Effects of Quercetin on Proliferation and H2O2-Induced Apoptosis of Intestinal Porcine Enterocyte Cells
Abstract
1. Introduction
2. Results
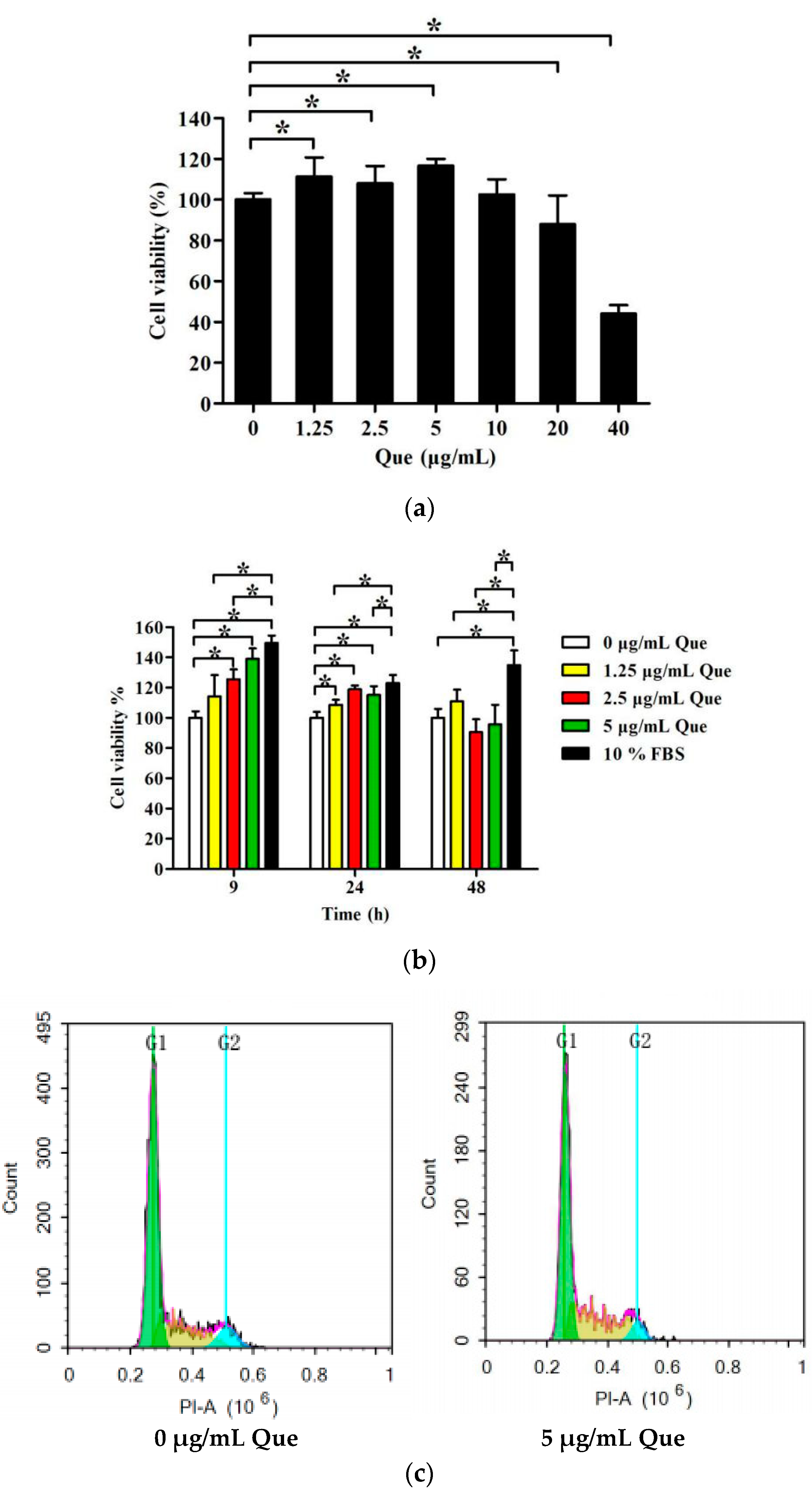
2.1. Effects of Que on IPEC-J2 Viability
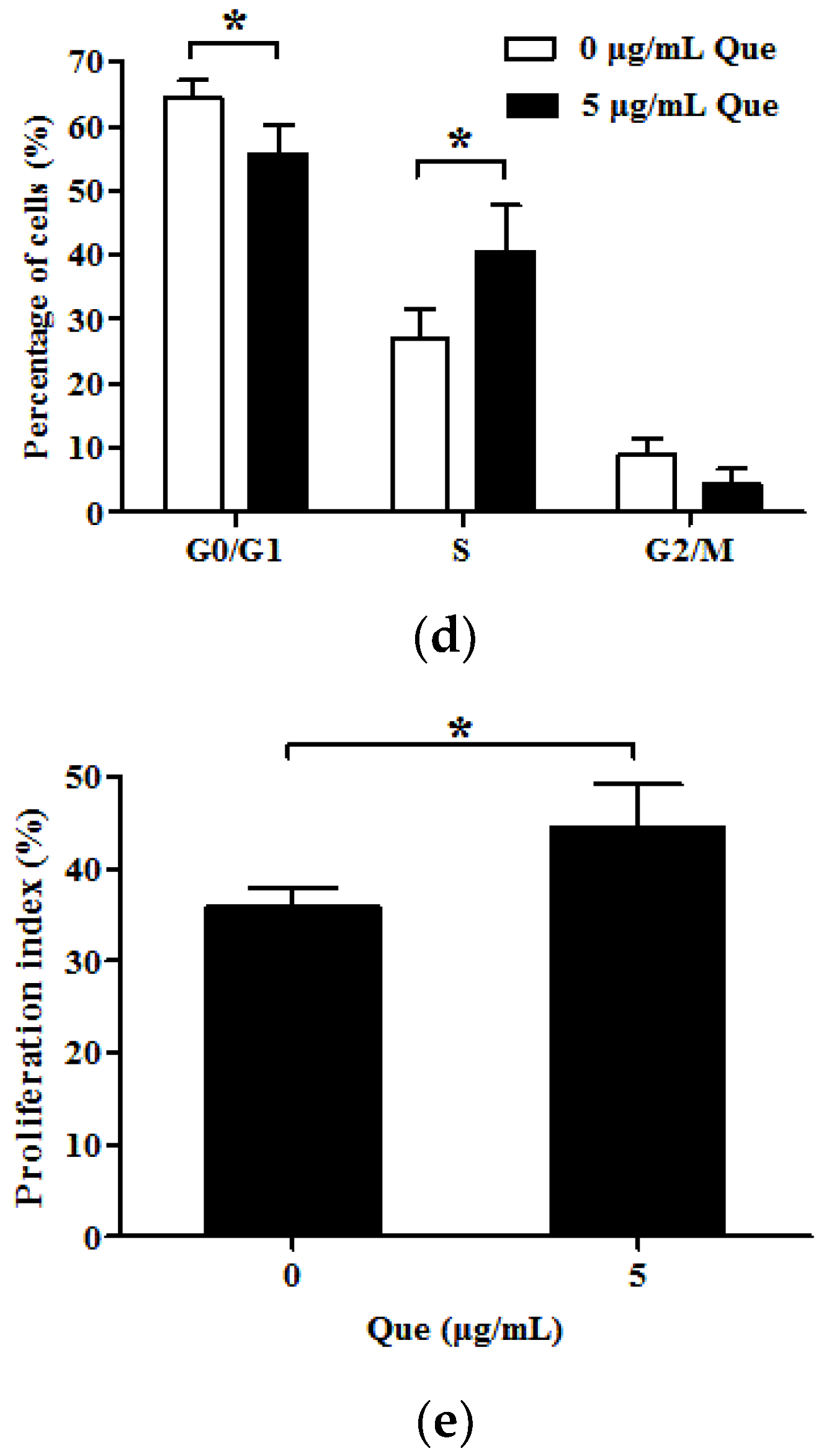
2.2. Effects of Que on Cell Cycle and Proliferation Index in IPEC-J2
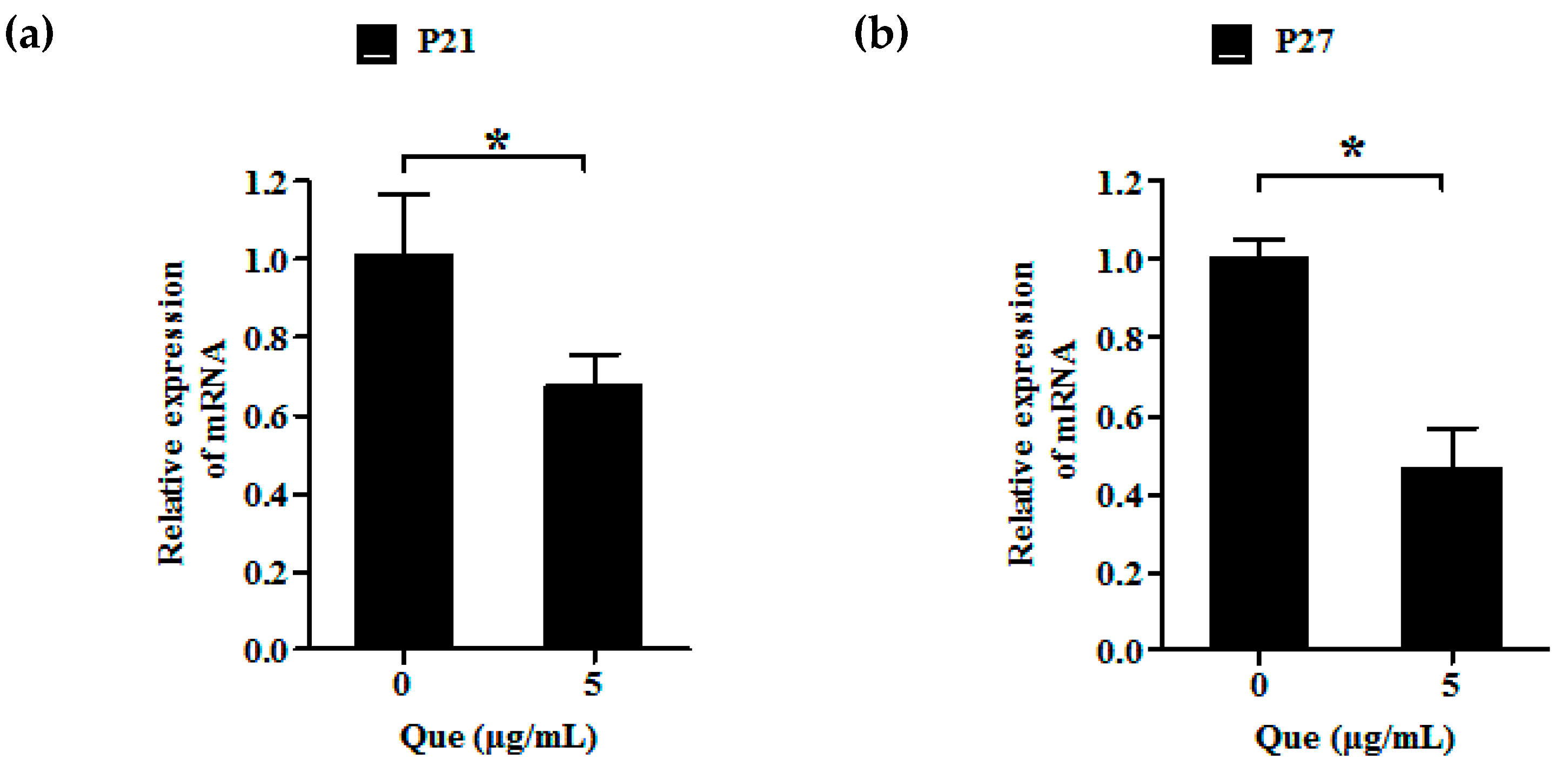
2.3. Effects of Que on mRNA Relative Expression Level of P21 and P27 of IPEC-J2
2.4. Effects of Que on Repair of IPEC-J2 after Scratching
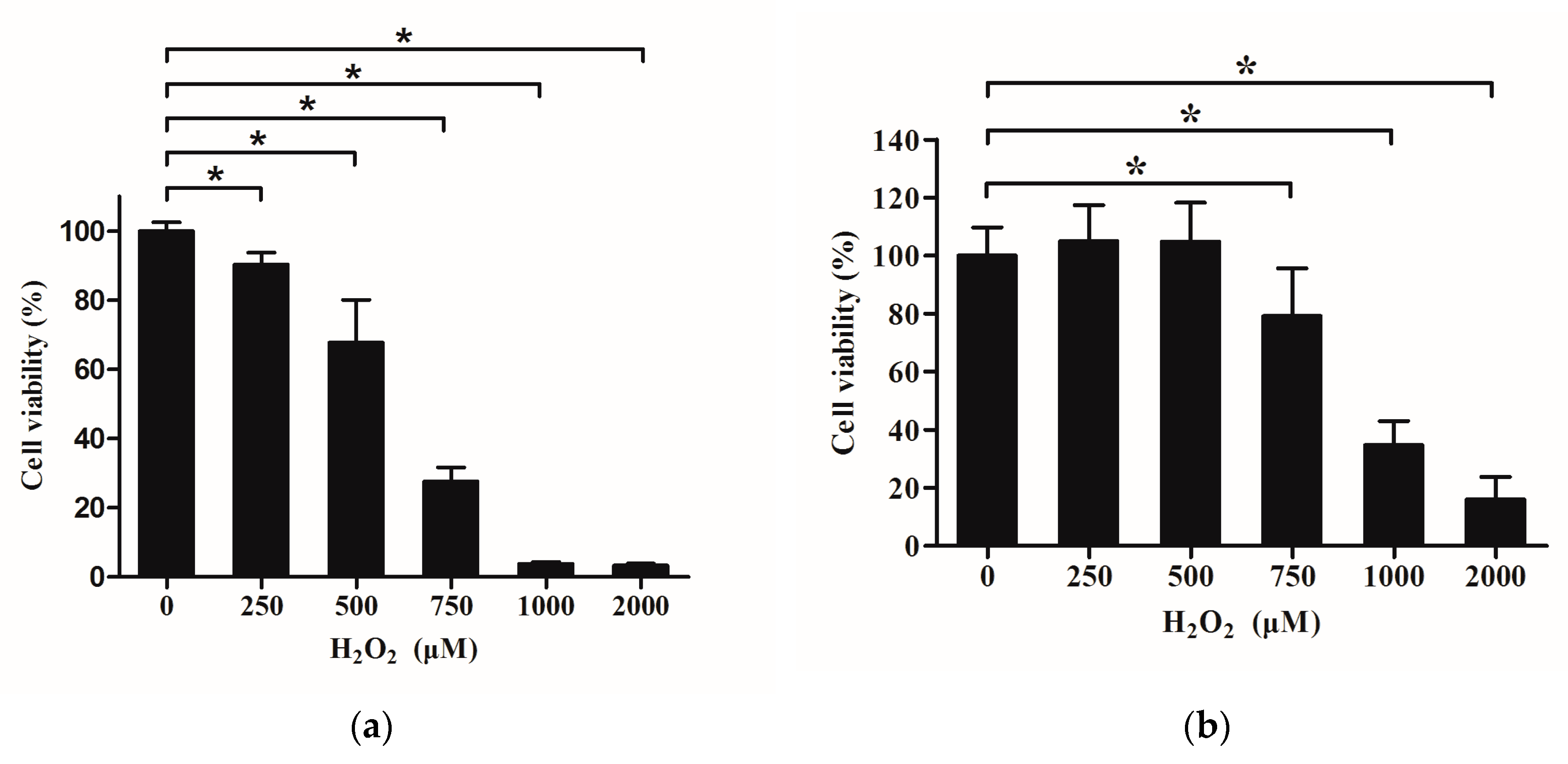
2.5. Effects of H2O2 on Viability of IPEC-J2
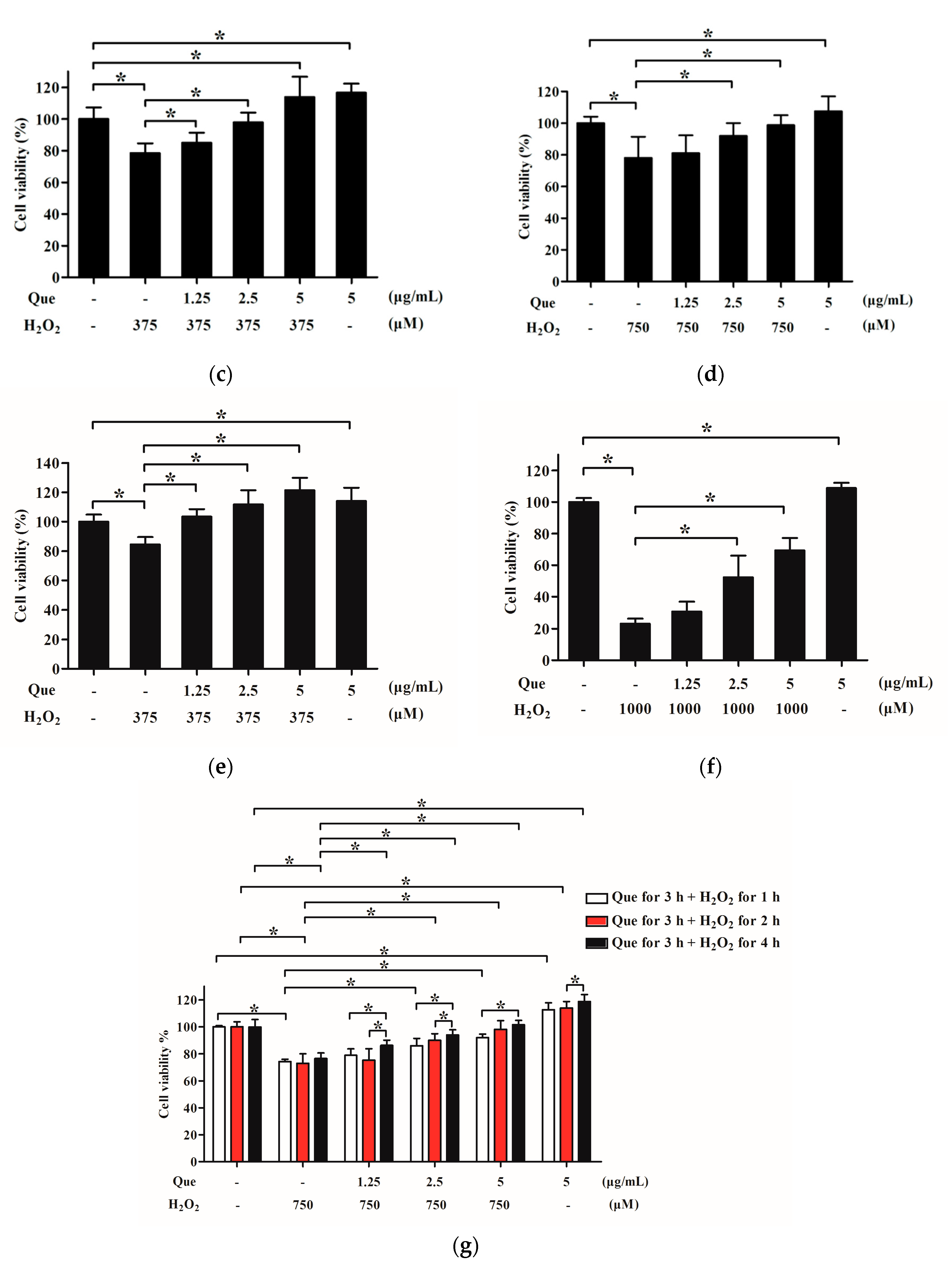
2.6. Effects of Que on Viability of IPEC-J2 Exposure to H2O2
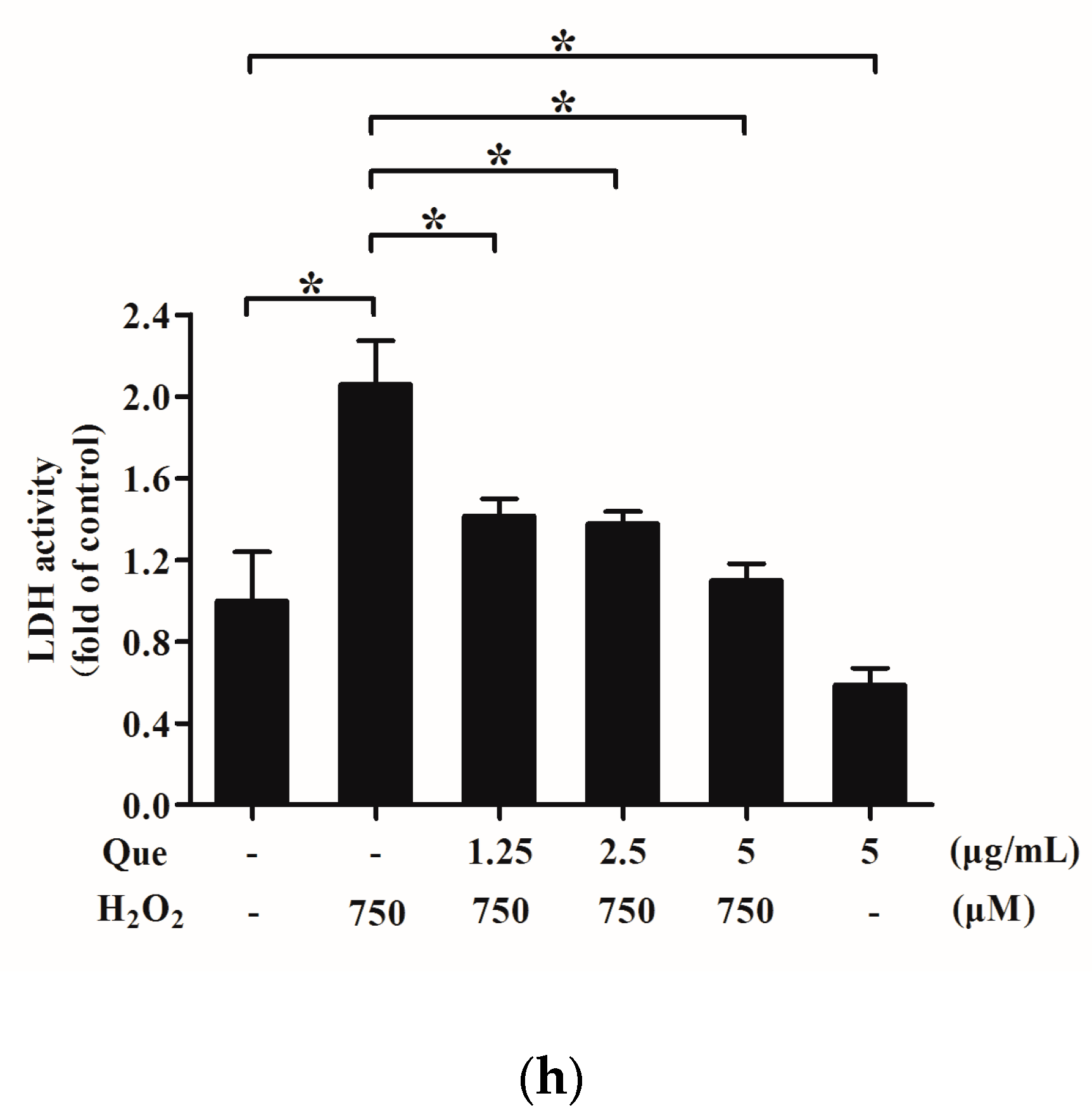
2.7. Effects of Que on Lactate Dehydrogenase (LDH) Activity in Culture Medium of IPEC-J2 Exposure to H2O2
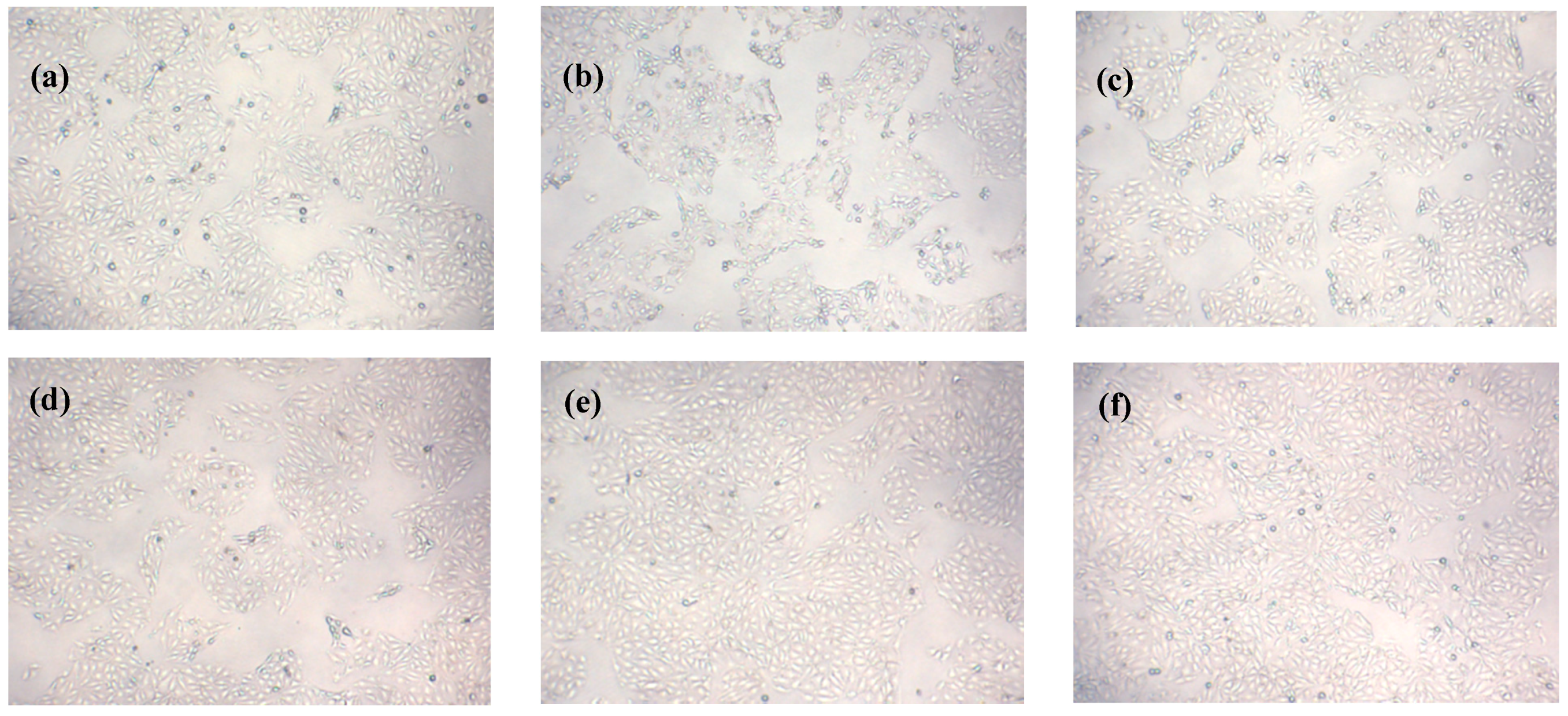
2.8. Effects of Que on Morphology of IPEC-J2 Cells Exposed to H2O2
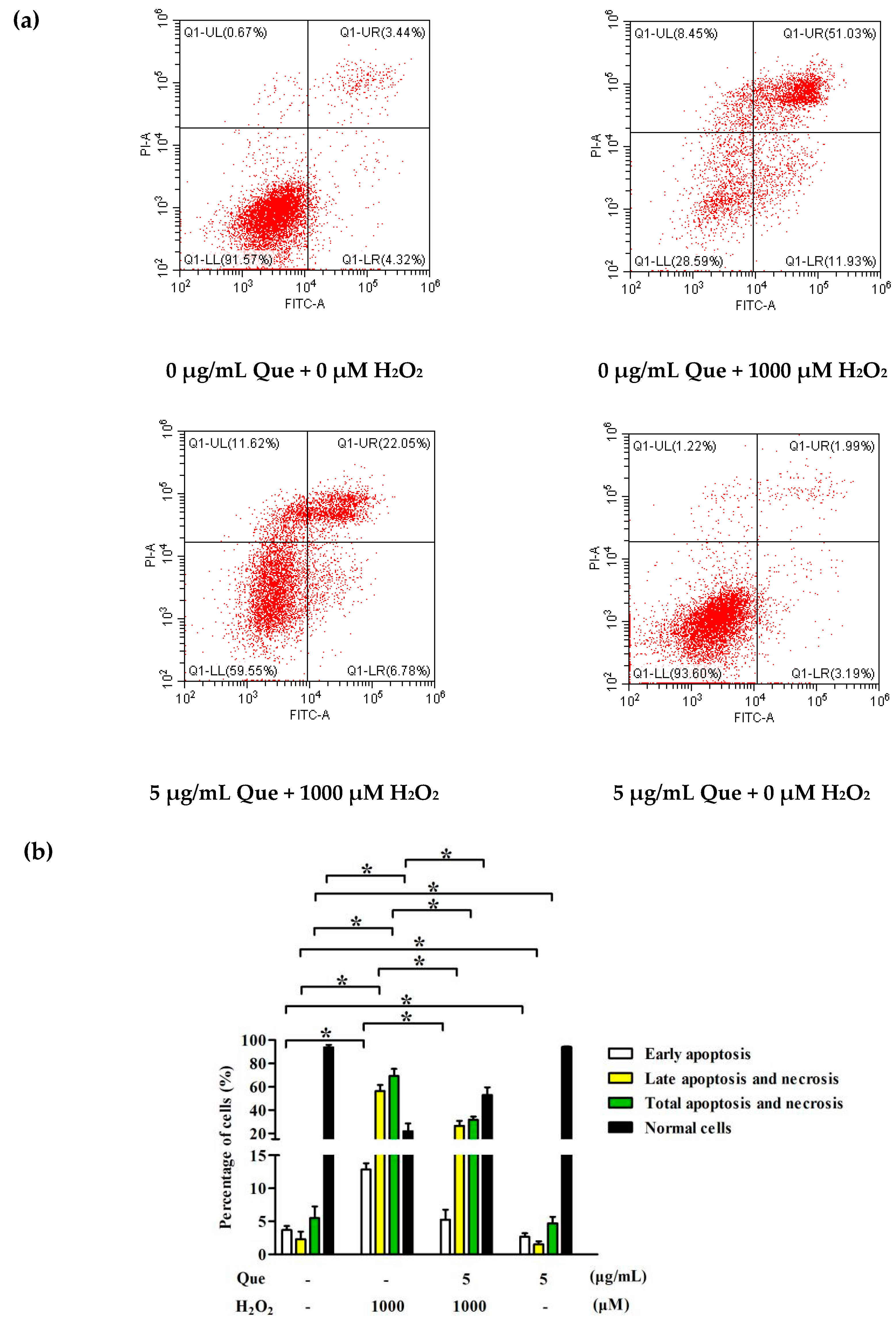
2.9. Effects of Que on Apoptosis of IPEC-J2 Exposure to H2O2
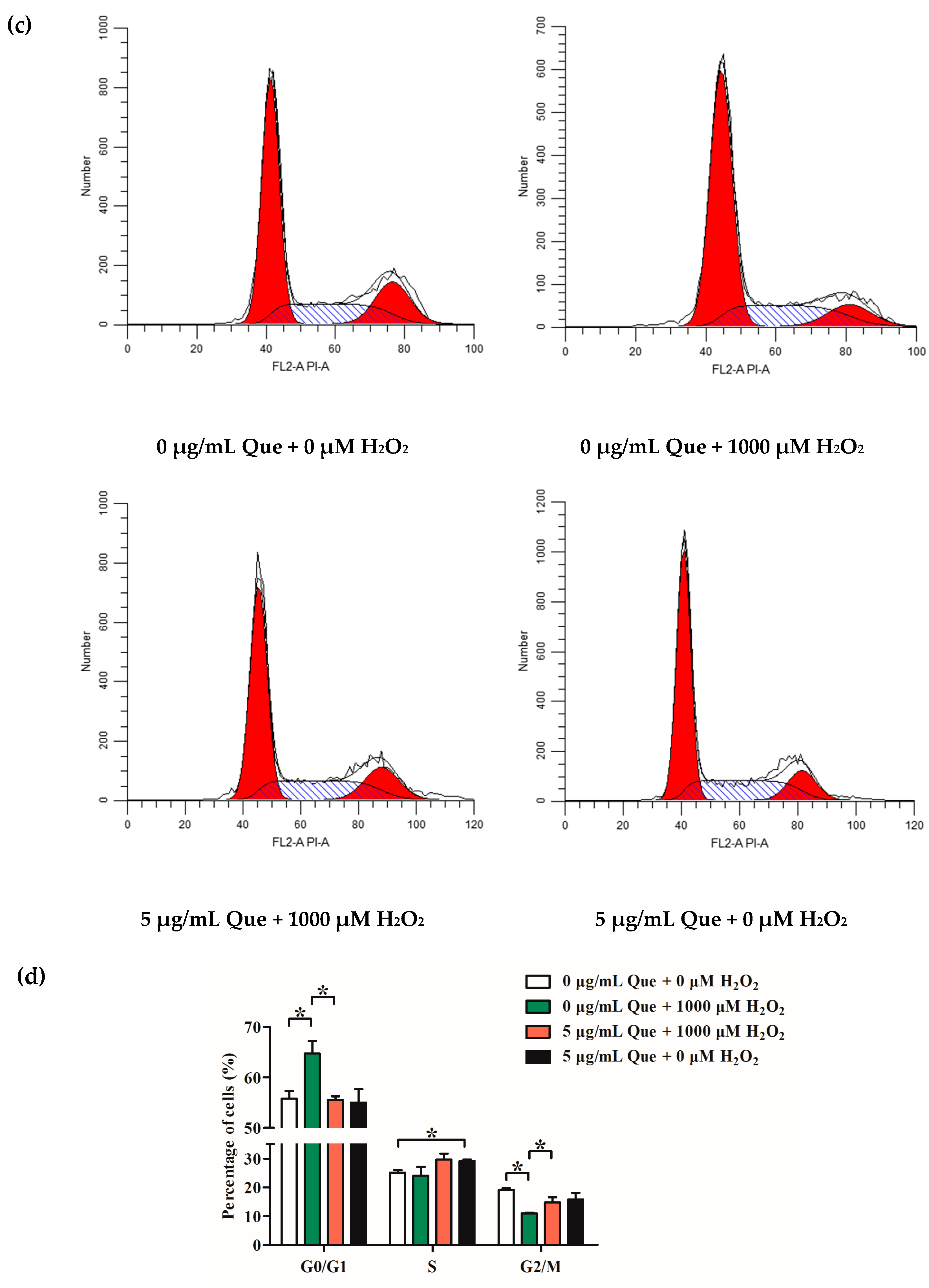
2.10. Effects of Que on Cell Cycle of IPEC-J2 Exposure to H2O2
2.11. Effects of Que on ROS Generation of IPEC-J2 Exposure to H2O2
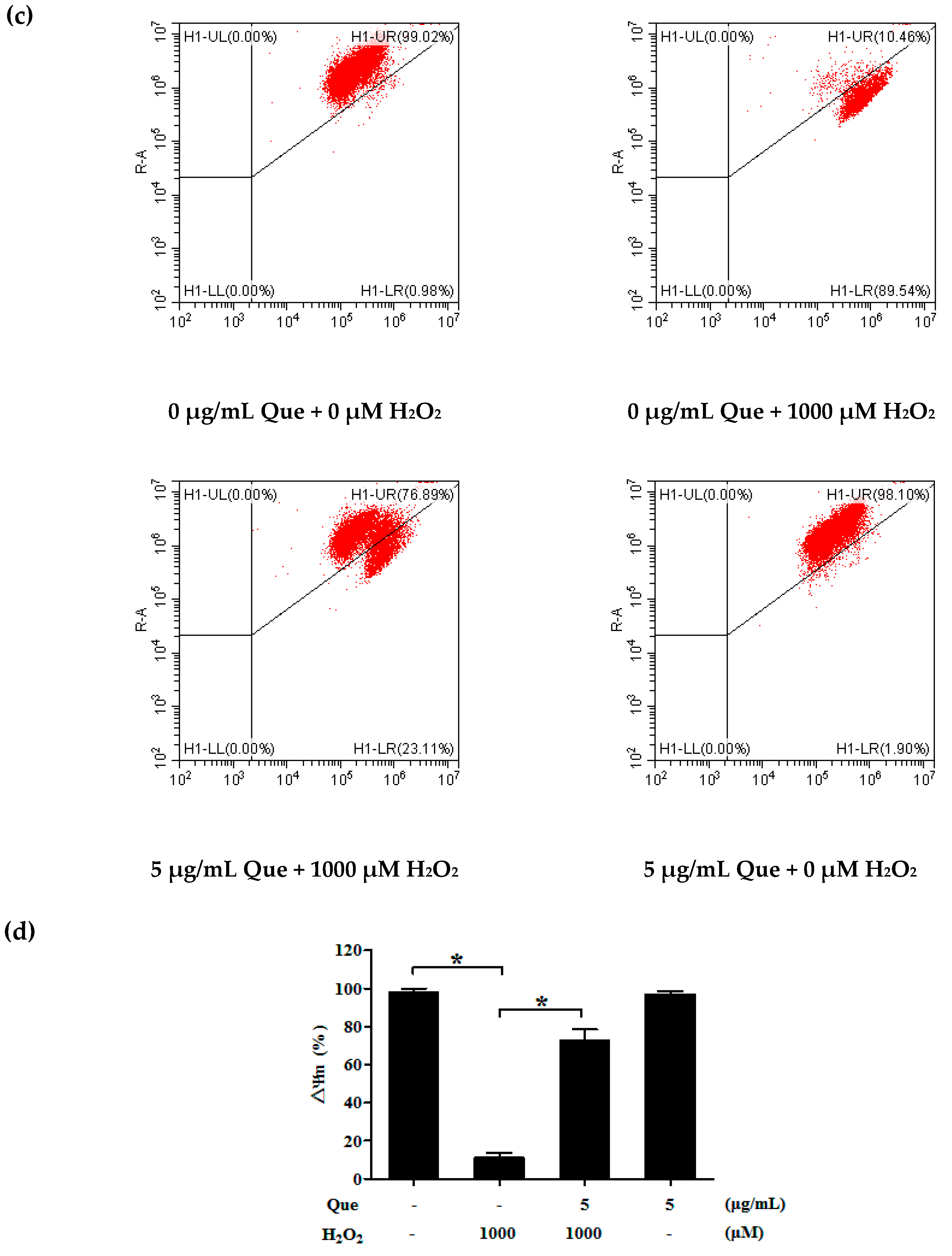
2.12. Effects of Que on Mitochondrial Membrane Potential (Δψm) of IPEC-J2 Exposure to H2O2
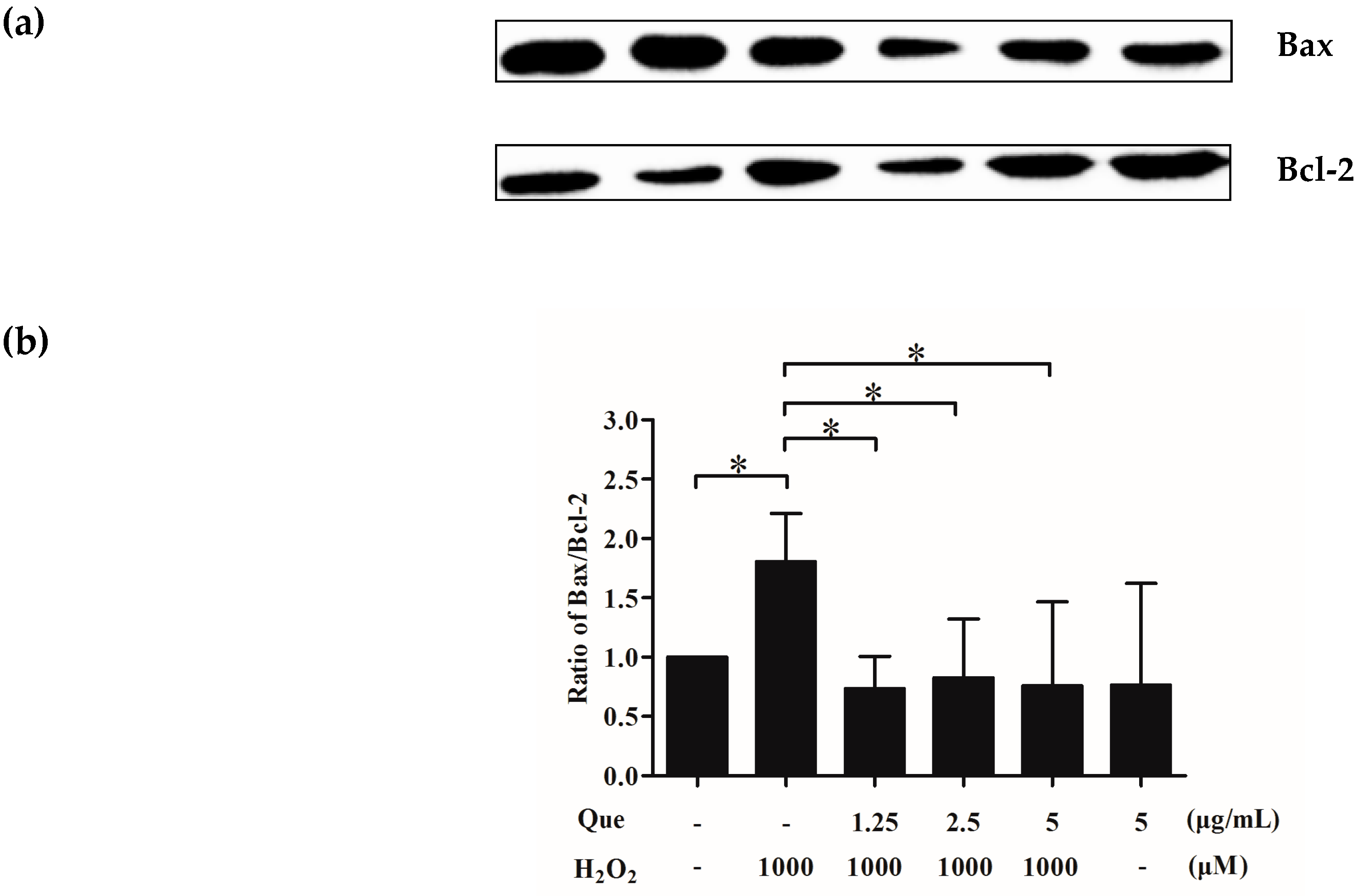
2.13. Effects of Que on Bax/Bcl-2 of IPEC-J2 Exposure to H2O2
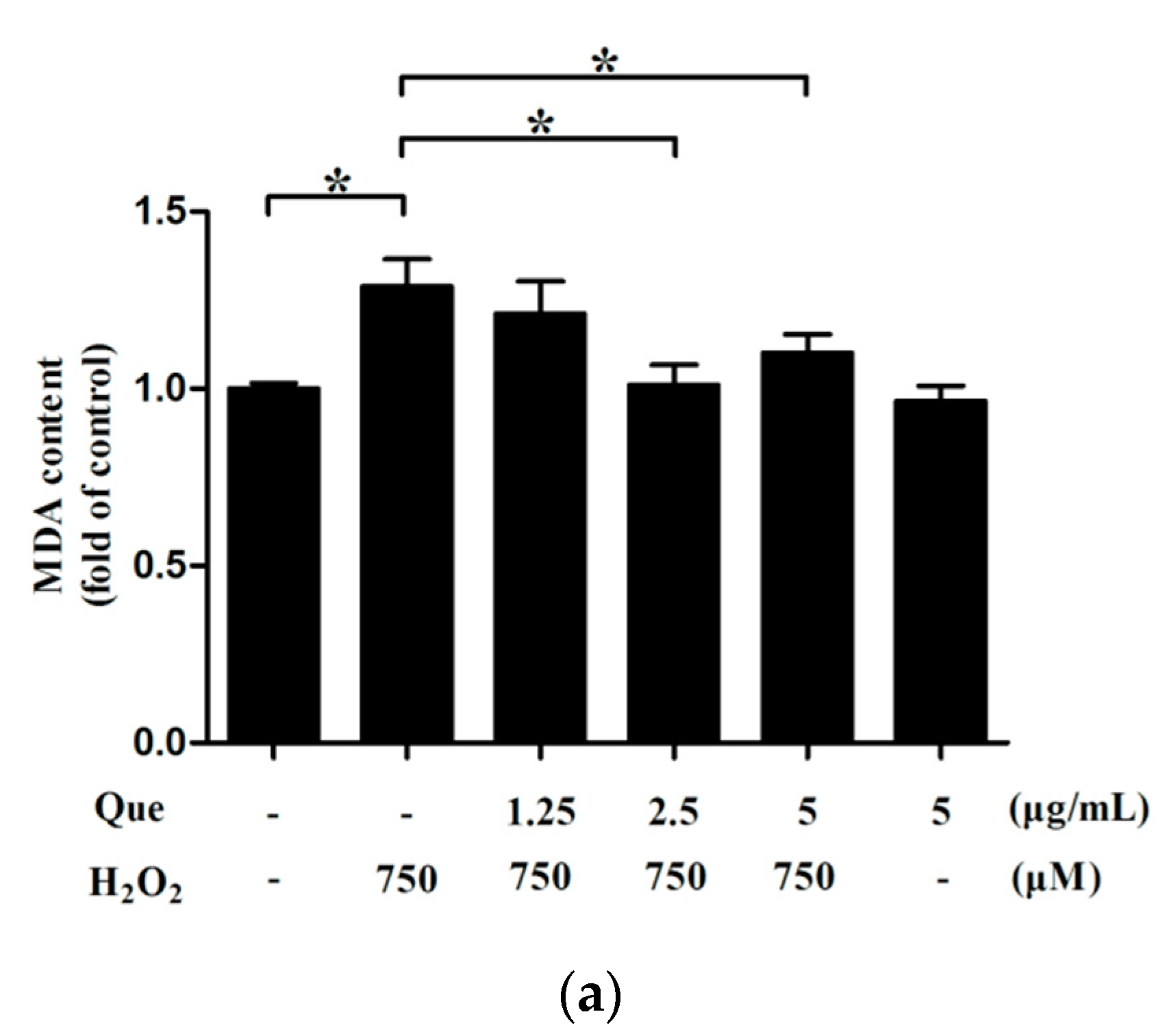
2.14. Effects of Que on Lipid Peroxidation of IPEC-J2 under Low-Degree Oxidative Damage
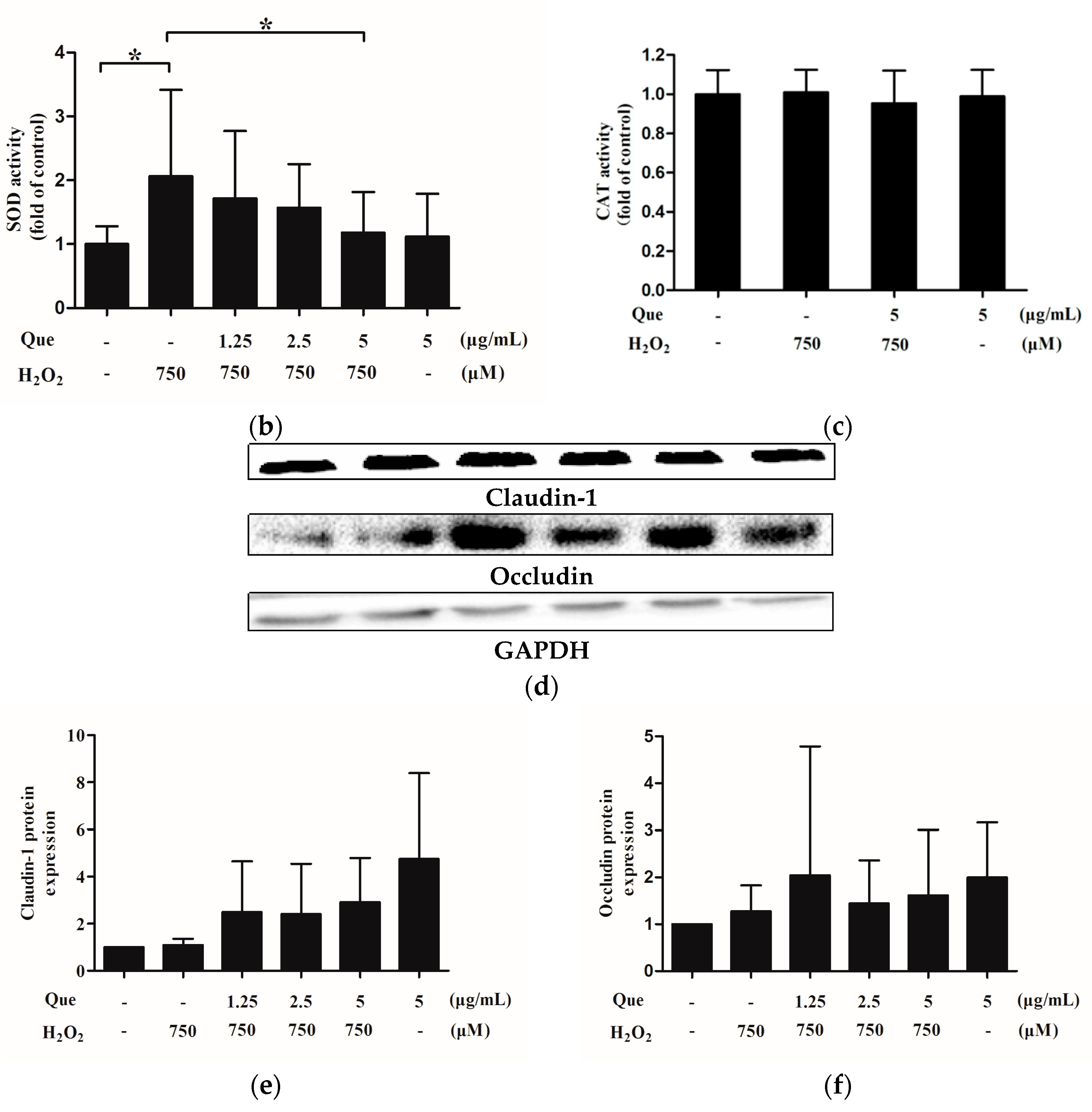
2.15. Effects of Que on Activity of Superoxide Dismutase (SOD) and Catalase (CAT) of IPEC-J2 under Low-Degree Oxidative Damage Conditions
2.16. Effects of Que on Claudin-1 and Occludin Expression of IPEC-J2 under Low-Degree Oxidative Damage Conditions
3. Discussion
4. Materials and Methods
4.1. Materials and Solution Preparation
4.2. Cells Culture
4.3. Cell Viability Assay
4.4. Measurement of Cell Cycle
4.5. Measurement of mRNA Relative Levels
4.6. Measurement of Cell Repair after Scratching
4.7. Determination of LDH Activity
4.8. Observation of Cell Morphology
4.9. Apoptosis Analysis
4.10. ROS Assay
4.11. Δψm Determination
4.12. Proteins Relative Expression Levels Assay
4.13. Determination of Level of Lipid Peroxidation (MDA), and Activities of SOD and CAT
