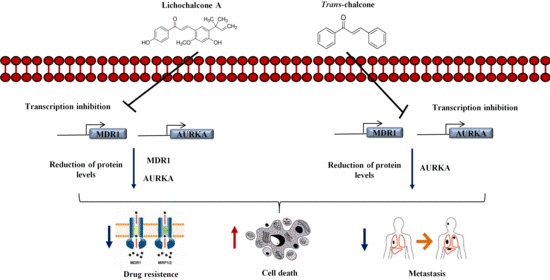
Chalcones Repressed the AURKA and MDR Proteins Involved in Metastasis and Multiple Drug Resistance in Breast Cancer Cell Lines
Abstract
1. Introduction
2. Results
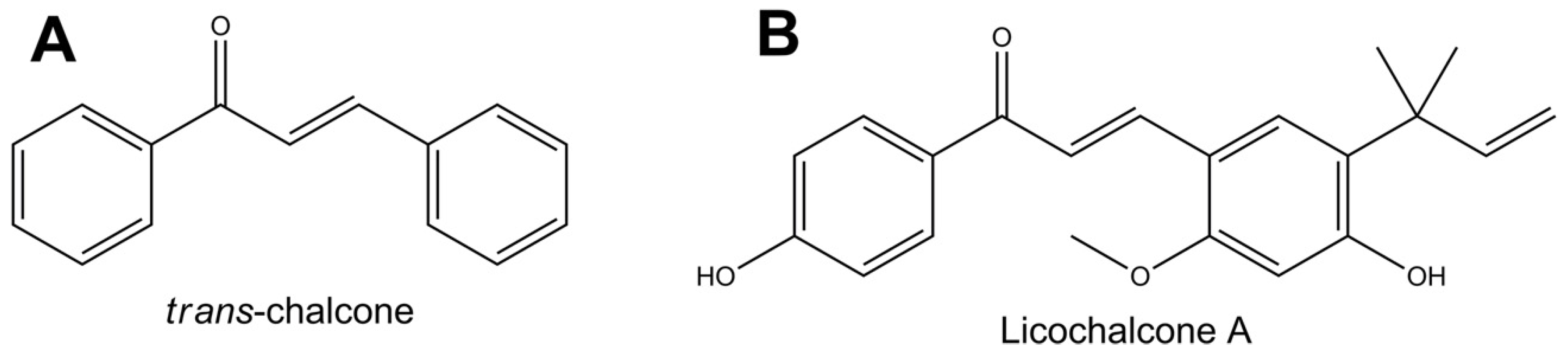
2.1. Cytotoxicity of Chalcones
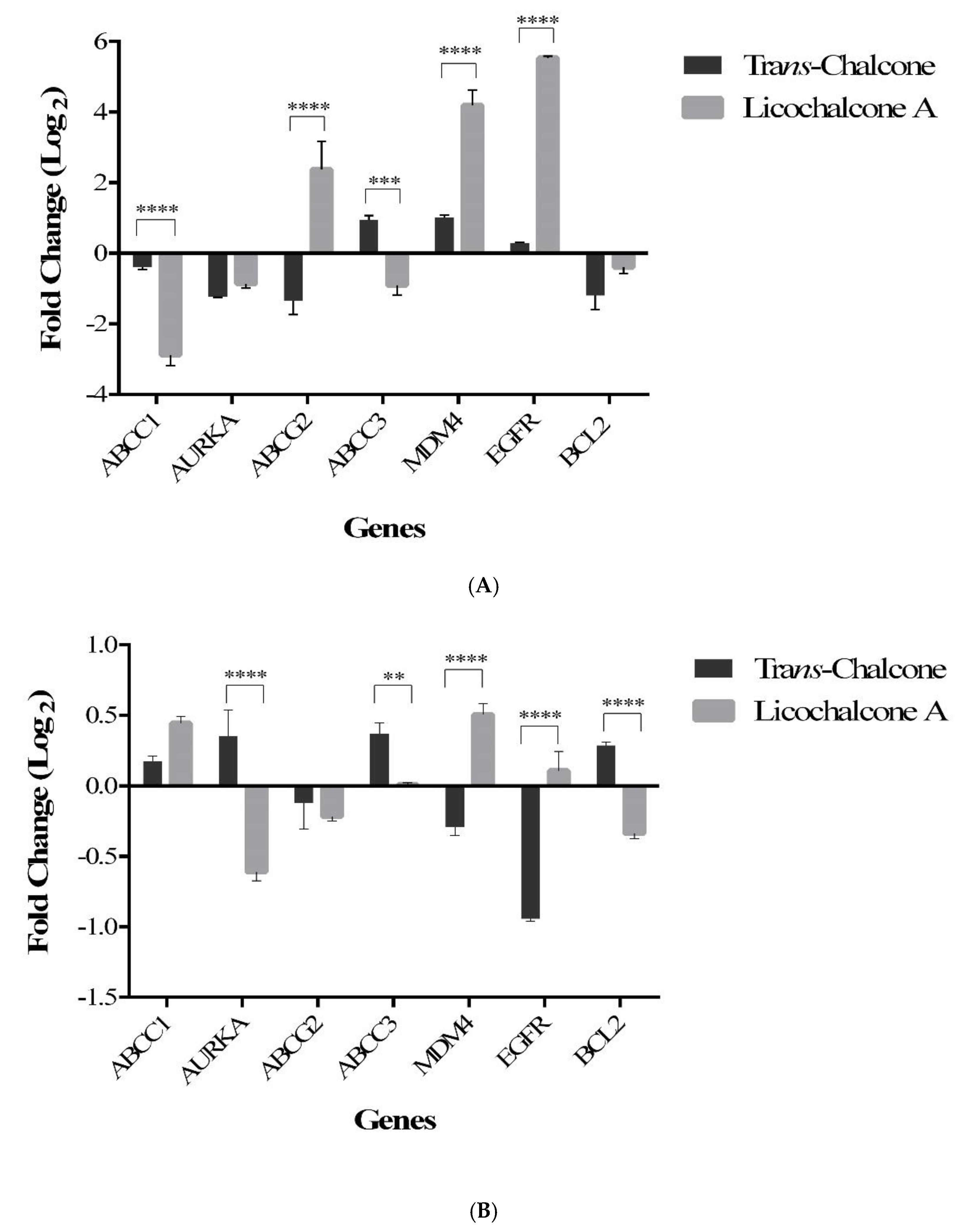
2.2. Trans-Chalcone and Licochalcone A Modulate Genes Involved in Drug Resistance and Metastasis in MCF-7 Cell
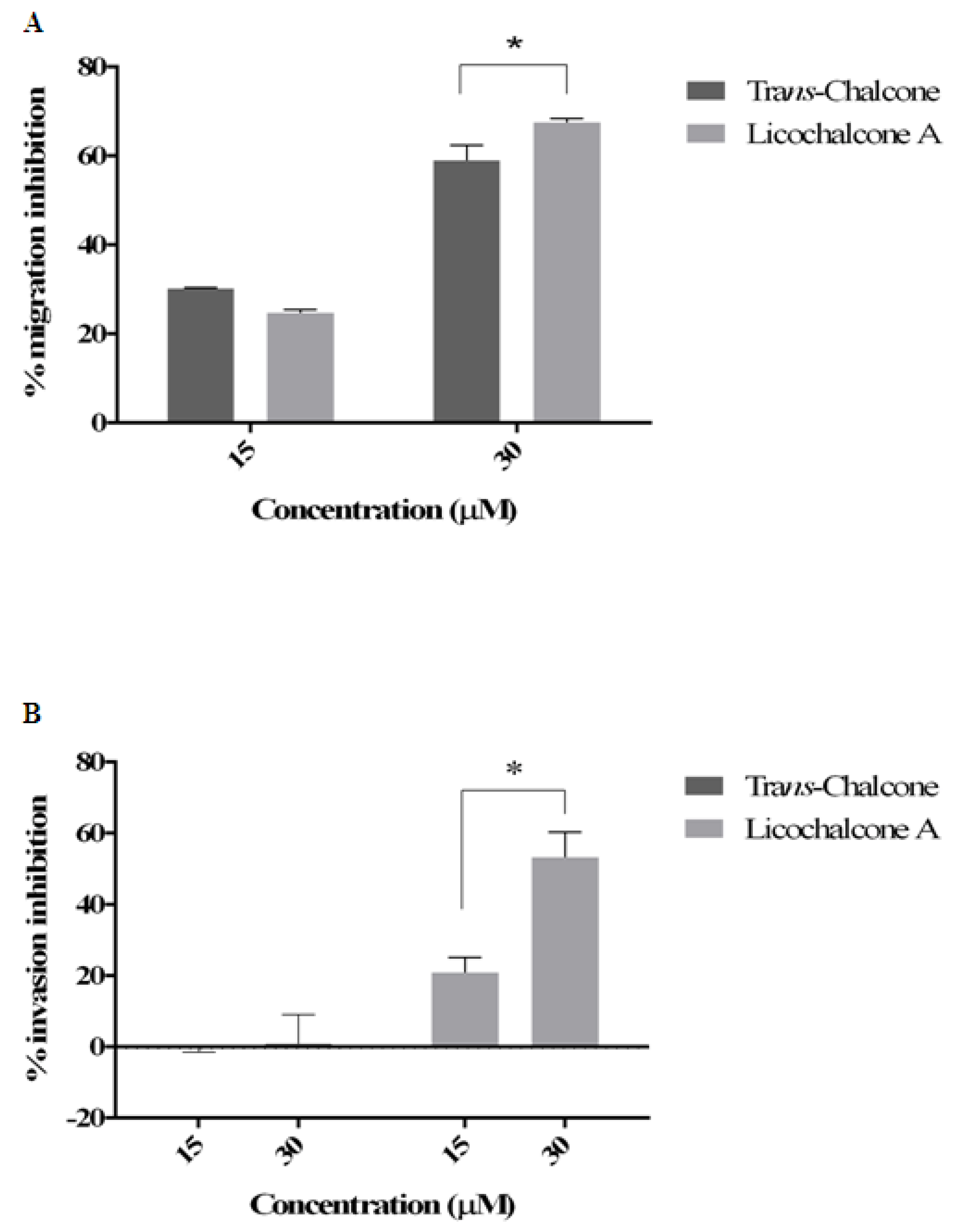
2.3. Effect of Chalcones on Cell Migration and Invasion
2.4. The Chalcones Act in AURKA and MDR-1 Protein
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Materials
5.2. Cell Culture
5.3. Viability Assay Conditions
5.4. RNA Extraction, cDNA Conversion and RT2 Profiler PCR Array
5.5. qRT-PCR
5.6. Migration and Invasion Assays
5.7. Western Blot Analysis
5.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25220842 (accessed on 1 August 2016).
- Ginsburg, O.; Bray, F.; Coleman, M.P.; Vanderpuye, V.; Eniu, A.; Kotha, S.R.; Sarker, M.; Huong, T.T.; Allemani, C.; Dvaladze, A.; et al. The global burden of women’s cancers: A grand challenge in global health. Lancet 2017, 389, 847–860. [Google Scholar] [CrossRef]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Irimia, D.; Toner, M. Spontaneous migration of cancer cells under conditions of mechanical confinement. Integr. Biol. 2009, 1, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Owen, S.; Davies, E.L.; Jiang, W.G.; Martin, T.A. The Effect of Aurora Kinase Inhibitor on Adhesion and Migration in Human Breast Cancer Cells and Clinical Implications. World J. Oncol. 2017, 8, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Howard-Anderson, J.; Ganz, P.A.; Bower, J.E.; Stanton, A.L. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: A systematic review. J. Natl. Cancer Inst. 2012, 104, 386–405. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.I.; Haber, M.; Henderson, M.J.; Norris, M.D. ABC transporters in cancer: More than just drug efflux pumps. Nat. Rev. Cancer 2010, 10, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Kunická, T.; Václavíková, R.; Hlaváč, V.; Vrána, D.; Pecha, V.; Rauš, K.; Trnková, M.; Kubáčková, K.; Ambruš, M.; Vodičková, L.; et al. Non-coding polymorphisms in nucleotide binding domain 1 in ABCC1 gene associate with transcript level and survival of patients with breast cancer. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Di, C.; Zhao, Y. Multiple drug resistance due to resistance to stem cells and stem cell treatment progress in cancer (Review). Exp. Ther. Med. 2014, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Bortolotto, L.F.B.; Barbosa, F.R.; Silva, G.; Bitencourt, T.A.; Beleboni, R.O.; Baek, S.J.; Marins, M.; Fachin, A.L. Cytotoxicity of trans-chalcone and licochalcone A against breast cancer cells is due to apoptosis induction and cell cycle arrest. Biomed. Pharmacother. 2016. [Google Scholar] [CrossRef] [PubMed]
- Haupt, S.; Vijayakumaran, R.; Miranda, P.J.; Burgess, A.; Lim, E.; Haupt, Y. The role of MDM 2 and MDM 4 in breast cancer development and prevention. J. Mol. Cell Biol. 2017, 9, 53–61. [Google Scholar] [PubMed]
- Hsueh, K.-W.; Fu, S.-L.; Huang, C.-Y.F.; Lin, C.-H. Aurora-A phosphorylates hnRNPK and disrupts its interaction with p53. FEBS Lett. 2011, 585, 2671–2675. [Google Scholar] [CrossRef] [PubMed]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Badve, S.; Dabbs, D.J.; Schnitt, S.J.; Baehner, F.L.; Decker, T.; Eusebi, V.; Fox, S.B.; Ichihara, S.; Jacquemier, J.; Lakhani, S.R.; et al. Basal-like and triple-negative breast cancers: A critical review with an emphasis on the implications for pathologists and oncologists. Mod. Pathol. 2011, 24, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Naga Prasad, S.V.; Mutharasan, R.K.; Naik, T.J.; et al. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Investig. 2014, 124, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.; Marins, M.; Fachin, A.L.; Lee, S.-H.; Baek, S.J. Anti-cancer activity of trans-chalcone in osteosarcoma: Involvement of Sp1 and p53. Mol. Carcinog. 