Development of Antibacterial and Antifungal Triazole Chromium(III) and Cobalt(II) Complexes: Synthesis and Biological Activity Evaluations
Abstract
1. Introduction
2. Discussion
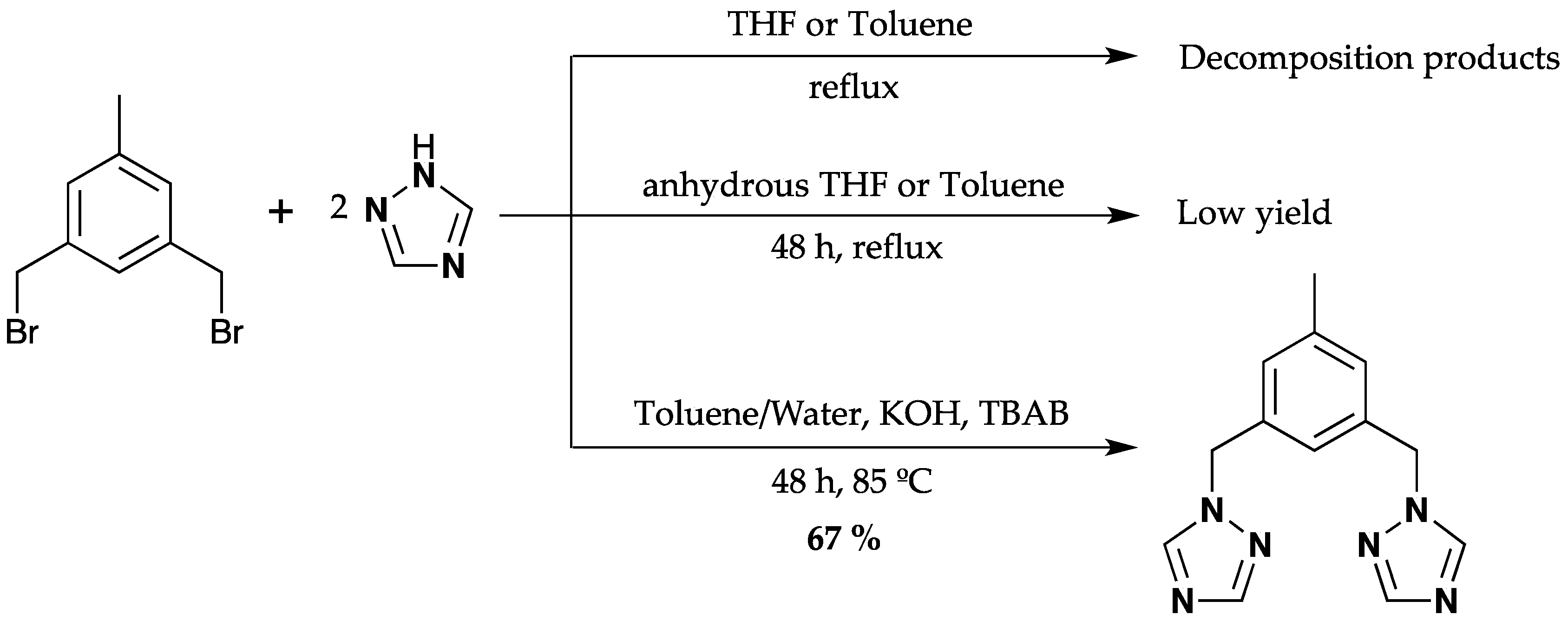
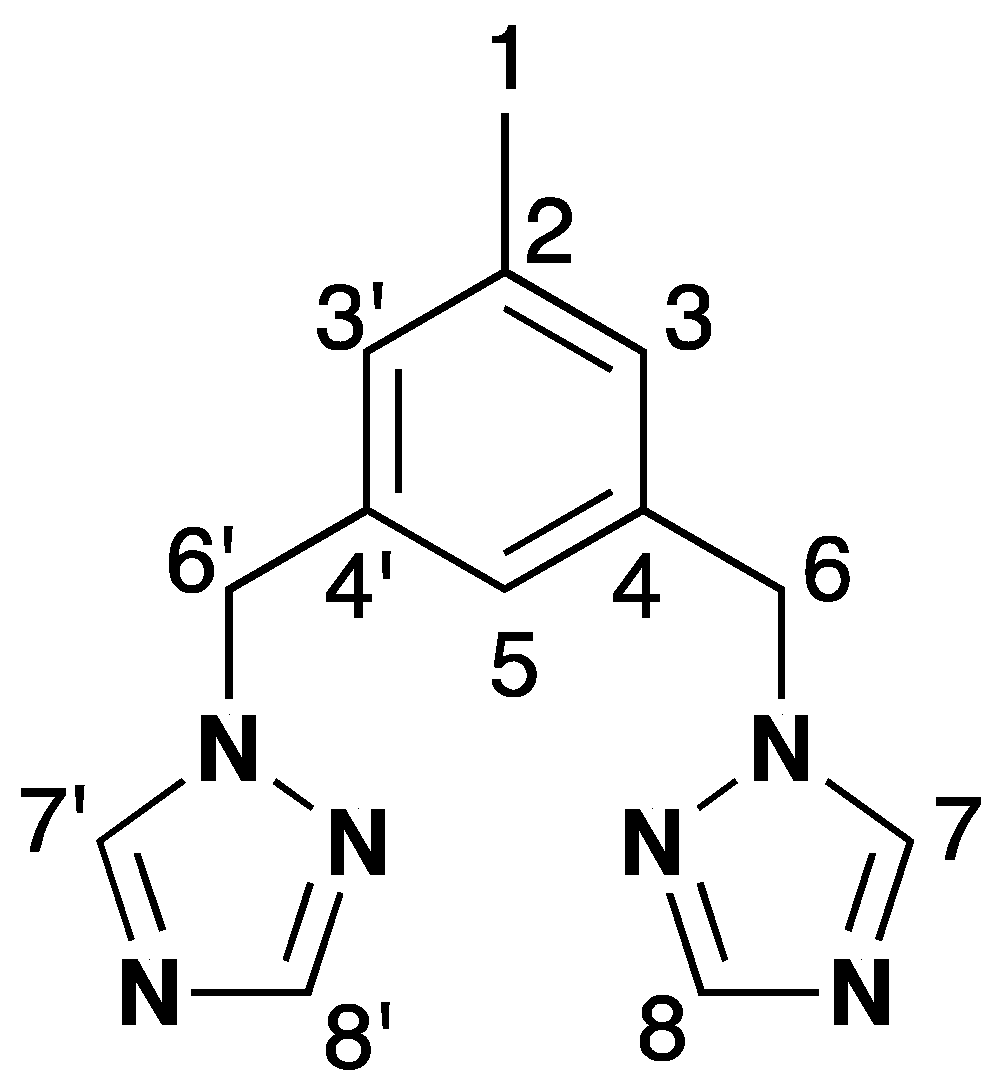
2.1. Synthesis and Characterization of Ligand (1)
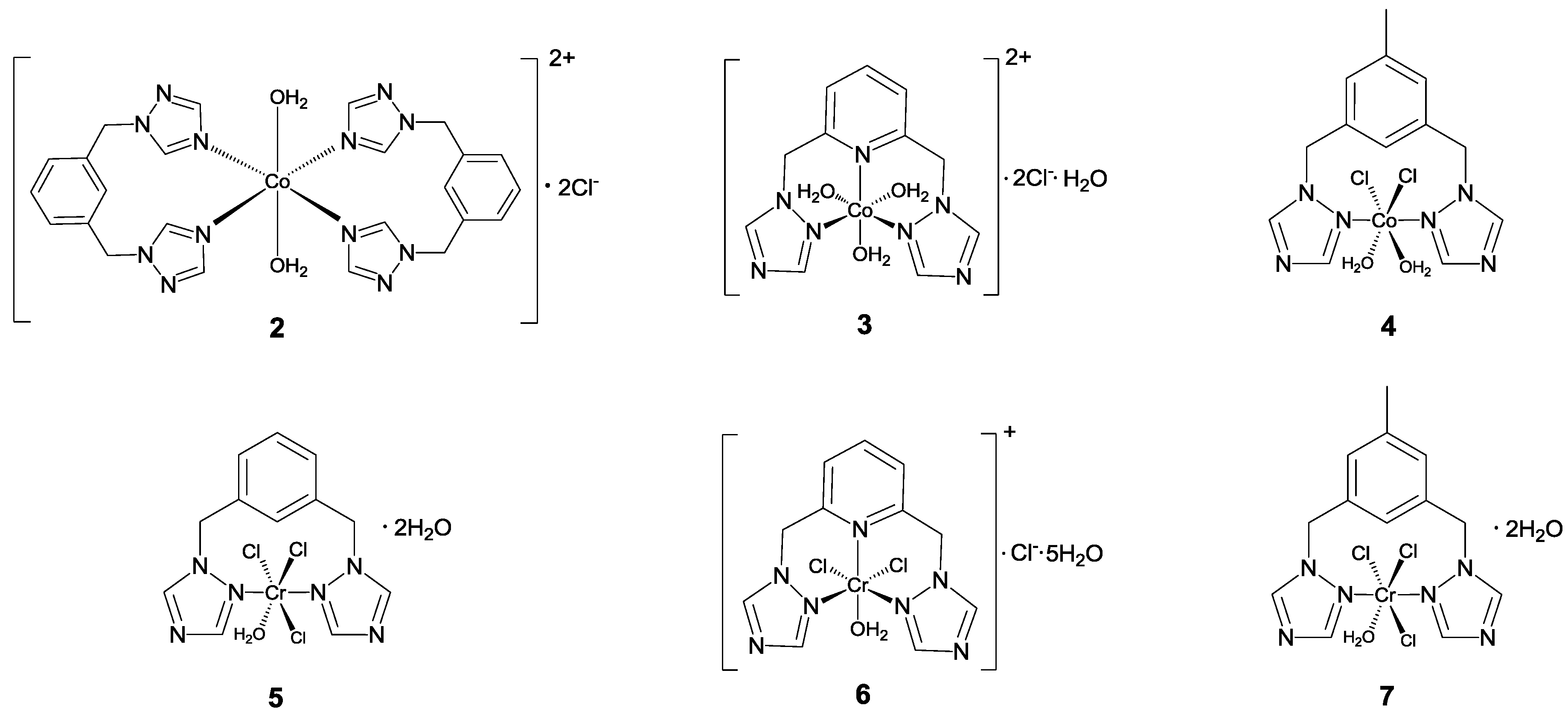
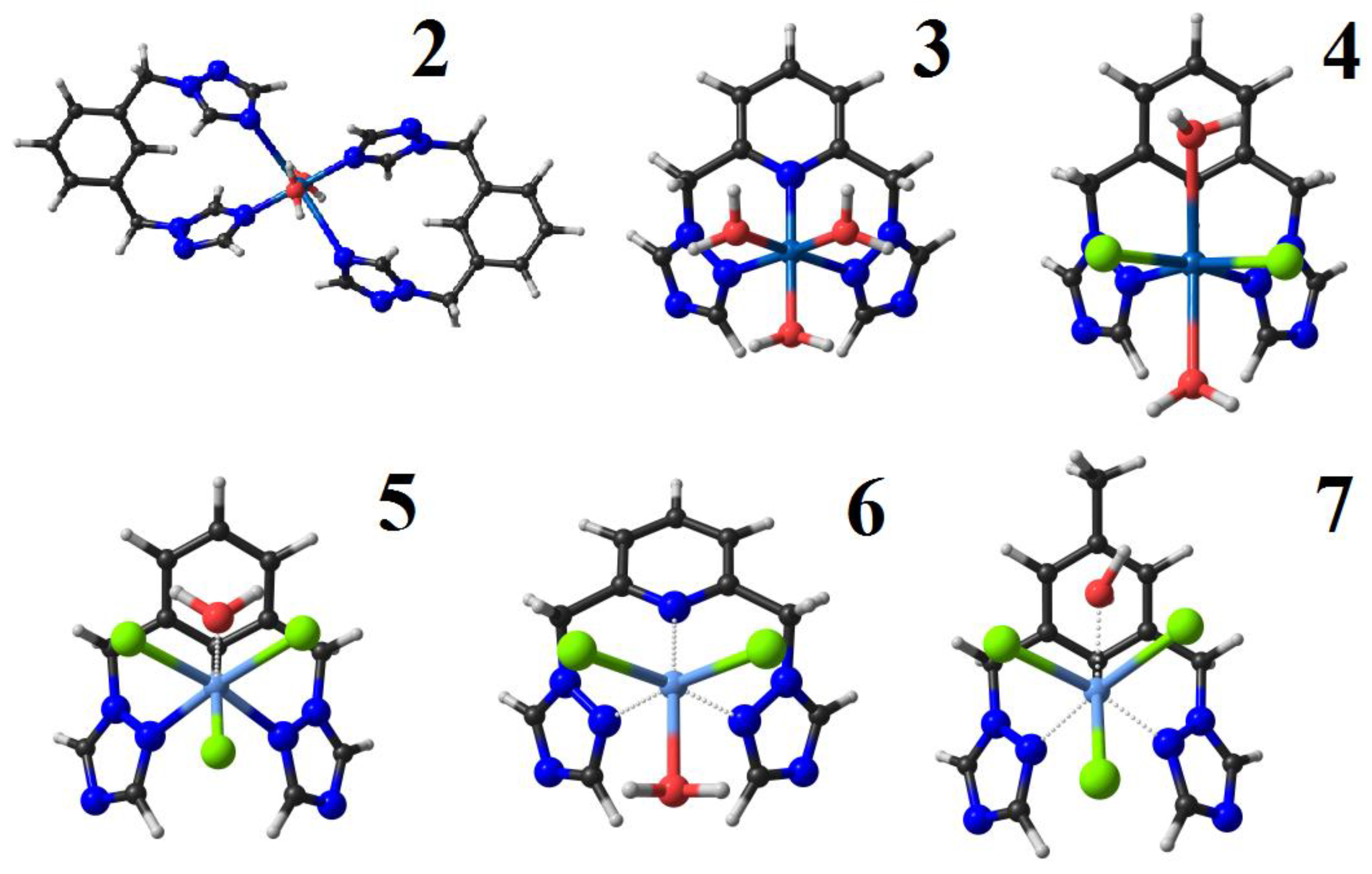
2.2. Synthesis and Characterization of Metal Complexes
2.2.1. FTIR Spectroscopy
2.2.2. Raman Spectroscopy
2.2.3. UV/Vis Spectroscopy
2.2.4. Thermal Analysis
2.3. Biological Activity
2.3.1. Antibacterial Activity
2.3.2. Antifungal Activity
2.4. Quantum-Chemical Calculations
3. Materials and Methods
3.1. General Information
3.2. Synthesis of 3,5-Bis(1,2,4-triazol-1-ylmethyl)toluene (1)
3.3. Synthesis of the Complexes
3.3.1. Synthesis of [Co{1,3-bis((1,2,4-triazol-1-ylmethyl)benzene-N,N}(H2O)2]Cl2 (2)
3.3.2. Synthesis of [Co{2,6-bis((1,2,4-triazol-1-ylmetil)pyridine-N,N,N}(H2O)3] Cl2·H2O (3)
3.3.3. Synthesis of [Co{3,5-bis((1,2,4-triazol-1-ylmethy)toluene-N,N}(H2O)2Cl2] (4)
3.3.4. Synthesis of [Cr {1,3-Bis(1,2,4-triazol-1ylmethyl)benzene-N,N}(H2O)Cl3]·2H2O (5)
3.3.5. Synthesis of [Cr{2,6-bis((1,2,4-triazol-1-ylmethyl)pyridine-N,N,N}(H2O)Cl2]Cl·5H2O (6)
3.3.6. Synthesis of [Cr{3,5-Bis(1,2,4-triazol-1-ylmethyl)toluene-N,N}(H2O)Cl3] 2H2O (7)
3.4. Biological Studies
3.4.1. Microorganisms and Mammalian Cells
3.4.2. Evaluation of the Antimicrobial Activity
3.4.3. Cytotoxicity Test on Mammalian Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. 2016. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 7 August 2018).
- Crump, J.A.; Ramadhani, H.O.; Morrissey, A.B.; Saganda, W.; Mwako, M.S.; Yang, L.Y.; Chow, S.C.; Morpeth, S.C.; Reyburn, H.; Njau, B.N.; et al. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected adults and adolescents in northern Tanzania. Clin. Infect. Dis. 2011, 52, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.D.; Warnock, D.W. Fungal Infection: Diagnosis and Management; John Wiley & Sons: West Sussex, UK, 2012. [Google Scholar]
- Browning, D.F.; Busby, S.J. Local and Global Regulation of Transcription Initiation in Bacteria. Nat. Rev. Microbiol. 2016, 14, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Vaziri, I.; Hanna, H.A.; Boktour, M.; Thornby, J.; Hachem, R.; Bodey, G.P.; Raad, I.I. Risk Factors for Candida Tropicalis Fungemia in Patients with Cancer. Clin. Infect. Dis. 2001, 33, 1676–1681. [Google Scholar] [CrossRef] [PubMed]