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hu, J.; Chen, L.; Zheng, W.; Shi, M.; Liu, L.; Xie, C.; Wang, X.; Niu, Y.; Hou, Q.; Xu, X.; et al. Lactobacillus frumenti facilitates intestinal epithelial barrier function maintenance in early-weaned piglets. Front. Microbiol. 2018, 9, 897. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yin, J.; Wu, M.; Liao, P.; Deng, D.; Liu, G.; Wen, Q.; Wang, Y.; Qiu, W.; Liu, Y.; et al. Dietary glutamate supplementation ameliorates mycotoxin-induced abnormalities in the intestinal structure and expression of amino acid transporters in young pigs. PLoS ONE 2014, 9, e112357. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Y.; Sun, L.H.; Lin, Y.C.; Ma, X.Y.; Zheng, C.T.; Zhou, G.L.; Chen, F.; Zou, S.T. Effects of dietary glycyl-glutamine on growth performance, small intestinal integrity, andimmune responses of weaning piglets challenged with lipopolysaccharide. J. Anim. Sci. 2009, 87, 4050–4056. [Google Scholar] [CrossRef] [PubMed]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of two mycotoxins deoxynivalenol and fumonisin on pig intestinal health. Porcine Health Manag. 2016, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Pohl, C.S.; Medland, J.E.; Mackey, E.; Edwards, L.L.; Bagley, K.D.; De, W.M.P.; Williams, K.J.; Moeser, A.J. Early weaning stress induces chronic functional diarrhea, intestinal barrier defects, and increased mast cell activity in a porcine model of early life adversity. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Lv, Y.; Ren, S.; Shao, M.; Shen, T.; Huang, K.; Zhou, J.; Yan, L.; Song, S. Zearalenone (ZEA)-induced intestinal inflammation is mediated by the NLRP3 inflammasome. Chemosphere 2018, 190, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.L.; Liu, Y.L.; Xie, X.L.; Huang, J.J.; Hou, Y.Q. Effect of L-arginine on intestinal mucosal immune barrier function in weaned pigs after Escherichia coli LPS challenge. Innate Immun. 2013, 19, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Mariani, V.; Palermo, S.; Fiorentini, S.; Lanubile, A.; Giuffra, E. Gene expression study of two widely used pig intestinal epithelial cell lines: IPEC-J2 and IPI-2I. Vet. Immunol. Immunopathol. 2009, 131, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Brosnahan, A.J.; Brown, D.R. Porcine IPEC-J2 intestinal epithelial cells in microbiological investigations. Vet. Microbiol. 