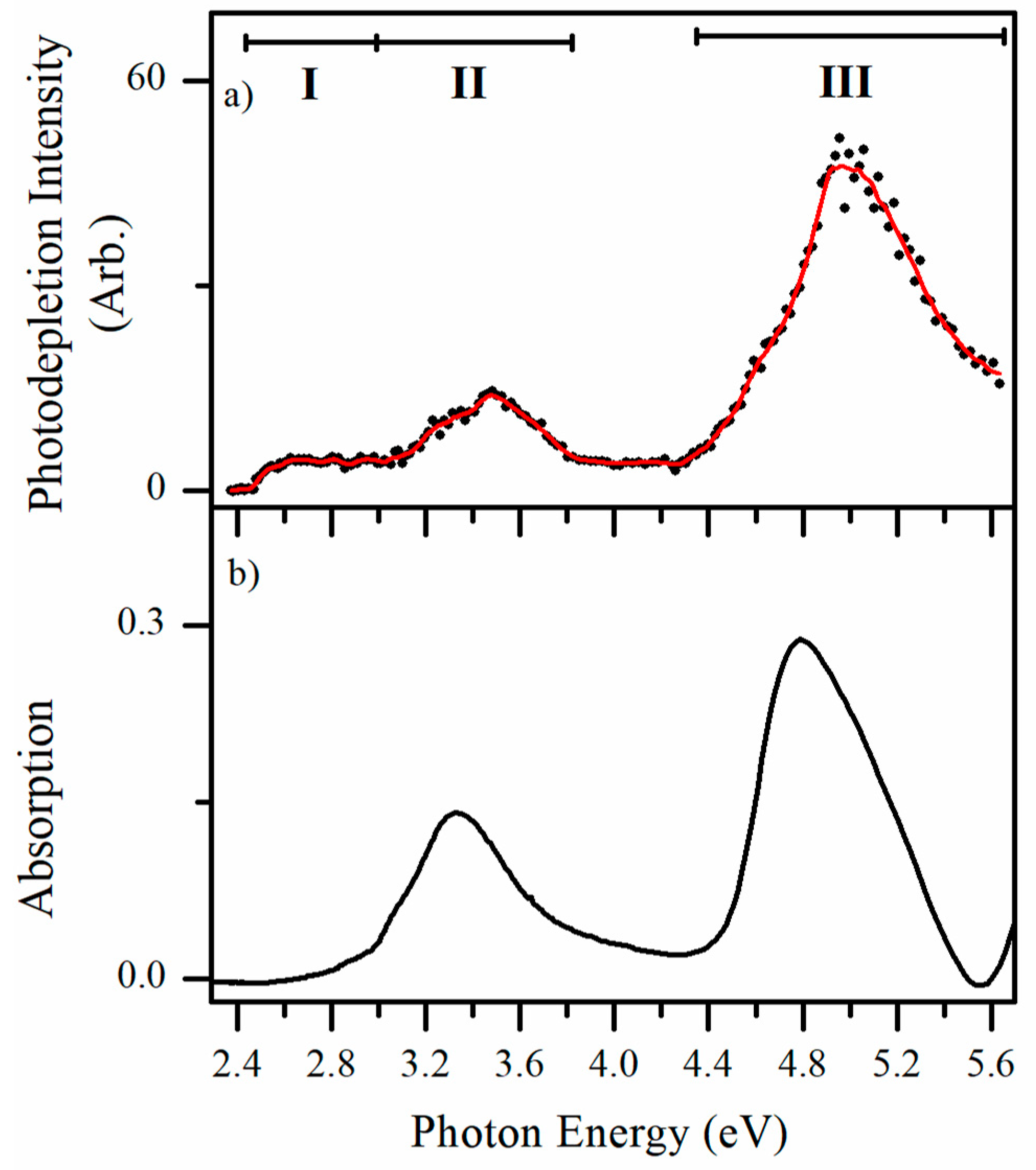
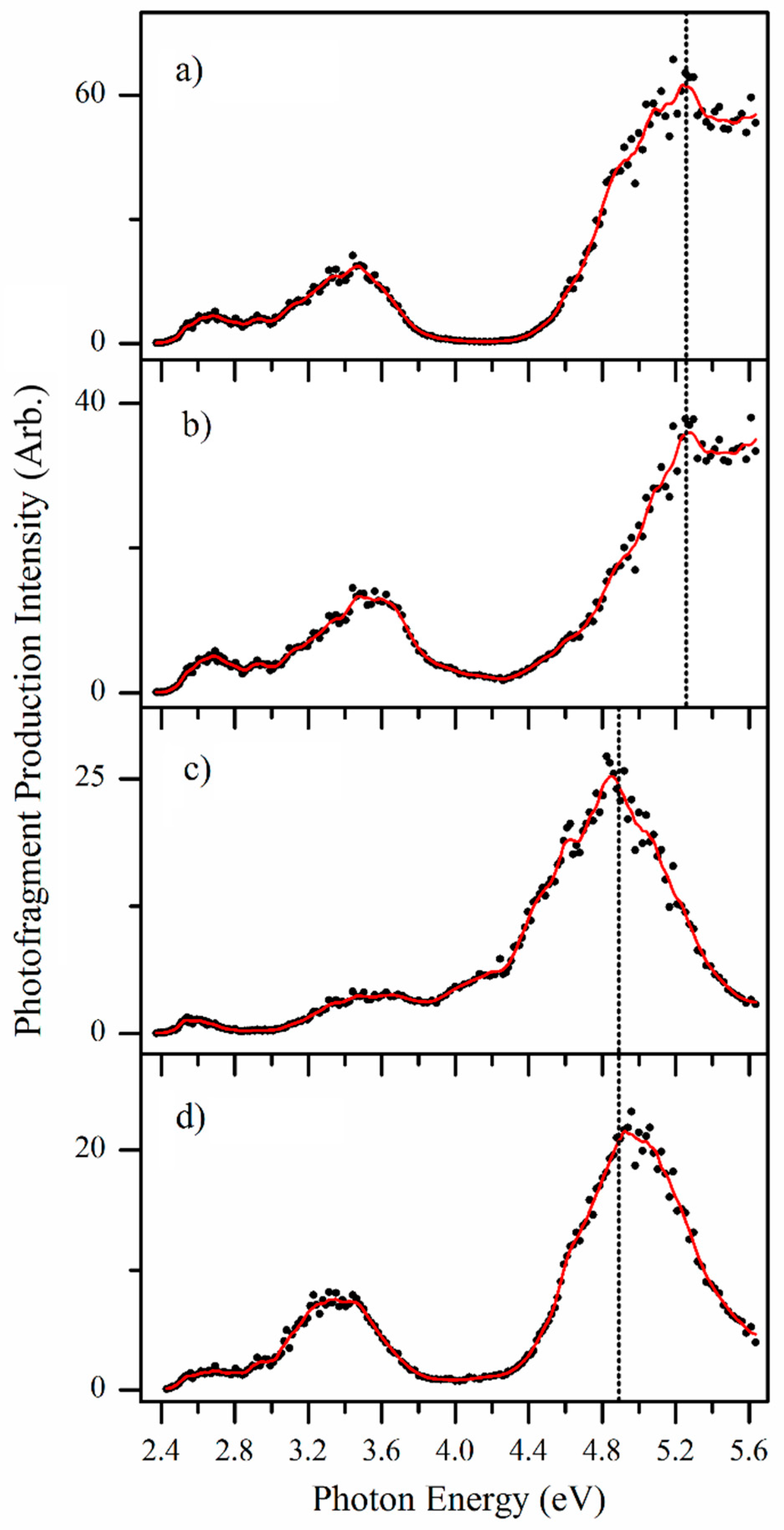
2.2.3. Photofragmentation Action Spectroscopy
3. Discussion
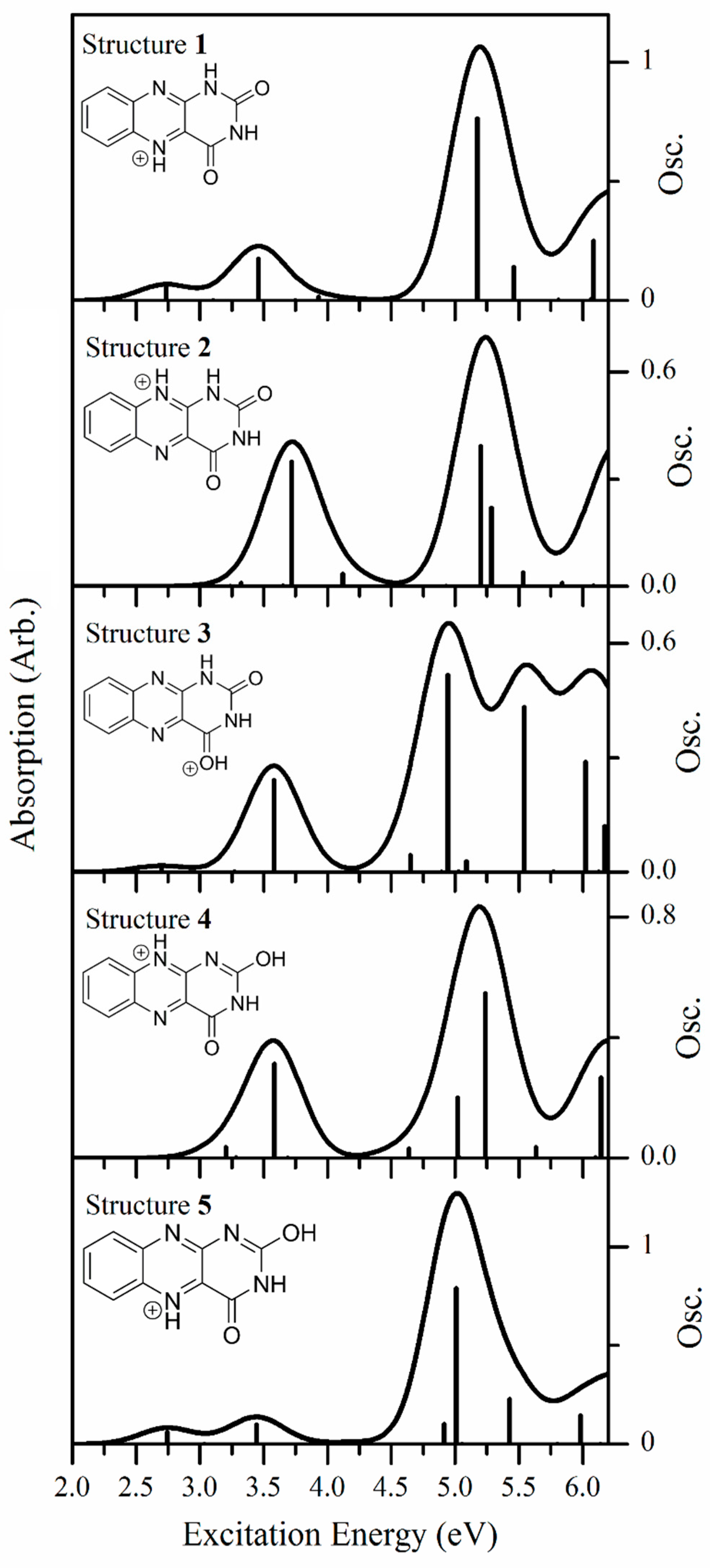
3.1. Assignment of the Protomers of AL∙H+
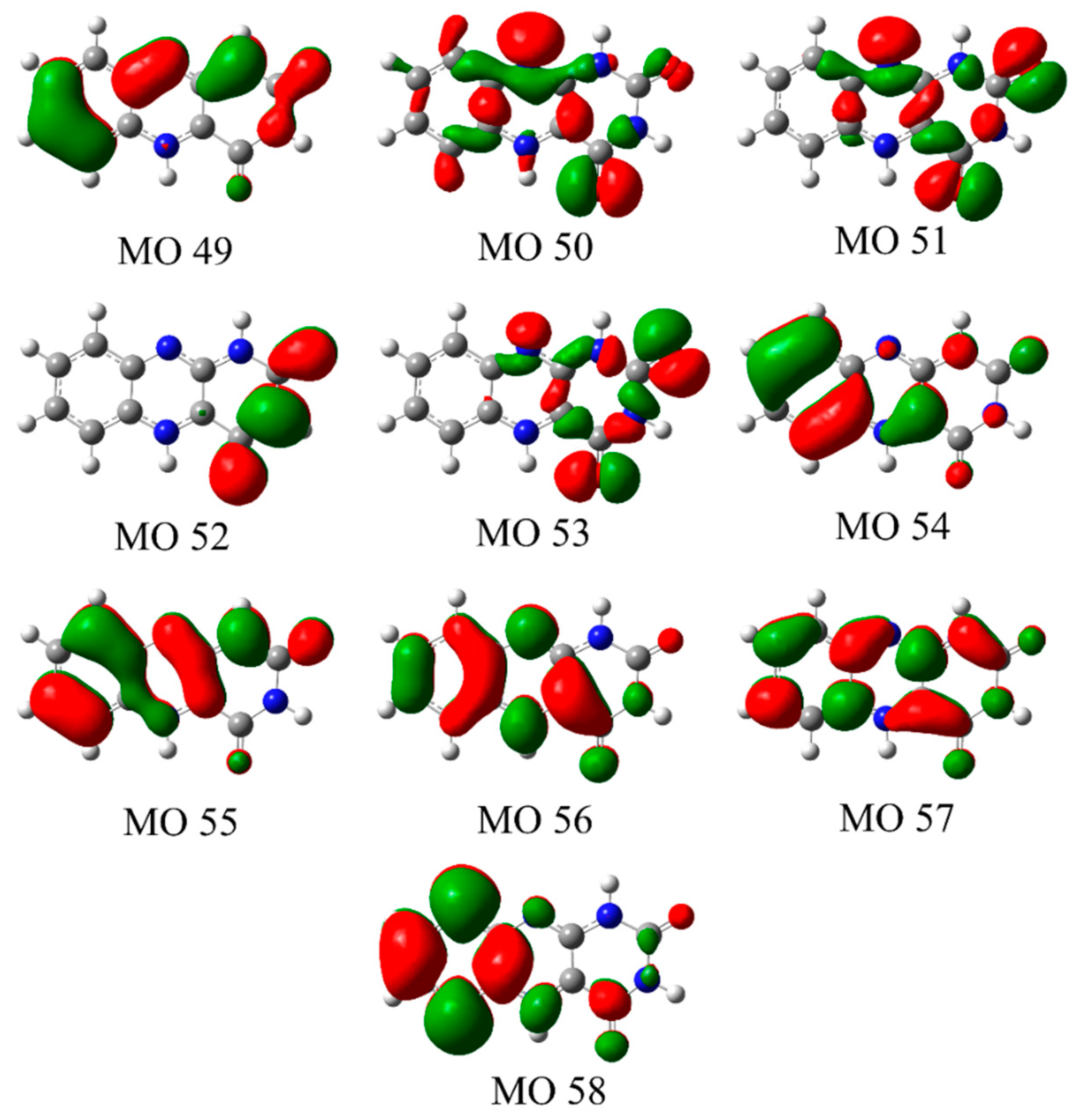
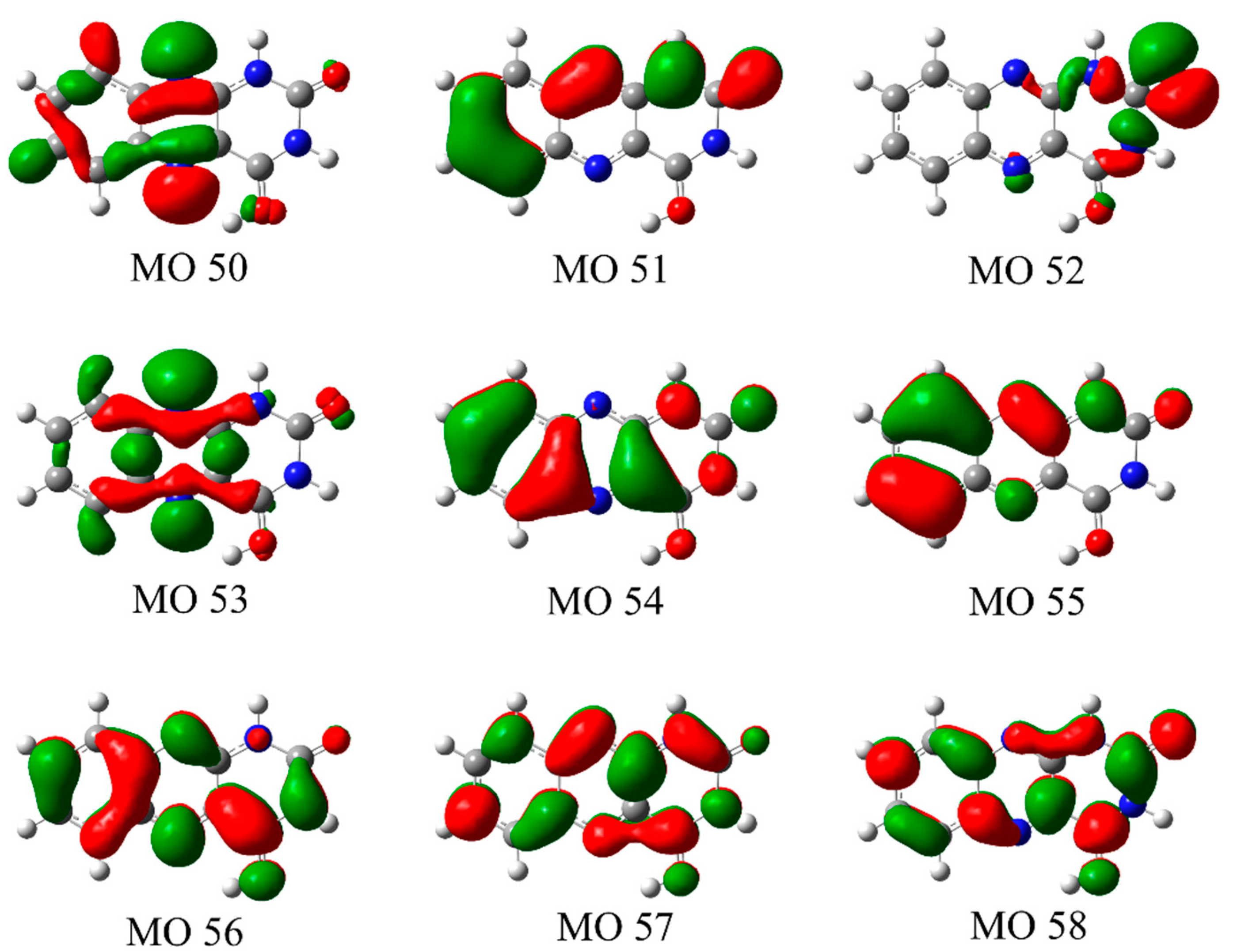
3.2. Nature of the Electronic Transitions of AL∙H+
3.3. Comparison of Protonated Lumichrome with Protonated Alloxazine
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Walsh, C. Flavin coenzymes: At the crossroads of biological redox chemistry. Acc. Chem. Res. 1980, 13, 148–155. [Google Scholar] [CrossRef]
- Conrad, K.S.; Manahan, C.C.; Crane, B.R. Photochemistry of flavoprotein light sensors. Nat. Chem. Biol. 2014, 10, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Losi, A. Flavin-based Blue-light Photosensors: A Photobiophysics Update. Photochem. Photobiol. 2007, 83, 1283–1300. [Google Scholar] [CrossRef] [PubMed]
- Penzkofer, A. Absorption and emission spectroscopic investigation of alloxazine in aqueous solutions and comparison with lumichrome. J. Photochem. Photobiol. A Chem. 2016, 314, 114–124. [Google Scholar] [CrossRef]
- Tyagi, A.; Penzkofer, A. pH dependence of the absorption and emission behaviour of lumiflavin in aqueous solution. J. Photochem. Photobiol. A Chem. 2010, 215, 108–117. [Google Scholar] [CrossRef]
- Kondo, M.; Nappa, J.; Ronayne, K.L.; Stelling, A.L.; Tonge, P.J.; Meech, S.R. Ultrafast Vibrational Spectroscopy of the Flavin Chromophore. J. Phys. Chem. B 2006, 110, 20107–20110. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, S.; Marian, C.M. Effects of protonation and deprotonation on the excitation energies of lumiflavin. Chem. Phys. Lett. 2008, 463, 400–404. [Google Scholar] [CrossRef]