- Kothavade, R.J.; Kura, M.M.; Valand, A.G.; Panthaki, M.H. Candida Tropicalis: Its Prevalence, Pathogenicity and Increasing Resistance to Fluconazole. J. Med. Microbiol. 2010, 59, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida Glabrata, Candida Parapsilosis and Candida Tropicalis: Biology, Epidemiology, Pathogenicity and Antifungal Resistance. FEMS Microbiol. Rev. 2012, 36, 288–305. [Google Scholar] [CrossRef] [PubMed]
- Bihan, C.L.; Zahar, J.R.; Timsit, J.F. Staphylococcus aureus Transmission in the Intensive Care Unit: The Potential Role of the Healthcare Worker Carriage. Ann. Infect. 2017, 1, 1–5. [Google Scholar] [CrossRef]
- Price, J.R.; Cole, K.; Bexley, A.; Kostiou, V.; Eyre, D.W.; Golubchik, T.; Wilson, D.J.; Crook, D.W.; Walker, A.S.; Peto, T.E. Transmission of Staphylococcus aureus between Health-Care Workers, the Environment, and Patients in an Intensive Care Unit: A Longitudinal Cohort Study Based on Whole-Genome Sequencing. Lancet Infect. Dis. 2017, 17, 207–214. [Google Scholar] [CrossRef]
- Recker, M.; Laabei, M.; Toleman, M.S.; Reuter, S.; Saunderson, R.B.; Blane, B.; Török, M.E.; Ouadi, K.; Stevens, E.; Yokoyama, M.; et al. Clonal Differences in Staphylococcus aureus bacteraemia-Associated Mortality. Nat. Microbiol. 2017, 2, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Pierce, R.; Lessler, J.; Popoola, V.O.; Milstone, A.M. Meticillin-Resistant Staphylococcus aureus (MRSA) Acquisition Risk in an Endemic Neonatal Intensive Care Unit with an Active Surveillance Culture and Decolonization Programme. J. Hosp. Infect. 2017, 95, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Kathiravan, M.K.; Salake, A.B.; Chothe, A.S.; Dudhe, P.B.; Watode, R.P.; Mukta, M.S.; Gadhwe, S. The Biology and Chemistry of Antifungal Agents: A Review. Bioorg. Med. Chem. 2012, 20, 5678–5698. [Google Scholar] [CrossRef] [PubMed]
- Bendaha, H.; Yu, L.; Touzani, R.; Souane, R.; Giaever, G.; Nislow, C.; Boone, C.; El Kadiri, S.; Brown, G.W.; Bellaoui, M. New azole antifungal agents with novel modes of action: Synthesis and biological studies of new tridentate ligands based on pyrazole and triazole. Eur. J. Med Chem. 2011, 46, 4117–4124. [Google Scholar] [CrossRef] [PubMed]