2012, 156, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Goossens, J.; Pasmans, F.; Verbrugghe, E.; Vandenbroucke, V.; De Baere, S.; Meyer, E.; Haesebrouck, F.; De Backer, P.; Croubels, S. Porcine intestinal epithelial barrier disruption by the Fusarium mycotoxins deoxynivalenol and T-2 toxin promotes transepithelial passage of doxycycline and paromomycin. BMC Vet. Res. 2012, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Ye, L.; Hou, Q.; Yu, Q. Effects of arginine on intestinal epithelial cell integrity and nutrient uptake. Br. J. Nutr. 2016, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Tian, G.; Chen, D.W.; Yao, Y.; He, J.; Zheng, P.; Mao, X.B.; Yu, J.; Huang, Z.Q.; Yu, B. Involvement of PKA signalling in anti-inflammatory effects of chitosan oligosaccharides in IPEC-J2 porcine epithelial cells. J. Anim. Physiol. Anim. Nutr. 2018, 102, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Oswald, I.P. Role of intestinal epithelial cells in the innate immune defence of the pig intestine. Vet. Res. 2006, 37, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, S.; Xiong, X.; Su, D.; He, J. Effects of Macleaya cordata alkaloids on intestinal porcine epithelial cells proliferation. Chin. J. Anim. Nutr. 2014, 26, 1632–1637. [Google Scholar] [CrossRef]
- Sukhotnik, I.; Agam, M.; Shamir, R.; Shehadeh, N.; Lurie, M.; Coran, A.G.; Shiloni, E.; Mogilner, J. Oral glutamine prevents gut mucosal injury and improves mucosal recovery following lipopolysaccharide endotoxemia in a rat. J. Surg. Res. 2007, 143, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Dua, T.K.; Dewanjee, S.; Khanra, R.; Joardar, S.; Barma, S.; Das, S.; Zia-Ul-Haq, M.; De Feo, V. Cytoprotective and antioxidant effects of an edible herb, Enhydra fluctuans Lour. (Asteraceae), against experimentally induced lead acetate intoxication. PLoS ONE 2016, 11, e0148757. [Google Scholar] [CrossRef] [PubMed]
- Ramyaa, P.; Padma, V.V. Ochratoxin-induced toxicity, oxidative stress and apoptosis ameliorated by quercetin-modulation by Nrf2. Food Chem. Toxicol. 2013, 62, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhao, Y.; Farouk, M.H.; Bao, N.; Wang, T.; Qin, G. Integrins were involved in soybean agglutinin induced cell apoptosis in IPEC-J2. Int. J. Mol. Sci. 2018, 19, 587. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, Y.; Li, C. Autophagy plays a positive role in zinc-induced apoptosis in intestinal porcine epithelial cells. Toxicol. In Vitro 2017, 44, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Shen, T.; Ding, Q.; Lv, Y.; Li, L.; Huang, K.; Yan, L.; Song, S. Zearalenone induces ROS-mediated mitochondrial damage in porcine IPEC-J2 cells. J. Biochem. Mol. Toxicol. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Zhang, H.; Ying, Z.; Li, Y.; Zhou, L.; Wang, F.; Zhang, L.; Wang, T. Effects of dietary L-methionine supplementation on intestinal integrity and oxidative status in intrauterine growth-retarded weanling piglets. Eur. J. Nutr. 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Sheng, Y.C.; Zheng, Z.Y.; Shi, L.; Wang, Z.T. The involvement of p62-Keap1-Nrf2 antioxidative signaling pathway and JNK in the protection of natural flavonoid quercetin against hepatotoxicity. Free Radic. Biol. Med. 2015, 85, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.B.; Mir, H.; Kapur, N.; Gales, D.N.; Carriere, P.P.; Singh, S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J. Surg. Oncol. 2018, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Hrelia, S. Quercetin reduces inflammatory responses in LPS-stimulated cardiomyoblasts. Oxid. Med. Cell. Longev. 2012, 2012, 837104. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Guo, C.; Wei, J.; Yang, J.; Jiang, Y. Study on the absorbtion of quercetin and rutin at different segments of intestine. Chin. J. Hyg. Res. 2002, 31, 55–57. [Google Scholar]
- Wang, H.L.; Liu, N.; Liu, Z.Q.; Liu, Z.Y.; Zhang, H.Q.; Zhou, Q.L. Absorbtion of quercetin and rutin across human intestinal epithelial by using Caco 2 cells. Chin. J. Jilin Univ. Med. Ed. 2007, 1, 33–39. [Google Scholar] [CrossRef]
- Ben, S.I.; Boussabbeh, M.; Graiet, I.; Rhouma, A.; Bacha, H.; Essefi, S.A. Quercetin protects HCT116 cells from Dichlorvos-induced oxidative stress and apoptosis. Cell Stress Chaperones 2016, 21, 179–186. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Han, Z.; Tang, F. Protective effects of Semen Crotonis Pulveratum on trinitrobenzene sulphonic acid-induced colitis in rats and H2O2-induced intestinal cell apoptosis in vitro. Int. J. Mol. Med. 2015, 35, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhu, L.; Chen, X.; Sheng, Y.; Guo, Q.; Bao, J.; Xu, J. X/XO or H2O2 induced IPEC-J2 cell as a new in vitro model for studying apoptosis in post-weaning piglets. Cytotechnology 2016, 68, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.N.; Mei, Q.B.; Wang, Z.P.; Zhang, Y.; Liu, L.; Liu, X.Y. Promoting effects of Rheum tanguticum polysaccharide on IEC-6 cell proliferation, migration and its possible mechanism. Chin. Pharmacol. Bull. 2008, 24, 303–307. [Google Scholar]