2015, 55, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, X.; Wang, Y.; Sun, Q.; Chen, M.; Liu, S.; Zou, X. Licochalcone A suppresses hexokinase 2-mediated tumor glycolysis in gastric cancer via downregulation of the Akt signaling pathway. Oncol. Rep. 2018, 39, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, Y.; Bublik, D.R.; Pilpel, Y.; Oren, M. miR-661 downregulates both Mdm2 and Mdm4 to activate p53. Cell Death Differ. 2014, 21, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Hole, S.; Pedersen, A.M.; Lykkesfeldt, A.E.; Yde, C.W. Aurora kinase A and B as new treatment targets in aromatase inhibitor-resistant breast cancer cells. Breast Cancer Res. Treat. 2015, 149, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.M.; Man, C.; Jin, Y.; Jin, C.; Guan, X.Y.; Wang, Q.; Wan, T.S.K.; Cheung, A.L.M.; Tsao, S.W. Amplification and overexpression of Aurora kinase A (AURKA) in immortalized human ovarian epithelial (HOSE) cells. Mol. Carcinog. 2005, 43, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Umene, K.; Banno, K.; Kisu, I.; Yanokura, M.; Nogami, Y.; Tsuji, K.; Masuda, K.; Ueki, A.; Kobayashi, Y.; Yamagami, W.; et al. Aurora kinase inhibitors: Potential molecular-targeted drugs for gynecologic malignant tumors (Review). Biomed. Rep. 2013. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, L.; Shan, Y.; Cai, C.; Wang, S.; Zhang, H. AURKA promotes cell migration and invasion of head and neck squamous cell carcinoma through regulation of the AURKA/Akt/FAK signaling pathway. Oncol. Lett. 2016, 11, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Katayama, H.; Wang, J.; Li, S.A.; Hong, Y.; Radvanyi, L.; Li, J.J.; Sen, S. Estrogen-Induced Aurora Kinase-A (AURKA) Gene Expression is Activated by GATA-3 in Estrogen Receptor-Positive Breast Cancer Cells. Horm. Cancer 2010, 1, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Wang, L.; Yin, L.; Zhou, J.; Huo, M. Co-delivery of hydrophobic paclitaxel and hydrophilic AURKA specific siRNA by redox-sensitive micelles for effective treatment of breast cancer. Biomaterials 2015, 61, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wilson, J.M.; Harvel, N.; Liu, J.; Pei, L.; Huang, S.; Hawthorn, L. A systematic evaluation of miRNA: MRNA interactions involved in the migration and invasion of breast cancer