- Shrinivas, D.J.; Sheshagiri, R.D.; Uttam, A.M.; Tejraj, A.; Venkatrao, H.K.; Andanappa, K.G. Enoyl ACP Reductase as Effective Target for the Synthesized Novel Antitubercular Drugs: A-State-of-the-Art. Mini Rev. Med. Chem. 2014, 14, 678–693. [Google Scholar] [CrossRef]
- Singh, K.; Singh, D.P.; Barwa, M.S.; Tyagi, P.; Mirza, Y. Antibacterial Co(II), Ni(II), Cu(II) and Zn(II) Complexes of Schiff bases Derived from Fluorobenzaldehyde and Triazoles. J. Enzyme Inhib. Med. Chem. 2006, 21, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Holla, B.S.; Poojary, K.N.; Rao, B.S.; Shivananda, M.K. New bis-aminomercaptotriazoles and bis-triazolothiadiazoles as possible anticancer agents. Eur. J. Med. Chem. 2002, 37, 511–517. [Google Scholar] [CrossRef]
- El-Gammal, O.A.; Bekheit, M.M.; Tahoon, M. Synthesis, Characterization and Biological Activity of 2-Acetylpyridine-α-Naphthoxyacetylhydrazone Its Metal Complexes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 135, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, J.; Ibarra, L.; Yepes, D.; García-Huertas, P.; Macías, M.A.; Triana-Chavez, O.; Nagles, E.; Suescun, L.; Muñoz-Castro, A. Synthesis, crystal structure, catalytic and anti-Trypanosomacruzi activity of a new chromium(III) complex containing bis(3,5-dimethylpyrazol-1-yl)methane. J. Mol. Struct. 2017, 1146, 365–372. [Google Scholar] [CrossRef]
- Alaghaz, A.-N.M.A.; Ammar, R.A. New Dimeric Cyclodiphosph(V)Azane Complexes of Cr(III), Co(II), Ni(II), Cu(II), and Zn(II): Preparation, Characterization and Biological Activity Studies. Eur. J. Med. Chem. 2010, 45, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, M.T.H.; Ali, M.A.; Wee, D.J.; Azahari, K.; Silong, S.; Crouse, K.A. Complexes of a Tridentate ONS Schiff Base. Synthesis and Biological Properties. Transit. Met. Chem. 2000, 25, 456–460. [Google Scholar] [CrossRef]
- Mohamed, G.G.; Zayed, M.A.; Abdallah, S.M. Metal Complexes of a Novel Schiff Base Derived from Sulphametrole and Varelaldehyde. Synthesis, Spectral, Thermal Characterization and Biological Activity. J. Mol. Struct. 2010, 979, 62–71. [Google Scholar] [CrossRef]
- Emara, A.A.A. Structural, Spectral and Biological Studies of Binuclear Tetradentate Metal Complexes of N3O Schiff Base Ligand Synthesized from 4,6-Diacetylresorcinol and Diethylenetriamine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 77, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Kljun, J.; Scott, A.J.; LanišnikRižner, T.; Keiser, J.; Turel, I. Synthesis and Biological Evaluation of Organoruthenium Complexes with Azole Antifungal Agents. First Crystal Structure of a Tioconazole Metal Complex. Organometallics 2014, 33, 1594–1601. [Google Scholar] [CrossRef]