- Palócz, O.; Pászti-Gere, E.; Gálfi, P.; Farkas, O. Chlorogenic acid combined with Lactobacillus plantarum 2142 reduced LPS-induced intestinal inflammation and oxidative stress in IPEC-J2 cells. PLoS ONE 2016, 11, e0166642. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P. Control models of cell cycle transit, exit, and arrest. Biochem. Cell Biol. 1988, 66, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.F.; Ding, X.M.; Peng, X.; Li, X.C.; Fang, J. Effects of chitosan oligosaccharides supplementation on the cell cycle of immune organs in broilers. Kafkas. Univ. Vet. Fak. Derg. 2017, 23, 1003–1006. [Google Scholar] [CrossRef]
- Blagosklonny, M.V.; Pardee, A.B. The restriction point of the cell cycle. Cell Cycle 2012, 1, 102–109. [Google Scholar] [CrossRef]
- Wu, J.H.; Wei, W.; Wu, S.B. Effect of zinc-deficiency on cell cycle, DNA and protein contents of thymus and spleen. Ying Yang Xue Bao 1994, 16, 269–273. [Google Scholar] [CrossRef]
- Liu, G.; Ren, X.; Gao, C.; Zhang, W. Acylglycerol kinase promotes the proliferation and cell cycle progression of oral squamous cellcarcinoma. Mol. Med. Rep. 2015, 12, 2225–2230. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, Y.C. Cyclin-dependent kinase inhibitors in mammal cells. Prog. Physiol. Sci. 1996, 27, 107–112. [Google Scholar]
- Wang, X.H.; Xie, M.S.; Xu, G.X. Expression of p21 and p27 in rabbit lens epithelial cells. Int. Eye Sci. 2012, 12, 1629–1632. [Google Scholar]
- Wu, Y.S.; Lin, Y.P.; Lu, T.X.; Lin, Y.; Huang, Y.M.; Huang, M.Y. Effects of Jiangu granule containing serum on the cyclins in rat’s osteoblast at G1 phase. Chin. J. Integr. Tradit. West. Med. 2010, 30, 966–969. [Google Scholar]
- Qiu, Y.; Ma, X.; Yang, X.; Wang, L.; Jiang, Z. Effect of sodium butyrate on cell proliferation and cell cycle in porcine intestinal epithelial (IPEC-J2) cells. In Vitro Cell. Dev. Biol. Anim. 2017, 53, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Guasti, L.; Squizzato, A.; Moretto, P.; Vigetti, D.; Ageno, W.; Dentali, F.; Maresca, A.M.; Campiotti, L.; Grandi, A.M.; Passi, A. In vitro effects of Apixaban on 5 different cancer cell lines. PLoS ONE 2017, 12, e0185035. [Google Scholar] [CrossRef] [PubMed]
- Vergauwen, H.; Tambuyzer, B.; Jennes, K.; Degroote, J.; Wang, W.; De Smet, S.; Michiels, J.; Van Ginneken, C. Trolox and ascorbic acid reduce direct and indirect oxidative stress in the IPEC-J2 cells, an in vitro model for the porcine gastrointestinal tract. PLoS ONE 2015, 10, e0120485. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhong, Z.; Xu, Z.; Chen, L.; Wang, Y. No protective effect of curcumin on hydrogen peroxide-induced cytotoxicity in HepG2 cells. Pharmacol. Rep. 2011, 63, 724–732. [Google Scholar] [CrossRef]