- Salzmann, S.; Tatchen, J.; Marian, C.M. The photophysics of flavins: What makes the difference between gas phase and aqueous solution? J. Photochem. Photobiol. A Chem. 2008, 198, 221–231. [Google Scholar] [CrossRef]
- Salzmann, S.; Marian, C.M. The photophysics of alloxazine: A quantum chemical investigation in vacuum and solution. Photochem. Photobiol. Sci. 2009, 8, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Neiss, C.; Saalfrank, P.; Parac, M.; Grimme, S. Quantum Chemical Calculation of Excited States of Flavin-Related Molecules. J. Phys. Chem. A 2003, 107, 140–147. [Google Scholar] [CrossRef]
- Zanetti-Polzi, L.; Aschi, M.; Daidone, I.; Amadei, A. Theoretical modeling of the absorption spectrum of aqueous riboflavin. Chem. Phys. Lett. 2017, 669, 119–124. [Google Scholar] [CrossRef]
- Chang, X.P.; Xie, X.Y.; Lin, S.Y.; Cui, G. QM/MM Study on Mechanistic Photophysics of Alloxazine Chromophore in Aqueous Solution. J. Phys. Chem. A 2016, 120, 6129–6136. [Google Scholar] [CrossRef] [PubMed]
- Stockett, M.H. Photo-induced proton-coupled electron transfer and dissociation of isolated flavin adenine dinucleotide mono-anions. Phys. Chem. Chem. Phys. 2017, 19, 25829–25833. [Google Scholar] [CrossRef] [PubMed]