- Bello-Vieda, N.J.; Pastrana, H.F.; Garavito, M.F.; Ávila, A.G.; Celis, A.M.; Muñoz-Castro, A.; Restrepo, S.; Hurtado, J.J. Antibacterial Activities of Azole Complexes Combined with Silver Nanoparticles. Molecules 2018, 23, 361. [Google Scholar] [CrossRef] [PubMed]
- Pahontu, E.; Fala, V.; Gulea, A.; Poirier, D.; Tapcov, V.; Rosu, T. Synthesis and Characterization of Some New Cu(II), Ni(II) and Zn(II) Complexes with Salicylidene Thiosemicarbazones: Antibacterial, Antifungal and in Vitro Antileukemia Activity. Molecules 2013, 18, 8812–8836. [Google Scholar] [CrossRef] [PubMed]
- Yousef, T.A.; Abu El-Reash, G.M.; Al-Jahdali, M.; El-Rakhawy, E.-B.R. Synthesis, spectral characterization and biological evaluation of Mn(II), Co(II), Ni(II), Cu(II), Zn(II) and Cd(II) complexes with thiosemicarbazone ending by pyrazole and pyridyl rings. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 129, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Castillo, K.F.; Bello-Vieda, N.J.; Nuñez-Dallos, N.G.; Pastrana, H.F.; Celis, A.M.; Restrepo, S.; Hurtado, J.J.; Ávila, A.G. Metal complex derivatives of azole: A study on their synthesis, characterization, and antibacterial and antifungal activities. J. Braz. Chem. Soc. 2016, 27, 2334–2347. [Google Scholar] [CrossRef]
- Sumrra, S.H.; Suleman, A.; Chohan, Z.H.; Zafar, M.N.; Raza, M.A.; Iqbal, T. Triazole Metal Based Complexes as Antibacterial/Antifungal Agents. Russ. J. General Chem. 2017, 87, 1281–1287. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, Y.; Puri, P.; Kumar, M.; Sharma, C. Cobalt, Nickel, Copper and Zinc Complexes with 1,3-Diphenyl-1H-Pyrazole-4-Carboxaldehyde Schiff Bases: Antimicrobial, Spectroscopic, Thermal and Fluorescence Studies. Eur. J. Med. Chem. 2012, 52, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Sundaraganesan, N.; Kavitha, E.; Sebastian, S.; Cornard, J.P.; Martel, M. Experimental FTIR, FT-IR (gas phase), FT-Raman and NMR spectra, hyperpolarizability studies and DFT calculations of 3,5-dimethylpyrazole. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 74, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Geary, W.J. The Use of Conductivity Measurements in Organic Solvents for the Characterization of Coordination Compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Katritzky, A.; Ramsden, C.; Joule, J.; Zhdankin, V. Handbook of Heterocyclic Chemistry, 3rd ed.; Katritzky, A.R., Ed.; Elsevier: Amsterdam, NY, USA, 2010. [Google Scholar]
- Sandoval-Rojas, A.P.; Ibarra, L.; Cortés, M.T.; Macías, M.A.; Suescun, L.; Hurtado, J.J. Synthesis and characterization of copper(II) complexes containing acetate and N,N-donor ligands, and their electrochemical behavior in dopamine detection. J. Electroanal. Chem. 2017, 805, 60–67. [Google Scholar] [CrossRef]
- Larkin, P.J. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation; Elsevier: Amsterdam, NY, USA, 2011. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: Chichester, UK, 2001. [Google Scholar]
- Billes, F.; Ziegler, I.; Mikosch, H. Vibrational Spectroscopic Study of Sodium-1,2,4-Triazole, an Important Intermediate Compound in the Synthesis of Several Active Substances. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 153, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Billes, F.; Endredi, H.; Keresztury, G. Vibrational Spectroscopy of Triazoles and Tetrazole. J. Mol. Struct. THEOCHEM 2000, 530, 183–200. [Google Scholar] [CrossRef]
- Cotton, F.A.; Wilkinson, G. Advanced Inorganic Chemistry, 6th ed.; John Wiley & Sons: London, UK, 1999. [Google Scholar]
- Heerding, D.A.; Chan, G.; DeWolf, W.E.; Fosberry, A.P.; Janson, C.A.; Jaworski, D.D.; McManus, E.; Miller, W.H.; Moore, T.D.; Payne, D.J.; et al. 1,4-Disubstituted imidazoles are potential antibacterial agents functioning as inhibitors of enoyl acyl carrier protein reductase (FabI). Bioorg. Med. Chem. Lett. 2001, 11, 2061–2065. [Google Scholar] [CrossRef]
- Kabbani, A.T.; Hammud, H.H.; Ghannoum, A.M. Preparation and antibacterial activity of copper and cobalt complexes of 4-chloro-3-nitrobenzoate with a nitrogen donor ligand. Chem. Pharm. Bull. (Tokyo) 2007, 55, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Collin, F.; Karkare, S.; Maxwell, A. Exploiting bacterial DNA gyrase as a drug target: Current state and perspectives. Appl. Microbiol. Biotechnol. 2011, 92, 479–497. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, H. Pyridine-3-Sulphonic Acid and Its Amide as Inhibitors of Bacterial Growth. Br. J. Exp. Pathol. 1940, 21, 136. [Google Scholar]
