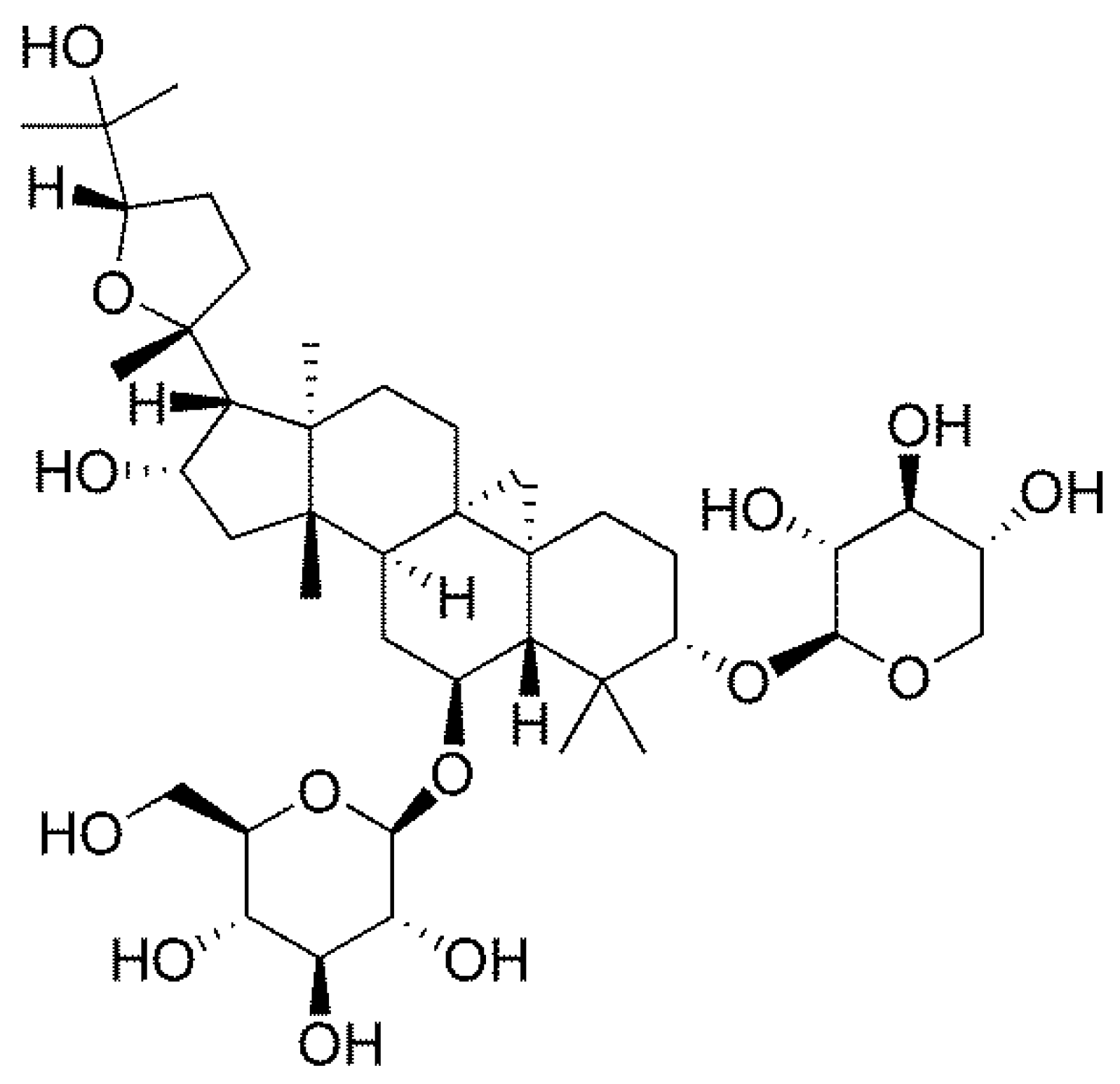
Astragaloside IV Attenuates Acetaminophen-Induced Liver Injuries in Mice by Activating the Nrf2 Signaling Pathway
Abstract
1. Introduction
2. Results
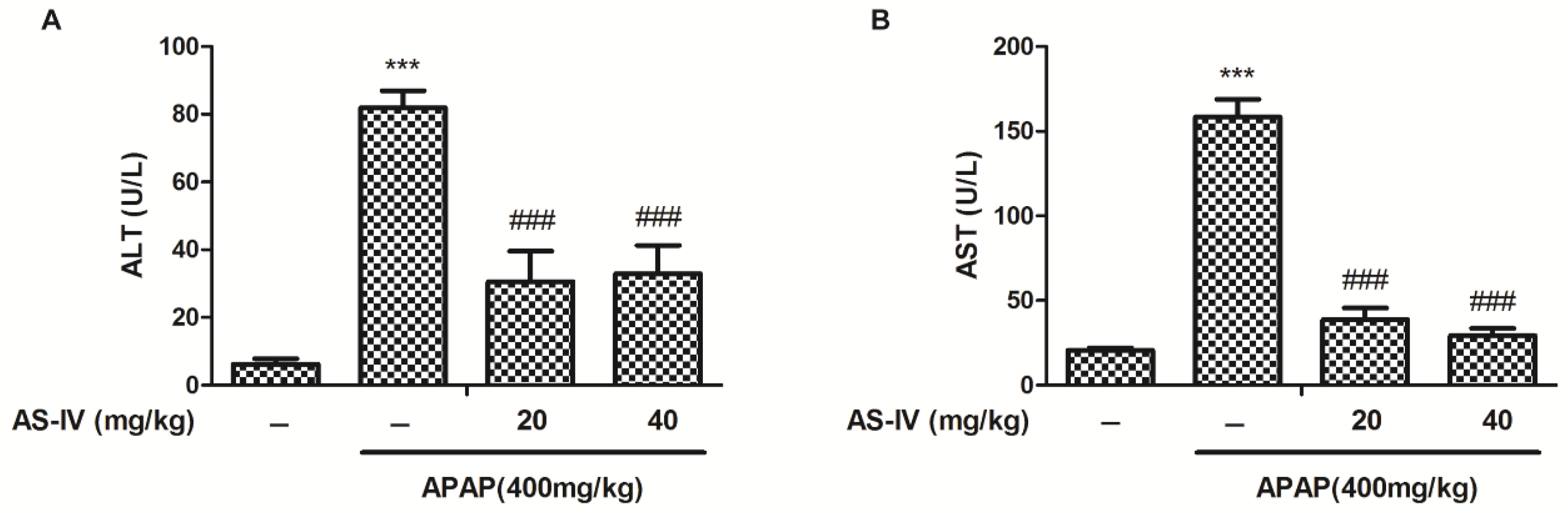
2.1. AS-IV Attenuates APAP-Induced Elevation of Serum Alanine/Aspartate Aminotransferase (ALT/AST)
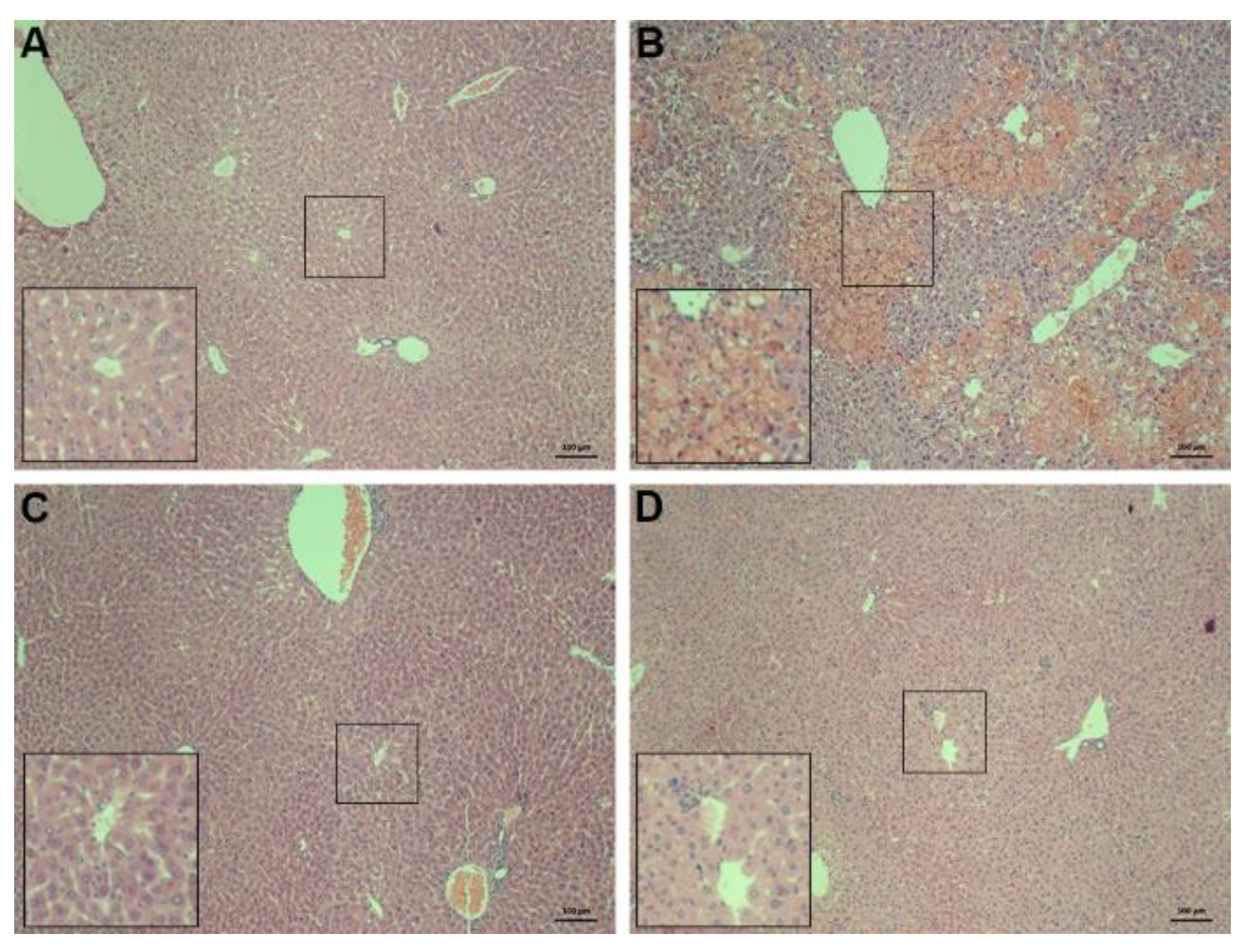
2.2. Histopathology of the Liver
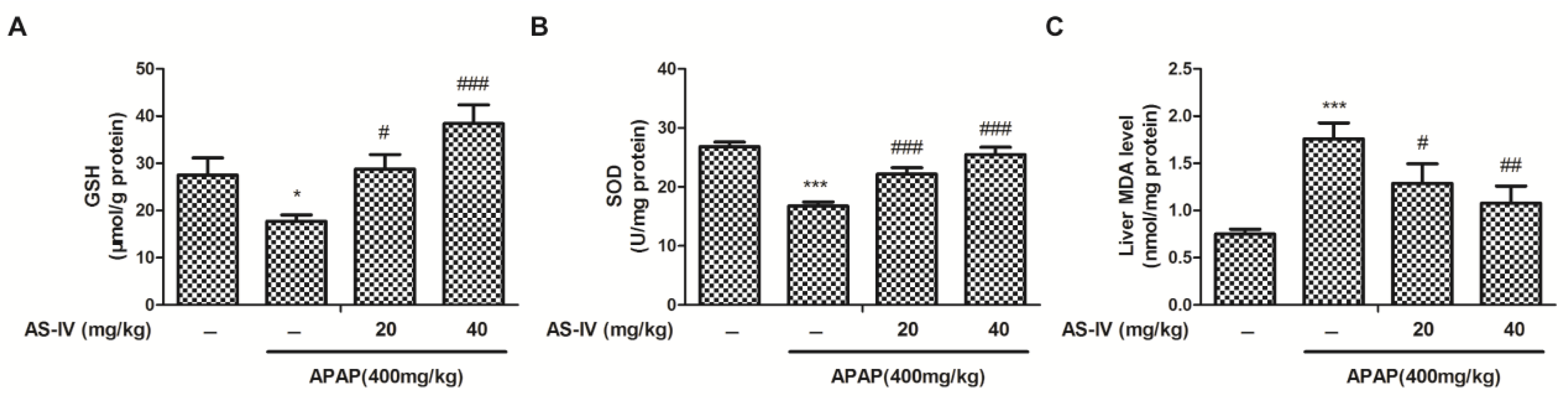
2.3. AS-IV Suppressed APAP-Induced Oxidative Stress
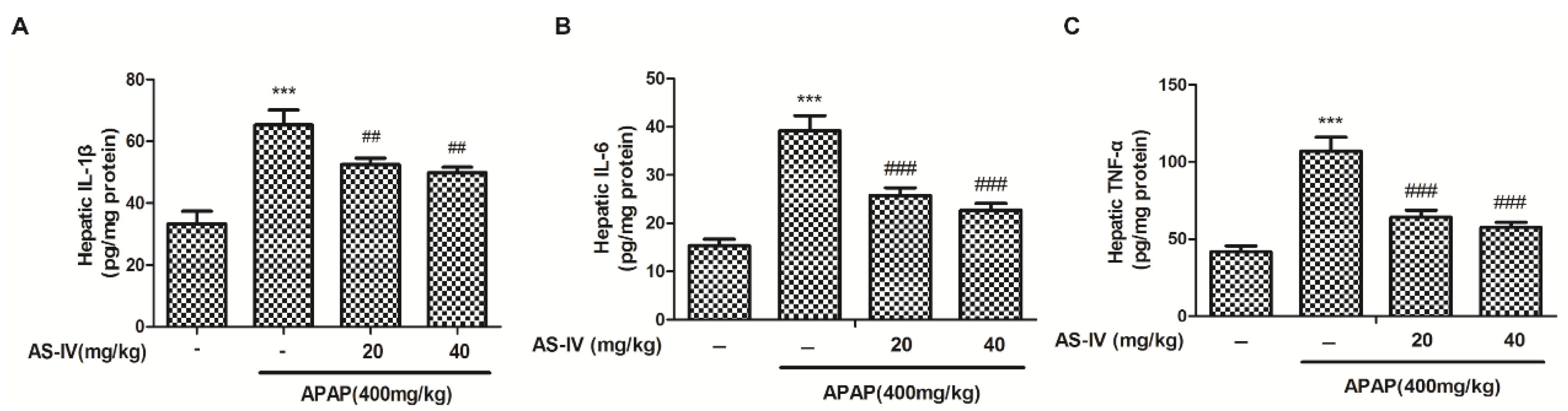
2.4. AS-IV Suppressed APAP-Induced Inflammatory Injury
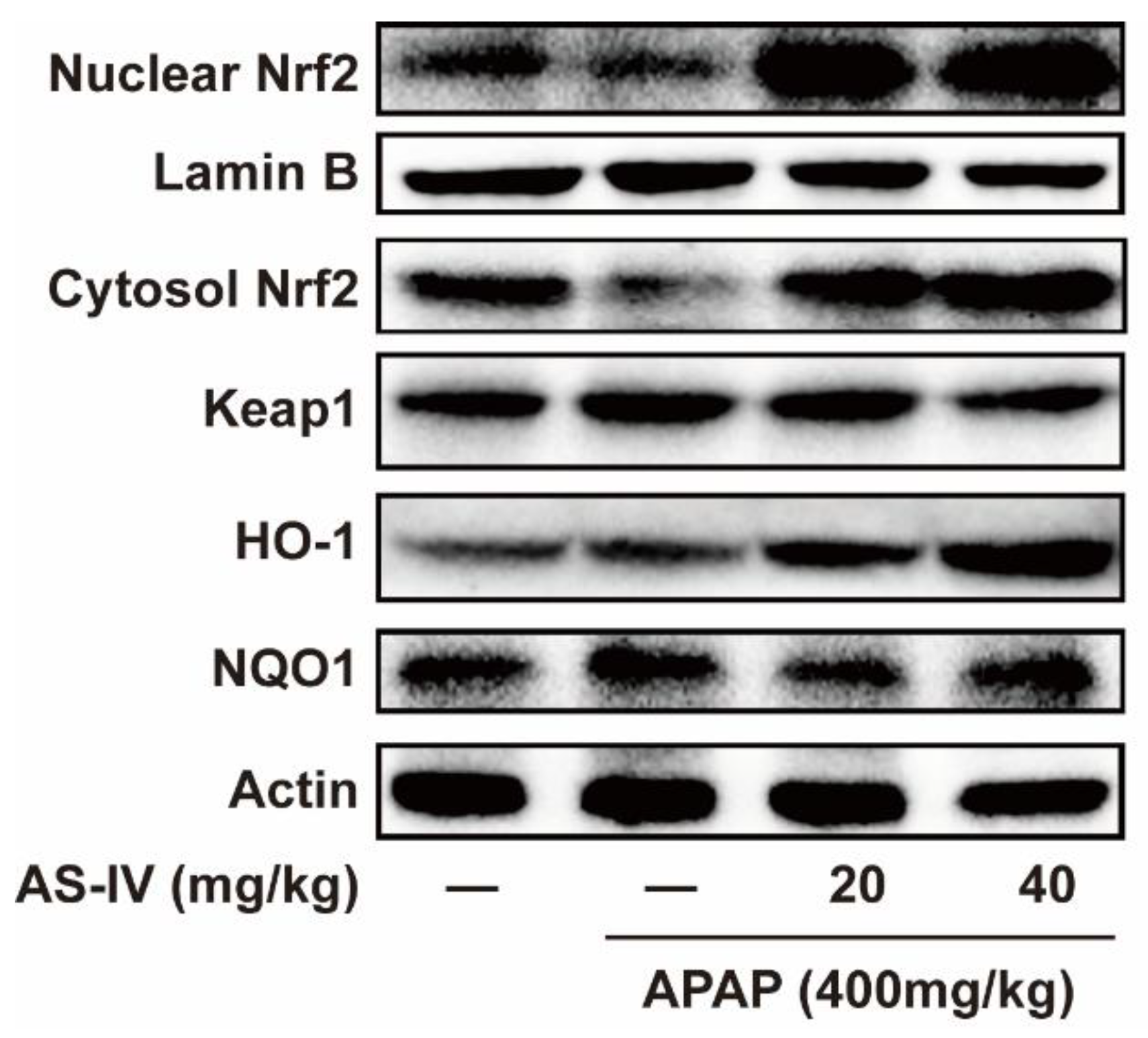
2.5. AS-IV StimulateNrf2 Pathway to Protect APAP-Induced Liver Injury
3. Discussion
4. Materials and Methods
4.1. Chemical Compounds and Reagents
4.2. Animals and Treatments
4.3. Biochemical Analysis of Serum ALT and AST Activities
4.4. Liver Histological Examination
4.5. Analysis of Liver GSH and MDA Amount
4.6. Analysis of Liver SOD Activity
4.7. Analysis of Serum and Hepatic TNF-α, IL-1β and IL-6 Levels
4.8. Protein Extraction
4.9. Western-Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALF | Acute liver failure | Keap1 | Kelch-like ECH-associated protein-1 |
| ALT/AST | Alanine/aspartate aminotransferase | MDA | Malondialdehyde |