- Guyon, L.; Tabarin, T.; Thuillier, B.; Antoine, R.; Broyer, M.; Boutou, V.; Wolf, J.-P.; Dugourd, P. Femtosecond pump-probe experiments on trapped flavin: Optical control of dissociation. J. Chem. Phys. 2008, 128, 075103. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Eriksson, L.A. Absorption Spectra of Riboflavin—A Difficult Case for Computational Chemistry. J. Phys. Chem. A 2010, 114, 10234–10242. [Google Scholar] [CrossRef] [PubMed]
- Matthews, E.; Dessent, C.E.H. Locating the Proton in Nicotinamide Protomers via Low-Resolution UV Action Spectroscopy of Electrosprayed Solutions. J. Phys. Chem. A 2016, 120, 9209–9216. [Google Scholar] [CrossRef] [PubMed]
- Matthews, E.; Dessent, C.E.H. Experiment and theory confirm that UV laser photodissociation spectroscopy can distinguish protomers formed via electrospray. Phys. Chem. Chem. Phys. 2017, 19, 17434–17440. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.N.; Coughlan, N.J.A.; Bieske, E.J. Protomer-Specific Photochemistry Investigated Using Ion Mobility Mass Spectrometry. J. Phys. Chem. A 2017, 121, 6021–6027. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.X.; Wang, X.B.; Wang, L.S.; Kass, S.R. Are Carboxyl Groups the Most Acidic Sites in Amino Acids? Gas-Phase Acidities, Photoelectron Spectra, and Computations on Tyrosine, p-Hydroxybenzoic Acid, and Their Conjugate Bases. J. Am. Chem. Soc. 2008, 131, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Schroder, D.; Budesinsky, M.; Roithova, J. Deprotonation of p-Hydroxybenzoic Acid: Does Electrospray Ionization Sample Solution or Gas-Phase Structures? J. Am. Chem. Soc. 2012, 134, 15897–15905. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.X.; Attygalle, A.B. Effect of Electrospray Ionization Source Conditions on the Tautomer Distribution of Deprotonated p-Hydroxybenzoic Acid in the Gas Phase. Anal. Chem. 2016, 88, 6035–6043. [Google Scholar] [CrossRef] [PubMed]