- Vergauwen, H.; Prims, S.; Degroote, J.; Wang, W.; Casteleyn, C.; Van Cruchten, S.; De Smet, S.; Michiels, J.; Van Ginneken, C. In Vitro Investigation of six antioxidants for pig diets. Antioxidants 2016, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, W.; Dong, M. Flavonoids of Kudzu root fermented by Eurtotium cristatum protected rat pheochromocytoma line 12 (PC12) cells against H2O2-induced apoptosis. Int. J. Mol. Sci. 2017, 18, 2754. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Z.H.; He, L.L.; Yao, Y.Q.; Liu, H.; Wang, L.X. Effect of autophagy inhibitor 3-MA on autophagy and apoptosis of “starving” myocardial cells. Chin. J. Wuhan Inst. Phys. Educ. 2014, 48, 61–66. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 19, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Bao, D.; Wang, J.; Pang, X.; Liu, H. Protective effect of quercetin against oxidative stress-induced cytotoxicity in rat pheochromocytoma (PC-12) cells. Molecules 2017, 22, 1122. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.H.; Wang, H.X.; Tang, F.T.; Lu, M.L.; Han, R.H.; Mei, M.; Hu, J. Protective effect of Astragaloside IV on dysfunction of human umbilical vein endothelial cells induced by hydrogen peroxide. Chin. J. Exp. Tradit. Med. Formulae 2015, 21, 101–104. [Google Scholar] [CrossRef]
- Sak, K. Dependence of DPPH radical scavenging activity of dietary flavonoid quercetin on reaction environment. Mini. Rev. Med. Chem. 2014, 14, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Ružić, I.; Skerget, M.; Knez, Z. Potential of phenolic antioxidants. Acta Chim. Slov. 2010, 57, 263–271. [Google Scholar] [PubMed]
- Xie, M.L.; Qian, Z.N.; Gu, Z.L.; Hu, T.X. Preliminary study on the anti-free-radical effects of quercetin and some Chinise herbs extracts. Chin. Suzhou Univ. J. Med. Sci. 1989, 341, 278–280. [Google Scholar]
- Je, J.Y.; Lee, D.B. Nelumbo nucifera leaves protect hydrogen peroxide-induced hepatic damage via antioxidant enzymes and HO-1/Nrf2 activation. Food Funct. 2015, 6, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Ji, L.; Zhang, S.; Xu, L.; Yin, L.; Li, L.; Zhao, Y.; Peng, J. Total flavonoids from Rosa Laevigata Michx fruit attenuates hydrogen peroxide induced injury in human umbilical vein endothelial cells. Food Chem. Toxicol. 2012, 50, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