| APAP | Acetaminophen | NAPQI | N-acetyl-p-benzoquinoneimine |
| AS-IV | Astragaloside IV | NF-κB | Nuclear factor kB |
| DILI | Drug-induced liver injury | NQO1 | NAD(P)H: quinone oxidoreductase 1 |
| GSH | Glutathione | Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| HO-1 | Heme oxygenase 1 | SOD | Superoxide dismutase |
| IL-1β, 6 | Interleukin 1β, 6 | TNF-α | Tumor necrosis factor α |
References
- Blieden, M.; Paramore, L.C.; Shah, D.; Ben-Joseph, R. A perspective on the epidemiology of acetaminophen exposure and toxicity in the United States. Expert Rev. Clin. Pharmacol. 2014, 7, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, S.; Bi, J.; Gu, J.; Deng, Y.; Liu, C. Astaxanthin pretreatment attenuates acetaminophen-induced liver injury in mice. Int. Immunopharmacol. 2017, 45, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, K.M.; Yang, J.H.; Cho, S.S.; Kim, J.Y.; Park, S.J.; Lee, S.K.; Ku, S.K.; Cho, I.J.; Ki, S.H. Sestrin2 protects against acetaminophen-induced liver injury. Chem. Biol. Interact. 2017, 269, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Hu, J.N.; Liu, Z.; Zhang, R.; He, Y.F.; Hou, W.; Wang, Z.Q.; Yang, G.; Li, W. Saponins (Ginsenosides) from the Leaves of Panax quinquefolius Ameliorated Acetaminophen-Induced Hepatotoxicity in Mice. J. Agric. Food Chem. 2017, 65, 3684–3692. [Google Scholar] [CrossRef] [PubMed]