- Galaverna, R.S.; Bataglion, G.A.; Heerdt, G.; de Sa, G.F.; Daroda, R.; Cunha, V.S.; Morgon, N.H.; Eberlin, M.N. Are Benzoic Acids Always More Acidic Than Phenols? The Case of ortho-, meta-, and para-Hydroxybenzoic Acids. Eur. J. Org. Chem. 2015, 2015, 2189–2196. [Google Scholar] [CrossRef]
- Steill, J.D.; Oomens, J. Gas-Phase Deprotonation of p-Hydroxybenzoic Acid Investigated by IR Spectroscopy: Solution-Phase Structure Is Retained upon ESI. J. Am. Chem. Soc. 2009, 131, 13570–13571. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, A.; Müller, D.; Günther, A.; Nieto, P.; Dopfer, O. Optical spectroscopy of isolated flavins: photodissociation of protonated lumichrome. Phys. Chem. Chem. Phys. 2018, 20, 7407–7414. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Luxford, T.F.M.; Yoshikawa, N.; Dessent, C.E.H. Solvent evaporation versus proton transfer in nucleobase–Pt(CN)4,62− dianion clusters: A collisional excitation and electronic laser photodissociation spectroscopy study. Phys. Chem. Chem. Phys. 2014, 16, 15490–15500. [Google Scholar] [CrossRef] [PubMed]
- Matthews, E.; Sen, A.; Yoshikawa, N.; Bergstrom, E.; Dessent, C.E.H. UV laser photoactivation of hexachloroplatinate bound to individual nucleobases in vacuo as molecular level probes of a model photopharmaceutical. Phys. Chem. Chem. Phys. 2016, 18, 15143–15152. [Google Scholar] [CrossRef] [PubMed]
