A Bifunctional Anti-Amyloid Blocks Oxidative Stress and the Accumulation of Intraneuronal Amyloid-Beta
Abstract
1. Introduction
2. Results
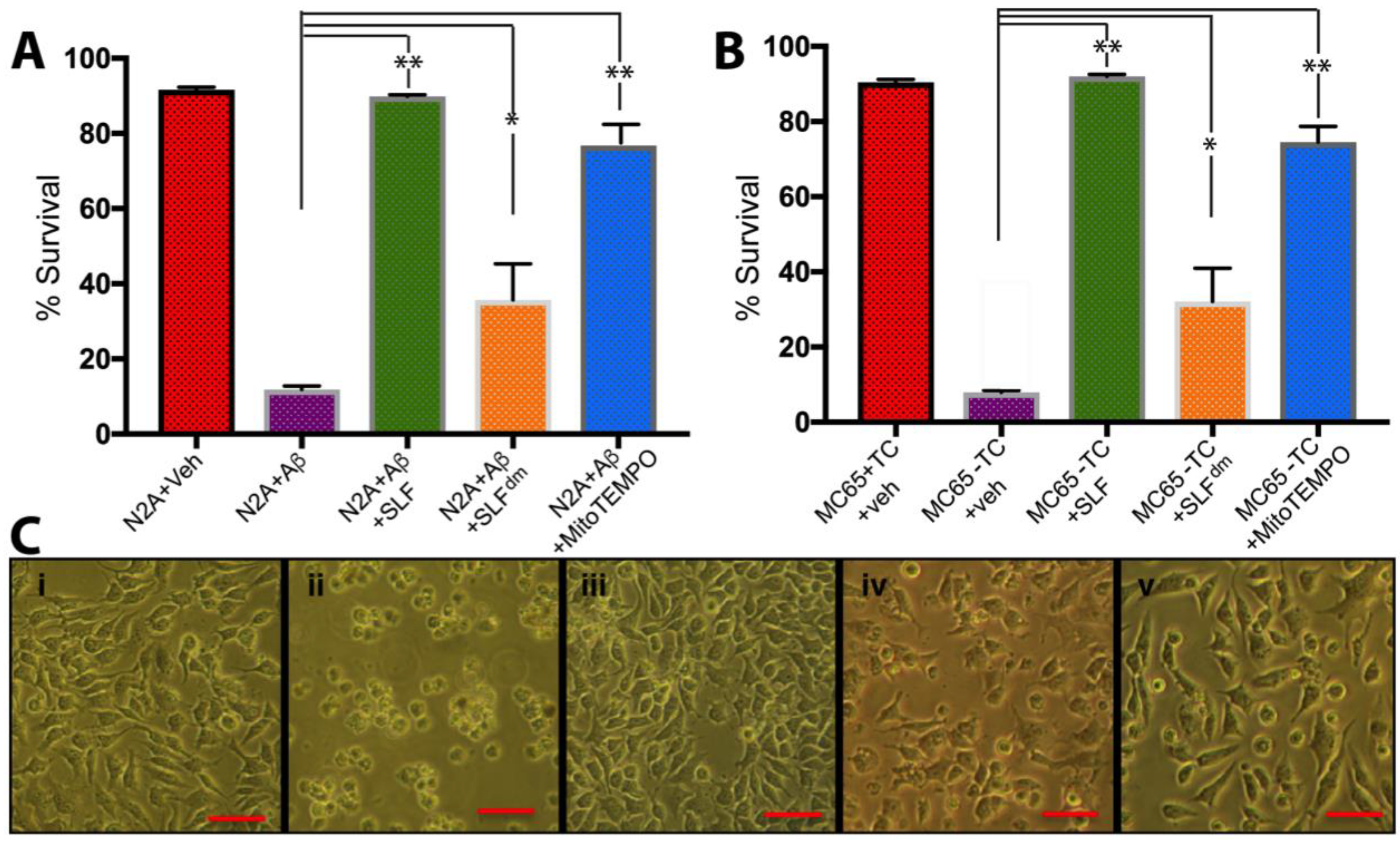