- Seto, S.W.; Chang, D.; Ko, W.M.; Zhou, X.; Kiat, H.; Bensoussan, A.; Lee, S.M.; Hoi, M.P.; Steiner, G.Z.; Liu, J. Sailuotong prevents hydrogen peroxide (H2O2)-induced injury in EA.hy926 cells. Int. J. Mol. Sci. 2017, 18, 95. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.R.; Yu, H.T.; Yang, Y.; Hang, L.; Yang, X.W.; Ding, S.H. Quercetin phospholipid complex significantly protects against oxidative injury in ARPE-19 cellsassociated with activation of Nrf2 pathway. Eur. J. Pharmacol. 2016, 770, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.W. Study on the Effects and Mechanisms of Lactobacillus Acidophilus on Oxidative Stress Induced by Oxidative Stress in Porcine Intestinal Epithelial Cells. Master’s Thesis, Jiangxi Agricultural University, Nanchang, China, 2017. [Google Scholar]
- Yu, C.H.; Liu, Z.Y.; Sun, L.S.; Li, Y.J.; Zhang, D.S.; Pan, R.T.; Sun, Z.L. Effect of Danofloxacin on reactive oxygen species production, lipid peroxidation and antioxidant enzyme activities in kidney tubular epithelial cell line, LLC-PK1. Basic Clin. Pharmacol. Toxicol. 2013, 113, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Fentoğlu, Ö.; Kırzıoğlu, F.Y.; Bulut, M.T.; Kumbul Doğuç, D.; Kulaç, E.; Önder, C.; Günhan, M. Evaluation of lipid peroxidation and oxidative DNA damage in patients with periodontitis and hyperlipidemia. J. Periodontol. 2015, 86, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, A.; Zeng, X.; Hou, C.; Liu, H.; Qiao, S. Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol. 2015, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Jiang, S.; Jiang, Z.; Zheng, C.; Gou, Z. Effects of equol on H2O2-induced oxidative stress in primary chicken intestinal epithelial cells. Poult. Sci. 2016, 95, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.; Wang, W.C.; Lin, C.X.; Fouad, A.M.; Chen, W.; Xia, W.G.; Wang, S.; Luo, X.; Zhang, W.H.; Yan, S.J.; et al. Effects of curcumin on performance, antioxidation, intestinal barrier and mitochondrial function in ducks fed corn contaminated with ochratoxin. Animal 2018, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zhang, Q.; Wang, C.; Wu, H.; Jiao, L.; Hong, Q.; Hu, C. LPS challenge increased intestinal permeability, disrupted mitochondrial function and triggered mitophagy of piglets. Innate Immun. 