- Schneider, D.; Parker, C.D. Effect of Pyridines on Phenotypic Properties of Bordetella Pertussis. Infect. Immun. 1982, 38, 548–553. [Google Scholar] [PubMed]
- Bagihalli, G.B.; Avaji, P.G.; Patil, S.A.; Badami, P.S. Synthesis, Spectral Characterization, in Vitro Antibacterial, Antifungal and Cytotoxic Activities of Co(II), Ni(II) and Cu(II) Complexes with 1,2,4-Triazole Schiff Bases. Eur. J. Med. Chem. 2008, 43, 2639–2649. [Google Scholar] [CrossRef] [PubMed]
- Poyraz, M.; Sari, M.; Demirci, F.; Kosar, M.; Demirayak, S.; Büyükgüngör, O. Synthesis, Crystal Structure and Biological Activity of 1-(1H-Benzoimidazol-2-Yl)-EthanoneThiosemicarbazone and Its Cobalt Complex. Polyhedron 2008, 27, 2091–2096. [Google Scholar] [CrossRef]
- Van den Bossche, H.; Willemsens, G.; Cools, W.; Marichal, P.; Lauwers, W. Hypothesis on the molecular basis of the antifungal activity of N-substituted imidazoles and triazoles. Biochem. Soc. Trans. 1983, 11, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Herbrecht, R. Voriconazole: Therapeutic review of a new azole antifungal. Expert Rev. Anti Infect. Ther. 2004, 2, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Chohan, Z.H.; Hanif, M. Design, Synthesis, and Biological Properties of Triazole Derived Compounds and Their Transition Metal Complexes. J. Enzyme Inhib. Med. Chem. 2010, 25, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Chohan, Z.H.; Hanif, M. Antibacterial and Antifungal Metal Based Triazole Schiff Bases. J. Enzyme Inhib. Med. Chem. 2013, 28, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Carcelli, M.; Mazza, P.; Pelizzi, C.; Zani, F. Antimicrobial and Genotoxic Activity of 2,6-Diacetylpyridine Bis(Acylhydrazones) and Their Complexes with Some First Transition Series Metal Ions. X-Ray Crystal Structure of a Dinuclear Copper(II) Complex. J. Inorg. Biochem. 1995, 57, 43–62. [Google Scholar] [CrossRef]
- Shreaz, S.; Sheikh, R.A.; Bhatia, R.; Neelofar, K.; Imran, S.; Hashmi, A.A.; Manzoor, N.; Basir, S.F.; Khan, L.A. Antifungal Activity of α-Methyl Trans Cinnamaldehyde, Its Ligand and Metal Complexes: Promising Growth and Ergosterol Inhibitors. BioMetals 2011, 24, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Bellú, S.; Hure, E.; Trapé, M.; Trossero, C.; Molina, G.; Drogo, C.; Williams, P.A.M.; Atria, A.M.; Muñoz Acevedo, J.C.; Zacchino, S.; et al. Synthesis, Structure and Antifungal Properties of Co(II)–sulfathiazolate Complexes. Polyhedron 2005, 24, 501–509. [Google Scholar] [CrossRef]