- Coen, M. Metabolic phenotyping applied to pre-clinical and clinical studies of acetaminophen metabolism and hepatotoxicity. Drug Metab. Rev. 2015, 47, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.W.; Yang, S.X.; Pan, Z.Z.; Zheng, B.; Wang, J.Z.; Lu, G.R.; Xue, Z.X.; Xu, C.L. Andrographolide ameliorates d-galactosamine/lipopolysaccharide-induced acute liver injury by activating Nrf2 signaling pathway. Oncotarget 2017, 8, 41202–41210. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, Y.; Zhang, X. Astragaloside IV Alleviates Lipopolysaccharide-Induced Acute Kidney Injury Through Down-Regulating Cytokines, CCR5 and p-ERK, and Elevating Anti-Oxidative Ability. Med. Sci. Monit. 2017, 23, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Frei, B. Astragaloside IV inhibits NF-kappa B activation and inflammatory gene expression in LPS-treated mice. Mediat. Inflamm. 2015, 2015, 274314. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yi, J.; Gong, X.; Ge, P.; Dai, J.; Lin, L.; Xing, Y.; Zhang, L. Anti-oxidative and anti-inflammatory benefits of the ribonucleoside analogue 5-azacitidine in mice with acetaminophen-induced toxic hepatitis. Int. Immunopharmacol. 