cells. J. Transl. Med. 2013, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Borel, F.; Han, R.; Visser, A.; Petry, H.; van Deventer, S.J.H.; Jansen, P.L.M.; Konstantinova, P. Adenosine triphosphate-binding cassette transporter genes up-regulation in untreated hepatocellular carcinoma is mediated by cellular microRNAs. Hepatology 2012, 55, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Komoto, T.T.; Bitencourt, T.A.; Silva, G.; Beleboni, R.O.; Marins, M.; Fachin, A.L.; Komoto, T.T.; Bitencourt, T.A.; Silva, G.; Beleboni, R.O.; et al. Gene Expression Response of Trichophyton rubrum during Coculture on Keratinocytes Exposed to Antifungal Agents. Evid. Based Complement. Altern. Med. 2015, 2015, 180535. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, T.A.; Macedo, C.; Franco, M.E.; Assis, A.F.; Komoto, T.T.; Stehling, E.G.; Beleboni, R.O.; Malavazi, I.; Marins, M.; Fachin, A.L. Transcription profile of Trichophyton rubrum conidia grown on keratin reveals the induction of an adhesin-like protein gene with a tandem repeat pattern. BMC Genom. 2016, 17, 249. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, P.B.; Baral, T.K.; Haller, H.; Dumler, I.; Acharya, K.; Kiyan, Y. Transcriptomic pathway analysis of urokinase receptor silenced breast cancer cells: A microarray study. Oncotarget 2017, 8, 101572–101590. [Google Scholar] [CrossRef] [PubMed]
- Sarper, M.; Cortes, E.; Lieberthal, T.J.; Del Río Hernández, A. ATRA modulates mechanical activation of TGF-β by pancreatic stellate cells. Sci. Rep. 2016, 6, 27639. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.; Lima, F.T.; Seba, V.; Mendes Lourenço, A.L.; Lucas, T.G.; De Andrade, B.V.; Torrezan, G.S.; Polaquini, C.R.; Garcia, M.E.; Couto, L.B.; et al. Curcumin analog CH-5 suppresses the proliferation, migration, and invasion of the human gastric cancer cell line HGC-27. Molecules 2018, 23, 279. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |



| Cell Lines | Trans-Chalcone | Licochalcone A |
|---|---|---|
| MCF-7 | 53.73 | 60.46 |
| BT-20 | 26.42 | 30.78 |
| Genes | Sequences (5′–3′) | Amplicon Size | References |
|---|---|---|---|