2.1. In Cultured Neurons, the Bifunctional Activity of SLF Offers Superior Protection against the Toxicity of both Exogenous Aβ and Aβ Peptide Generated Intracellularly
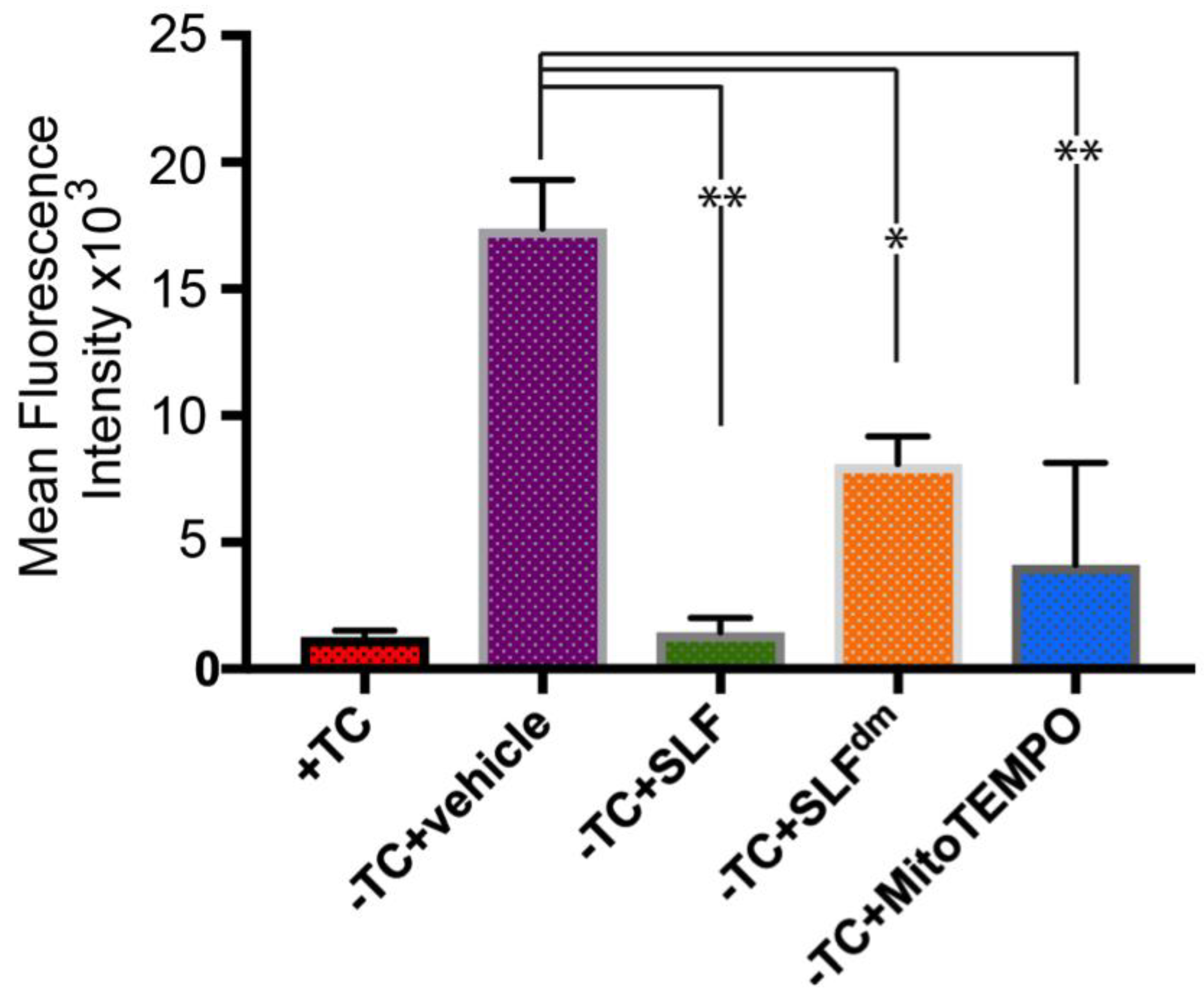