- Antoine, R.; Dugourd, P. Visible and ultraviolet spectroscopy of gas phase protein ions. Phys. Chem. Chem. Phys. 2011, 13, 16494–16509. [Google Scholar] [CrossRef] [PubMed]
- Wellman, S.M.J.; Jockusch, R.A. Moving in on the Action: An Experimental Comparison of Fluorescence Excitation and Photodissociation Action Spectroscopy. J. Phys. Chem. A 2015, 119, 6333–6338. [Google Scholar] [CrossRef] [PubMed]
- Cercola, R.; Matthews, E.; Dessent, C.E.H. Photoexcitation of Adenosine 5′-Triphosphate Anions in Vacuo: Probing the Influence of Charge State on the UV Photophysics of Adenine. J. Phys. Chem. B 2017, 121, 5553–5561. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Hornbeck, C.L.; Cronin, J.R. Alloxazines and isoalloxazines. Mass spectrometric analysis of riboflavin and related compounds. Org. Mass Spectrom. 1972, 6, 1383–1399. [Google Scholar] [CrossRef]
- Lucas, B.; Barat, M.; Fayeton, J.A.; Jouvet, C.; Çarçabal, P.; Grégoire, G. Statistical versus non-statistical photo-fragmentation of protonated GWG tri-peptide induced by UV excitation. Chem. Phys. 2008, 347, 324–330. [Google Scholar] [CrossRef]
- Boxford, W.E.; Pearce, J.K.; Dessent, C.E.H.D. Ionic fragmentation versus electron detachment in isolated transition metal complex dianions. Chem. Phys. Lett. 2004, 399, 465–470. [Google Scholar] [CrossRef]
- Muller, F. Spectroscopy And Photochemistry of Flavins and Flavoproteins. Photochem. Photobiol. 1981, 34, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Drossler, P.; Holzer, W.; Penzkofer, A.; Hegemann, P. pH dependence of the absorption and emission behaviour of riboflavin in aqueous solution. Chem. Phys. 2002, 28, 429–439. [Google Scholar] [CrossRef]