| ABCG2 | F: TTGATAGCCTCACCTTATTG | 88 | This paper |
| R: ACCAGCTGATTCAAAGTATC | |||
| ABCC1 | F: AGCAGAAAAATGTGTTAGGG | 176 | This paper |
| R: TACCCACTGGTAATACTTGG | |||
| ABCC3 | F: TTTTCTGGTGGTTCACAAAG | 92 | This paper |
| R: GATCTGTCCTCTTCCTTTAG | |||
| MDM4 | F: AGATGAAACATCTAGGCTG | 143 | This paper |
| R: CAATCCACCTGATTTGTCTG | |||
| EGFR | F: GAAAAGAAAGTTTGCCAAG | 195 | This paper |
| R: ATGAGGACATAACCAGCC | |||
| AURKA | F: TACAAAAGAATATCACGGG | 126 | [30] |
| R: AAGTACTTCTCTGAGCATTG | |||
| Bcl-2 | F: GATTGTGGCCTTCTTTGAC | 164 | This paper |
| R: GTTCCACAAAGGCATCC | |||
| GAPDH | F: ACAGTTGCCATGTAGACC | 99 | [31] |
| R: TTTTTGGTTGAGCACAGG |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komoto, T.T.; Bernardes, T.M.; Mesquita, T.B.; Bortolotto, L.F.B.; Silva, G.; Bitencourt, T.A.; Baek, S.J.; Marins, M.; Fachin, A.L. Chalcones Repressed the AURKA and MDR Proteins Involved in Metastasis and Multiple Drug Resistance in Breast Cancer Cell Lines. Molecules 2018, 23, 2018. https://doi.org/10.3390/molecules23082018
Komoto TT, Bernardes TM, Mesquita TB, Bortolotto LFB, Silva G, Bitencourt TA, Baek SJ, Marins M, Fachin AL. Chalcones Repressed the AURKA and MDR Proteins Involved in Metastasis and Multiple Drug Resistance in Breast Cancer Cell Lines. Molecules. 2018; 23(8):2018. https://doi.org/10.3390/molecules23082018
Chicago/Turabian StyleKomoto, Tatiana Takahasi, Tayná Minervina Bernardes, Thaís Balthazar Mesquita, Luis Felipe Buso Bortolotto, Gabriel Silva, Tamires Aparecida Bitencourt, Seung Joon Baek, Mozart Marins, and Ana Lúcia Fachin. 2018. "Chalcones Repressed the AURKA and MDR Proteins Involved in Metastasis and Multiple Drug Resistance in Breast Cancer Cell Lines" Molecules 23, no. 8: 2018. https://doi.org/10.3390/molecules23082018
APA StyleKomoto, T. T., Bernardes, T. M., Mesquita, T. B., Bortolotto, L. F. B., Silva, G., Bitencourt, T. A., Baek, S. J., Marins, M., & Fachin, A. L. (2018). Chalcones Repressed the AURKA and MDR Proteins Involved in Metastasis and Multiple Drug Resistance in Breast Cancer Cell Lines. Molecules, 23(8), 2018. https://doi.org/10.3390/molecules23082018