2.2. SLF’s Nitroxide Component Plays a Key Role in Decreasing Aβ-Induced Oxidative Stress in a Human Neuroblastoma Cell Line (MC65) Overexpressing the Amyloid Precursor Protein
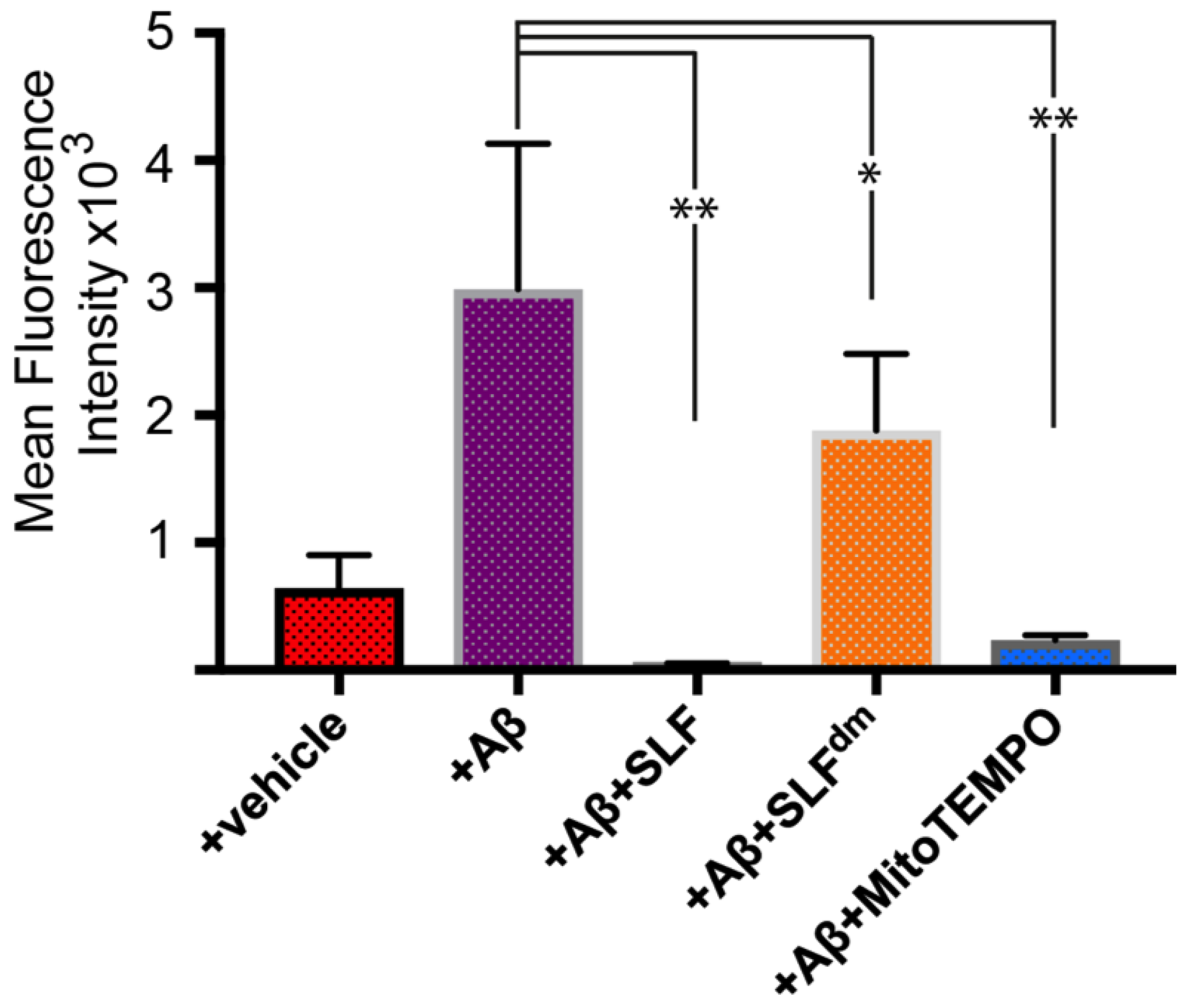