- Rodríguez-Argüelles, M.C.; Cao, R.; García-Deibe, A.M.; Pelizzi, C.; Sanmartín-Matalobos, J.; Zani, F. Antibacterial and Antifungal Activity of Metal(II) Complexes of Acylhydrazones of 3-Isatin and 3-(N-Methyl)Isatin. Polyhedron 2009, 28, 2187–2195. [Google Scholar] [CrossRef]
- Pea, F.; Lewis, R.E. Overview of antifungal dosing in invasive candidiasis. J. Antimicrob. Chemother. 2018, 73, i33–i43. [Google Scholar] [CrossRef] [PubMed]
- TeVelde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Lien, E.J.; Guo, Z.R.; Li, R.L.; Su, C.T. Use of Dipole Moment as a Parameter in Drug-Receptor Interaction and Quantitative Structure-Activity Relationship Studies. J. Pharm. Sci. 1982, 71, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; Pedretti, A. Molecular Fields to Assess Recognition Forces and Property Spaces. In Comprehensive Medicinal Chemistry II; Elsevier: New York, NY, USA, 2007; pp. 577–602. [Google Scholar] [CrossRef]
- Díez-Barra, E.; Guerra, J.; López-Solera, I.; Merino, S.; Rodríguez-López, J.; Sánchez-Verdú, P.; Tejeda, J. Novel Chiral and Achiral NCN Pincer Complexes Based on 1,3-Bis(1H-1,2,4-Triazol-1-Ylmethyl)Benzene. Organometallics 2003, 22, 541–547. [Google Scholar] [CrossRef]
- Kim, E.Y.; Song, Y.J.; Koo, H.G.; Lee, J.H.; Park, H.M.; Kim, C.; Kwon, T.-H.; Huh, S.; Kim, S.-J.; Kim, Y. 1-D, 2-D and 3-D Coordination Polymers Assembled from PolynuclearCoII Units Based on the Isophthalate(-2) Ligand. Polyhedron 2010, 29, 3335–3341. [Google Scholar] [CrossRef]
Sample Availability: Samples of all the compounds are available from the authors. |


| Compound | Conductivity (Ω−1 cm2 mol−1) | Type of Electrolyte (In Solution) |
|---|---|---|
| CoCl2·6H2O | 48.0 | 2:1 |
| 2 | 52.9 | 2:1 |
| 3 | 50.5 | 2:1 |
| 4 | 43.3 | No electrolyte |
| CrCl3·6H2O | 40.2 | 1:1 |
| 5 | 24.4 | No electrolyte |
| 6 | 30.3 | 1:1 |
| 7 | 27.4 | No electrolyte |
| Transition (nm) (ε(M−1 cm−1)) | ||||
|---|---|---|---|---|
| UV | Vis | |||
| Compound | ν1 | ν2 | ν1 | ν2 |
| L1 | 263(266) | |||
| 2 | 262(784) | 615(66) | 678(116) | |
| 5 | 260(3672) | |||
| L2 | 264(4423) | |||
| 3 | 264(3317) | 615(41) | 677(79) | |
| 6 | 263(7081) | |||
| 1 | 267(297) | 276(236) | ||