2018, 24, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Li, C.M.; Yan, H.C.; Fu, H.L.; Xu, G.F.; Wang, X.Q. Molecular cloning, sequence analysis, and function of the intestinal epithelial stem cell markerBmi1 in pig intestinal epithelial cells. J. Anim. Sci. 2014, 92, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dai, J.; Zhang, D.; Sun, Z.; Liu, Z. Oxidative stress inducting danofloxacin induces cell cycle arrest in LLC-PK1. Chin. J. Antibiot. 2016, 41, 388–395. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Trial NO. | Trial Designs |
|---|---|
| Trial 1 | IPEC-J2 were pre-cultured for 24 h. Cells were pre-incubated with Que for 2 h, then incubated with 375 μM H2O2 for 22 h. |
| Trial 2 | IPEC-J2 were pre-cultured for 24 h. Cells were pre-incubated with Que for 20 h, then incubated with 750 μM H2O2 for 4 h. |
| Trial 3 | IPEC-J2 were pre-cultured for 24 h. Cells were co-incubated with Que and 375 μM H2O2 for 24 h. |
| Trial 4 | IPEC-J2 were pre-cultured for 48 h. Cells were pre-incubated with Que for 3 h, then incubated with 1000 μM H2O2 for 4 h. |
| Trial 5 | IPEC-J2 were pre-cultured for 48 h. Cells were pre-incubated with Que for 3 h, then incubated with 750 μM H2O2 for 1 h. |
| Trial 6 | IPEC-J2 were pre-cultured for 48 h. Cells were pre-incubated with Que for 3 h, then incubated with 375 μM H2O2 for 2 h. |
| Trial 7 | IPEC-J2 were pre-cultured for 48 h. Cells were pre-incubated with Que for 3 h, then incubated with 375 μM H2O2 for 4 h. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Yuan, Q.; Xu, G.; Chen, H.; Lei, H.; Su, J. Effects of Quercetin on Proliferation and H2O2-Induced Apoptosis of Intestinal Porcine Enterocyte Cells. Molecules 2018, 23, 2012. https://doi.org/10.3390/molecules23082012
Chen Z, Yuan Q, Xu G, Chen H, Lei H, Su J. Effects of Quercetin on Proliferation and H2O2-Induced Apoptosis of Intestinal Porcine Enterocyte Cells. Molecules. 2018; 23(8):2012. https://doi.org/10.3390/molecules23082012
Chicago/Turabian StyleChen, Zhigang, Qiaoling Yuan, Guangren Xu, Huiyu Chen, Hongyu Lei, and Jianming Su. 2018. "Effects of Quercetin on Proliferation and H2O2-Induced Apoptosis of Intestinal Porcine Enterocyte Cells" Molecules 23, no. 8: 2012. https://doi.org/10.3390/molecules23082012
APA StyleChen, Z., Yuan, Q., Xu, G., Chen, H., Lei, H., & Su, J. (2018). Effects of Quercetin on Proliferation and H2O2-Induced Apoptosis of Intestinal Porcine Enterocyte Cells. Molecules, 23(8), 2012. https://doi.org/10.3390/molecules23082012