2.3. The Nitroxide Group of the SLF Compound Plays a Key Role in Decreasing Exogenous Aβ-Induced Oxidative Stress
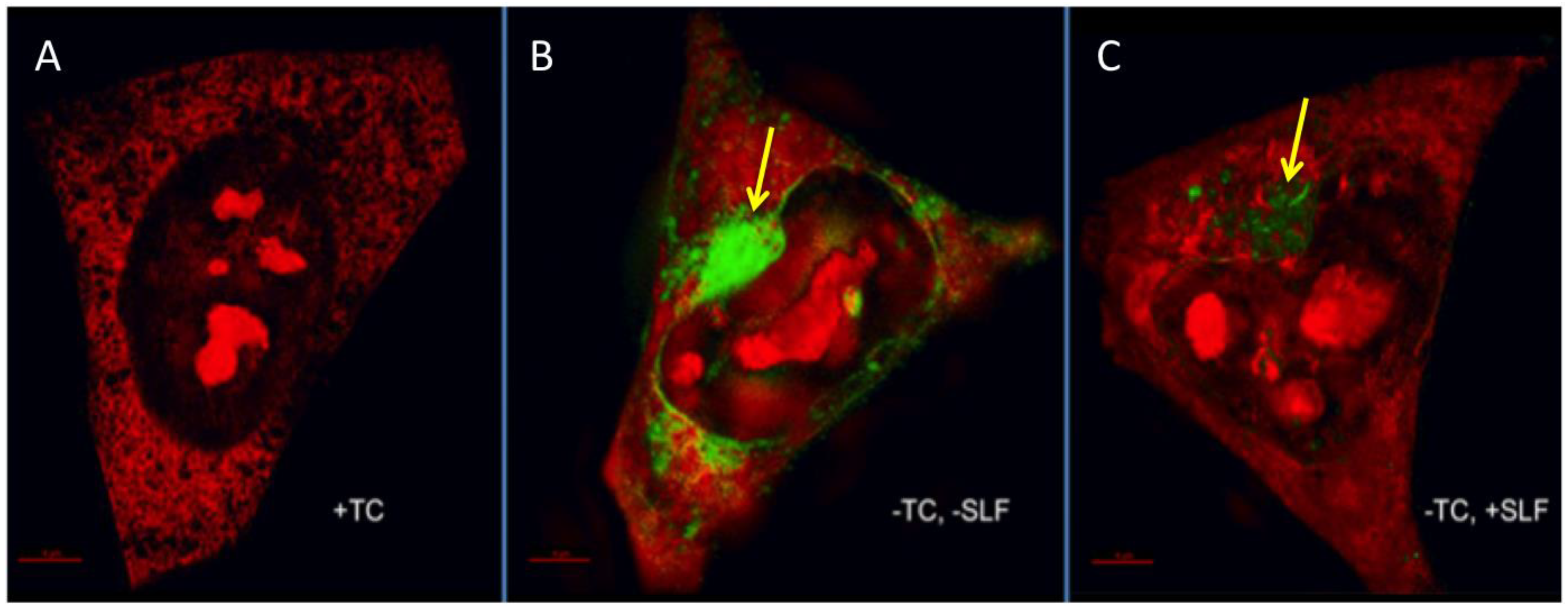
2.4. SLF Attenuates the Cytoplasmic Accumulation of Intracellular Aβ in a Human