| 4 | 268(556) | 276(478) | 615(7) | 675(26) |
| 7 | 259(2141) | |||
| CoCl2·6H2O | 238(73) | 266(64) | 614(41) | 678(81) |
| CrCl3·6H2O | 260(2764) | |||
| Compound | S. aureus (MIC) | S. typhimurium (MIC) | E. coli (MIC) | C. tropicalis (MIC) | C. albicans (MIC) | C. parasilopsis (MIC) | Vero Cells (CC50 ± SD) |
|---|---|---|---|---|---|---|---|
| L1 | >2000 | 1000 | >2000 | >2000 | >2000 | >2000 | >300 |
| L2 | >2000 | 1000 | >2000 | >2000 | >2000 | >2000 | >300 |
| 1 | >2000 | 2000 | >2000 | >2000 | >2000 | >2000 | >300 |
| 2 | 1000–2000 | 1000 | >2000 | 125 | 125 | 500 | 277.07 ± 1.86 |
| 3 | 250–500 | 1000 | 1000 | 31.25 | 125 | 125 | 68.76 ± 0.97 |
| 4 | 250–500 | 1000 | 2000 | 62.50 | 125 | 125 | 193.63 ± 6.51 |
| 5 | >2000 | 1000 | 2000 | 7.81 | 62.5 | 125 | >300 |
| 6 | >2000 | >2000 | >2000 | 7.81 | 62.5 | 62.5 | >300 |
| 7 | >2000 | >2000 | >2000 | 15.62 | 31.25 | 125 | 130.60 ± 3.57 |
| Ampicillin | 0.078–0.156 | ||||||
| Gentamicin | 2.50 | 0.625 | |||||
| Amphotericin B | 0.065 | 0.31 | 0.23 | ||||
| Itraconazole | 2.0 | 0.78 | 500 | 25.22 ± 3.51 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murcia, R.A.; Leal, S.M.; Roa, M.V.; Nagles, E.; Muñoz-Castro, A.; Hurtado, J.J. Development of Antibacterial and Antifungal Triazole Chromium(III) and Cobalt(II) Complexes: Synthesis and Biological Activity Evaluations. Molecules 2018, 23, 2013. https://doi.org/10.3390/molecules23082013
Murcia RA, Leal SM, Roa MV, Nagles E, Muñoz-Castro A, Hurtado JJ. Development of Antibacterial and Antifungal Triazole Chromium(III) and Cobalt(II) Complexes: Synthesis and Biological Activity Evaluations. Molecules. 2018; 23(8):2013. https://doi.org/10.3390/molecules23082013
Chicago/Turabian StyleMurcia, Ricardo A., Sandra M. Leal, Martha V. Roa, Edgar Nagles, Alvaro Muñoz-Castro, and John J. Hurtado. 2018. "Development of Antibacterial and Antifungal Triazole Chromium(III) and Cobalt(II) Complexes: Synthesis and Biological Activity Evaluations" Molecules 23, no. 8: 2013. https://doi.org/10.3390/molecules23082013
APA StyleMurcia, R. A., Leal, S. M., Roa, M. V., Nagles, E., Muñoz-Castro, A., & Hurtado, J. J. (2018). Development of Antibacterial and Antifungal Triazole Chromium(III) and Cobalt(II) Complexes: Synthesis and Biological Activity Evaluations. Molecules, 23(8), 2013. https://doi.org/10.3390/molecules23082013