2017, 48, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Bunchorntavakul, C.; Reddy, K.R. Acetaminophen (APAP or N-Acetyl-p-Aminophenol) and Acute Liver Failure. Clin. Liver Dis. 2018, 22, 325–346. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R.; Jaeschke, H. Mechanistic biomarkers in acetaminophen-induced hepatotoxicity and acute liver failure: From preclinical models to patients. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hou, X.; Xu, R.; Liu, C.; Tu, M. Research review on the pharmacological effects of astragaloside IV. Fundam. Clin. Pharmacol. 2017, 31, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, J.; Wang, C.; Qu, S.; Zhu, Y.; Yang, Z.; Wang, L. Acai (Euterpe oleracea Mart.) attenuates alcohol-induced liver injury in rats by alleviating oxidative stress and inflammatory response. Exp. Ther. Med. 2018, 15, 166–172. [Google Scholar] [PubMed]
- Balaban, Y.H.; Aka, C.; Koca-Caliskan, U. Liver immunology and herbal treatment. World J. Hepatol. 2017, 9, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Huang, Z.; Bai, Q.; Sheng, Y.; Hao, Z.; Wang, Z.; Ji, L. Natural product andrographolide alleviated APAP-induced liver fibrosis by activating Nrf2 antioxidant pathway. Toxicology 2018, 396–397, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Sheng, Y.; Lu, B.; Ji, L. The therapeutic detoxification of chlorogenic acid against acetaminophen-induced liver injury by ameliorating hepatic inflammation. Chem. Biol. Interact. 