- Olsen, J.V.; Macek, B.; Lange, O.; Makarov, A.; Horning, S.; Mann, M. Higher-energy C-trap dissociation for peptide modification analysis. Nat. Methods 2007, 9, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 2018, 148, 084304. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Matthews, E.; Cercola, R.; Mensa-Bonsu, G.; Neumark, D.; Dessent, C.E.H. Photoexcitation of iodide ion-pyrimidine clusters above the electron detachment threshold: Intracluster electron transfer versus nucleobase-centred excitations. J. Chem. Phys. 2018, 148, 084304. [Google Scholar] [CrossRef] [PubMed]
- Matsika, S.; Zhou, C.; Kotor, M.; Weinacht, T.C. Combing dissociative ionization with pump-probe spectroscopy and ab initio calculations to interpret dynamics and control through conical intersections. Faraday Discuss. 2011, 153, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Matsika, S.; Kotor, M.; Weinold, T.C. Fragmentation pathways in the uracil radical cation. J. Phys. Chem. A 2012, 116, 9217–9227. [Google Scholar] [CrossRef] [PubMed]
- Fennimore, M.A.; Matsika, S. Core excited and shape resonances in uracil. Phys. Chem. Chem. Phys. 2016, 18, 30536–30545. [Google Scholar] [CrossRef] [PubMed]
- Ashfold, M.N.R.; Bain, M.; Hansen, C.S.; Ingle, R.A.; Karsili, T.N.V.; Marchetti, B.; Murdock, D. Exploring the Dynamics of the Photoinduced Ring-Opening of Heterocyclic Molecules. J. Phys. Chem. Lett. 2017, 8, 3440–3451. [Google Scholar] [CrossRef] [PubMed]
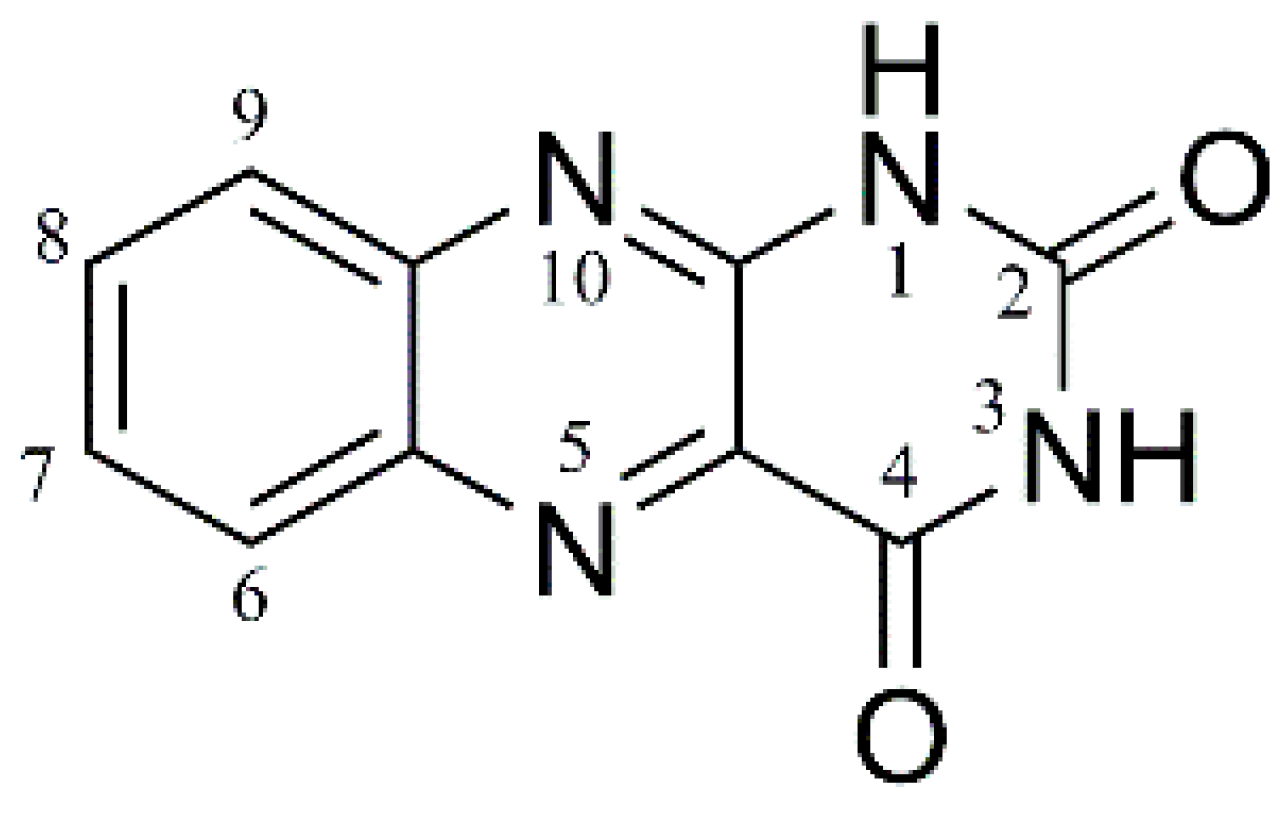
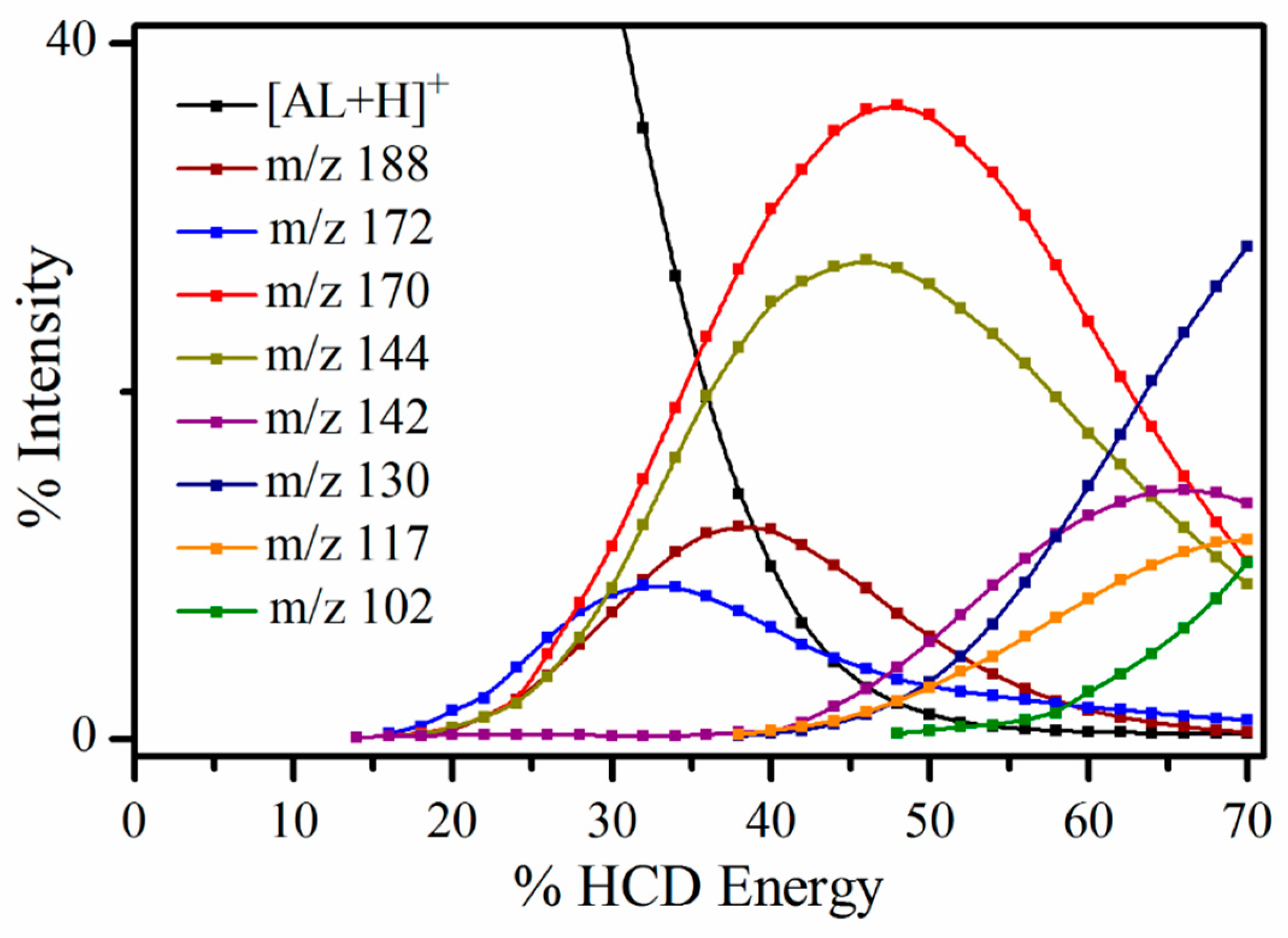