2015, 238, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Ma, Y.; Wang, Z.; Cai, Z.; Pang, C.; Wang, Z. Quercetin prevents pyrrolizidine alkaloid clivorine-induced liver injury in mice by elevating body defense capacity. PLoS ONE 2014, 9, e98970. [Google Scholar] [CrossRef] [PubMed]
- Saito, C.; Zwingmann, C.; Jaeschke, H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology 2010, 51, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, F.; Mathur, A.; Kakkar, P. Morin mitigates acetaminophen-induced liver injury by potentiating Nrf2 regulated survival mechanism through molecular intervention in PHLPP2-Akt-Gsk3beta axis. Apoptosis 2015, 20, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Gum, S.I.; Cho, M.K. Recent updates on acetaminophen hepatotoxicity: The role of nrf2 in hepatoprotection. Toxicol. Res. 2013, 29, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 2004, 36, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, M.; Xiong, Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Mol. Cell. Biol. 2005, 25, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Garcia, C.; Ferrer-Mayorga, G.; Gonzalez-Rodriguez, A.; Valverde, A.M.; Martin-Duce, A.; Velasco-Martin, J.P.; Regadera, J.; Fernandez, M.; Alemany, S. Sterile inflammation in acetaminophen-induced liver injury is mediated by Cot/tpl2. J. Biol. Chem. 2013, 288, 15342–15351. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Karin, M. Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Bolati, D.; Higashiyama, Y.; Nishijima, F.; Shimizu, K.; Niwa, T. Indoxyl sulfate upregulates renal expression of MCP-1 via production of ROS and activation of NF-kappaB, p53, ERK, and JNK in proximal tubular cells. Life Sci. 2012, 90, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Liu, J.; Bian, H.; Cai, J. Astragaloside IV ameliorates necrotizing enterocolitis by attenuating oxidative stress and suppressing inflammation via the vitamin D3-upregulated protein 1/NF-kappaB signaling pathway. Exp. Ther. Med. 2016, 12, 2702–2708. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Sun, X.; Gong, X.; Yang, Y.; Chen, C.; Shan, G.; Yao, Q. Astragaloside IV from Astragalus membranaceus ameliorates renal interstitial fibrosis by inhibiting inflammation via TLR4/NF-κB in vivo and in vitro. Int. Immunopharmacol. 2017, 42, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Zheng, Z.; Shi, L.; Sheng, Y.; Wei, H.; Wang, Z.; Ji, L. Caffeic acid prevents acetaminophen-induced liver injury by activating the Keap1-Nrf2 antioxidative defense system. Free Radic. Biol. Med. 2016, 91, 236–246. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Huang, W.; Wang, S.; Sun, K.; Zhang, W.; Ding, Y.; Zhang, L.; Tumen, B.; Ji, L.; Liu, C. Astragaloside IV Attenuates Acetaminophen-Induced Liver Injuries in Mice by Activating the Nrf2 Signaling Pathway. Molecules 2018, 23, 2032. https://doi.org/10.3390/molecules23082032
Li L, Huang W, Wang S, Sun K, Zhang W, Ding Y, Zhang L, Tumen B, Ji L, Liu C. Astragaloside IV Attenuates Acetaminophen-Induced Liver Injuries in Mice by Activating the Nrf2 Signaling Pathway. Molecules. 2018; 23(8):2032. https://doi.org/10.3390/molecules23082032
Chicago/Turabian StyleLi, Lei, Wenxiang Huang, Shoukai Wang, Kecheng Sun, Wenxue Zhang, Yanmei Ding, Le Zhang, Bayaer Tumen, Lili Ji, and Chang Liu. 2018. "Astragaloside IV Attenuates Acetaminophen-Induced Liver Injuries in Mice by Activating the Nrf2 Signaling Pathway" Molecules 23, no. 8: 2032. https://doi.org/10.3390/molecules23082032
APA StyleLi, L., Huang, W., Wang, S., Sun, K., Zhang, W., Ding, Y., Zhang, L., Tumen, B., Ji, L., & Liu, C. (2018). Astragaloside IV Attenuates Acetaminophen-Induced Liver Injuries in Mice by Activating the Nrf2 Signaling Pathway. Molecules, 23(8), 2032. https://doi.org/10.3390/molecules23082032