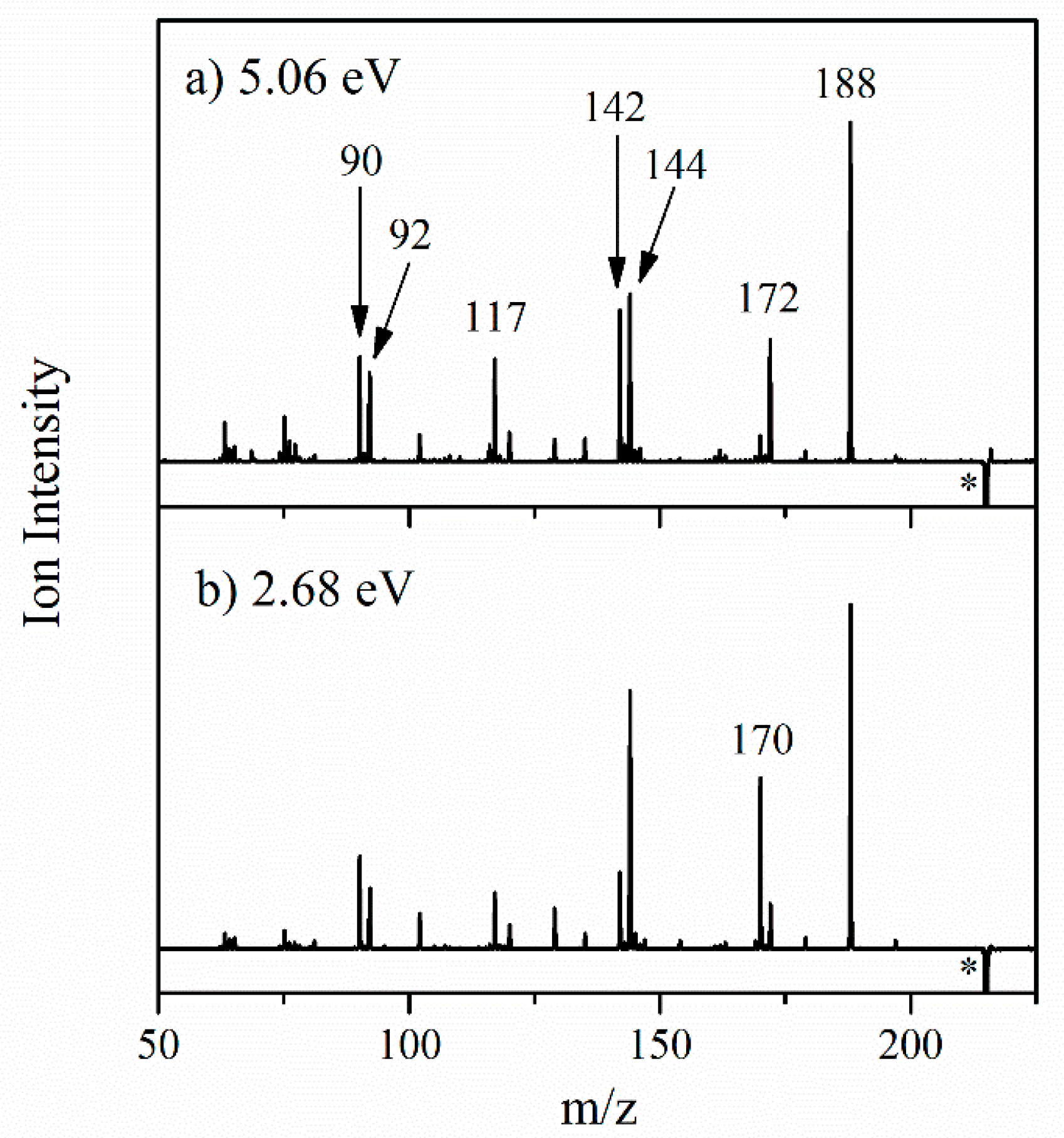
| Photofragment m/z | m/z Lost from AL∙H+ | HCD | 2.68 eV | 5.06 eV | Photofragment Results from Loss of Neutral |
|---|---|---|---|---|---|
| 188 | 27 | ✓ | ✓ (s) | ✓ (vs) | HCN |
| 172 | 43 | ✓ | ✓(w) | ✓ (s) | HNCO a |
| 170 | 45 | ✓ | ✓ (s) | ✓(w) | HCN + H2O |
| 144 | 71 | ✓ | ✓ (s) | ✓ (s) | (HNCO + CO) a Or, HCN + CO2 |
| 142 | 73 | ✓ | ✓(m) | ✓ (s) | HNCO + H2CO Or, HCN + H2O + CO |
| 130 | 85 | ✓ | ✓ (w) | ✓ (w) | HNCO + NCO |
| 117 | 98 | ✓ | ✓ (m) | ✓ (s) | (HNCO + CO + HCN) a |
| 92 | 123 | ✓ | ✓ (s) | ✓ (s) | HNCO + CO + (CN)2 |
| 90 | 125 | ✓ | ✓ (s) | ✓ (s) | HNCO + CO + 2HCN |
| Protomer | H1 | H2 | H3 | Rel. E Gaseous (kJ mol−1) | Rel. E Water (kJ mol−1) |
|---|---|---|---|---|---|
| 1 | N1 | N3 | N5 | 0.0 | 0.0 |
| 2 | N1 | N3 | N10 | 27.2 | 0.22 |
| 3 | N1 | N3 | O4 | 19.5 | 36.3 |
| 4 | O2 | N3 | N10 | 17.8 | 28.3 |
| 5 | O2 | N3 | N5 | 37.1 | 50.4 |
| State | Orbital Transitions | ΔE (eV) | f |
|---|---|---|---|
| S1 | (0.92) 55(π) → 56(π*) | 2.77 | 0.0542 |
| S2 | (0.84) 53(n) → 56(π*) | 3.09 | 0.0004 |
| S3 | (0.85) 54(π) → 56(π*) | 3.49 | 0.1650 |
| S4 | (0.61) 51(n) → 56(π*) + (0.25) 50(n) → 56(π*) | 3.74 | 0.0003 |
| S5 | (0.99) 52(π) → 56(π*) | 3.92 | 0.0129 |
| S6 | (0.72) 50(n) → 56(π*) + (0.23) 51(n) → 56(π*) | 4.02 | 0.0000 |
| S7 | (0.81) 49(π) → 56(π*) | 4.55 | 0.0017 |
| S8 | (0.81) 55(π) → 57(π*) | 5.21 | 0.7409 |
| S9 | (0.87) 53(n) → 57(π*) | 5.29 | 0.0000 |
| S10 | (0.66) 54(π) → 57(π*) + (0.23) 55(π) → 58(π*) | 5.50 | 0.1594 |
| S11 | (0.61) 51(n) → 57(π*) | 5.79 | 0.0000 |
| State | Orbital Transitions | ΔE (eV) | f |
|---|---|---|---|
| S1 | (0.99) 55(π) → 56(π*) | 2.74 | 0.0149 |
| S2 | (0.98) 53(n) → 56(π*) | 3.28 | 0.0010 |
| S3 | (0.88) 54(π) → 56(π*) | 3.62 | 0.2330 |
| S4 | (0.96) 52(n) → 56(π*) | 4.51 | 0.0001 |
| S5 | (0.72) 51(π) → 56(π*) + (0.23) 55(π) → 57(π*) | 4.69 | 0.0343 |
| S6 | (0.94) 50(n) → 56(π*) | 4.92 | 0.0001 |
| S7 | (0.59) 55(π) → 57(π*) + (0.20) 51(π) → 56(π*) | 5.02 | 0.5433 |
| S8 | (0.98) 53(n) → 57(π*) | 5.04 | 0.0005 |
| S9 | (0.78) 54(π) → 57(π*) | 5.14 | 0.0282 |
| S10 | (0.78) 55(π) → 58(π*) | 5.61 | 0.3970 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matthews, E.; Cercola, R.; Dessent, C.E.H. Protomer-Dependent Electronic Spectroscopy and Photochemistry of the Model Flavin Chromophore Alloxazine. Molecules 2018, 23, 2036. https://doi.org/10.3390/molecules23082036
Matthews E, Cercola R, Dessent CEH. Protomer-Dependent Electronic Spectroscopy and Photochemistry of the Model Flavin Chromophore Alloxazine. Molecules. 2018; 23(8):2036. https://doi.org/10.3390/molecules23082036
Chicago/Turabian StyleMatthews, Edward, Rosaria Cercola, and Caroline E. H. Dessent. 2018. "Protomer-Dependent Electronic Spectroscopy and Photochemistry of the Model Flavin Chromophore Alloxazine" Molecules 23, no. 8: 2036. https://doi.org/10.3390/molecules23082036
APA StyleMatthews, E., Cercola, R., & Dessent, C. E. H. (2018). Protomer-Dependent Electronic Spectroscopy and Photochemistry of the Model Flavin Chromophore Alloxazine. Molecules, 23(8), 2036. https://doi.org/10.3390/molecules23082036