Neuroblastoma Cell Line Overexpressing the Amyloid Precursor Protein Shown by Super-Resolution Structured Illumination Imaging
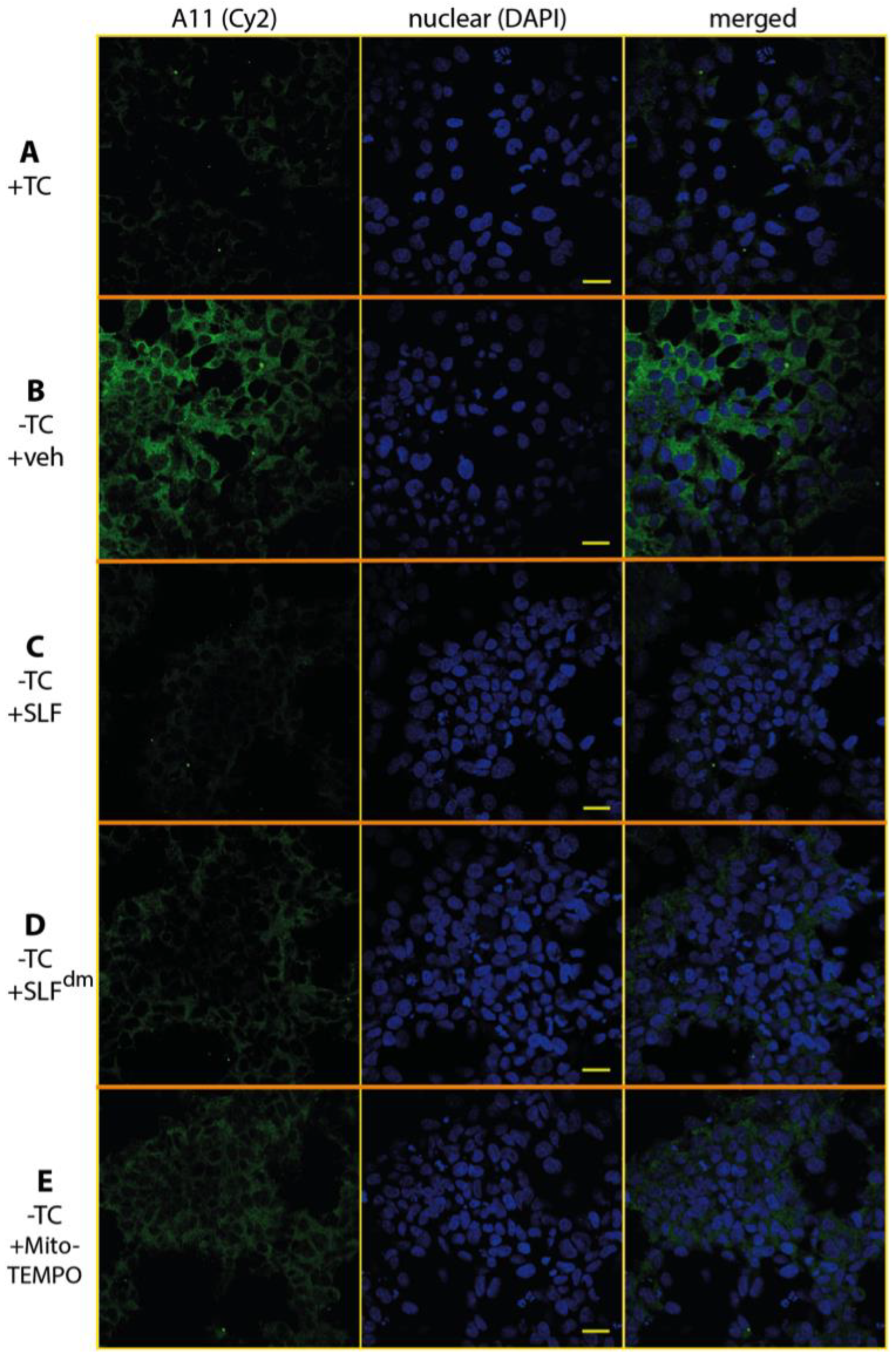
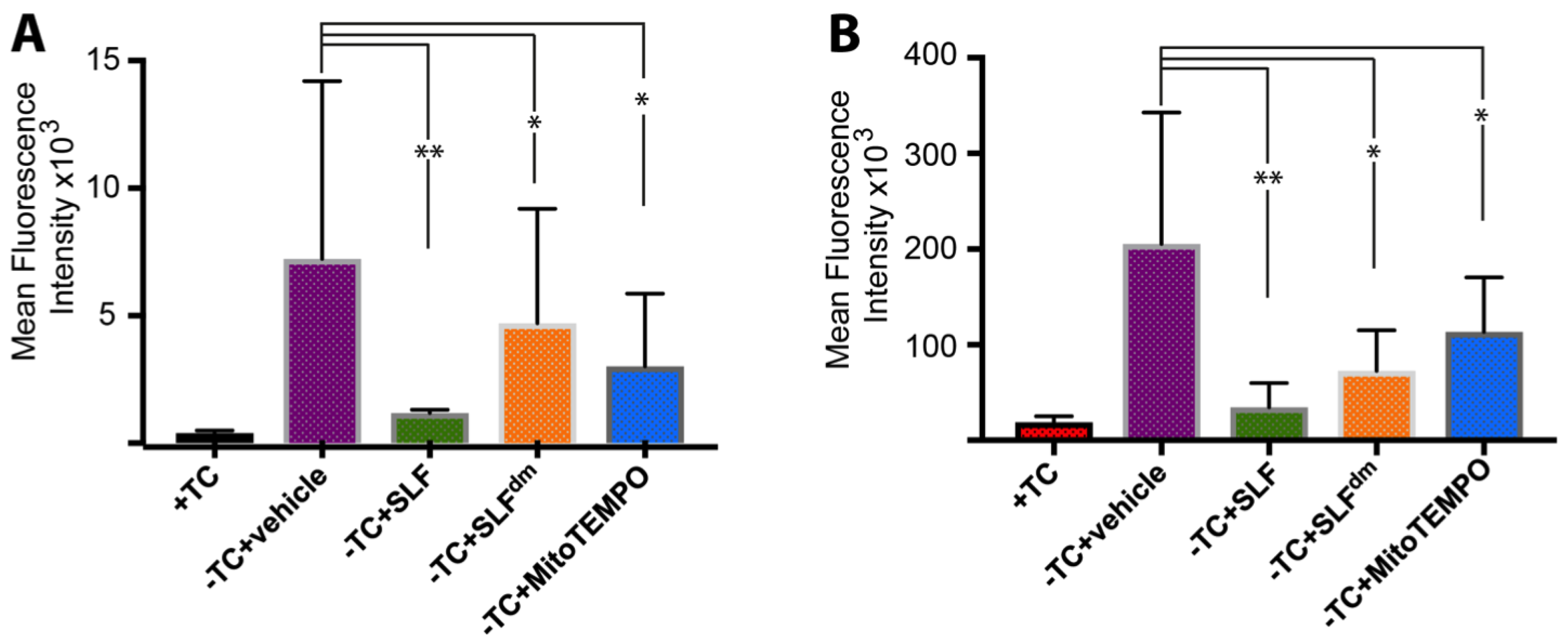
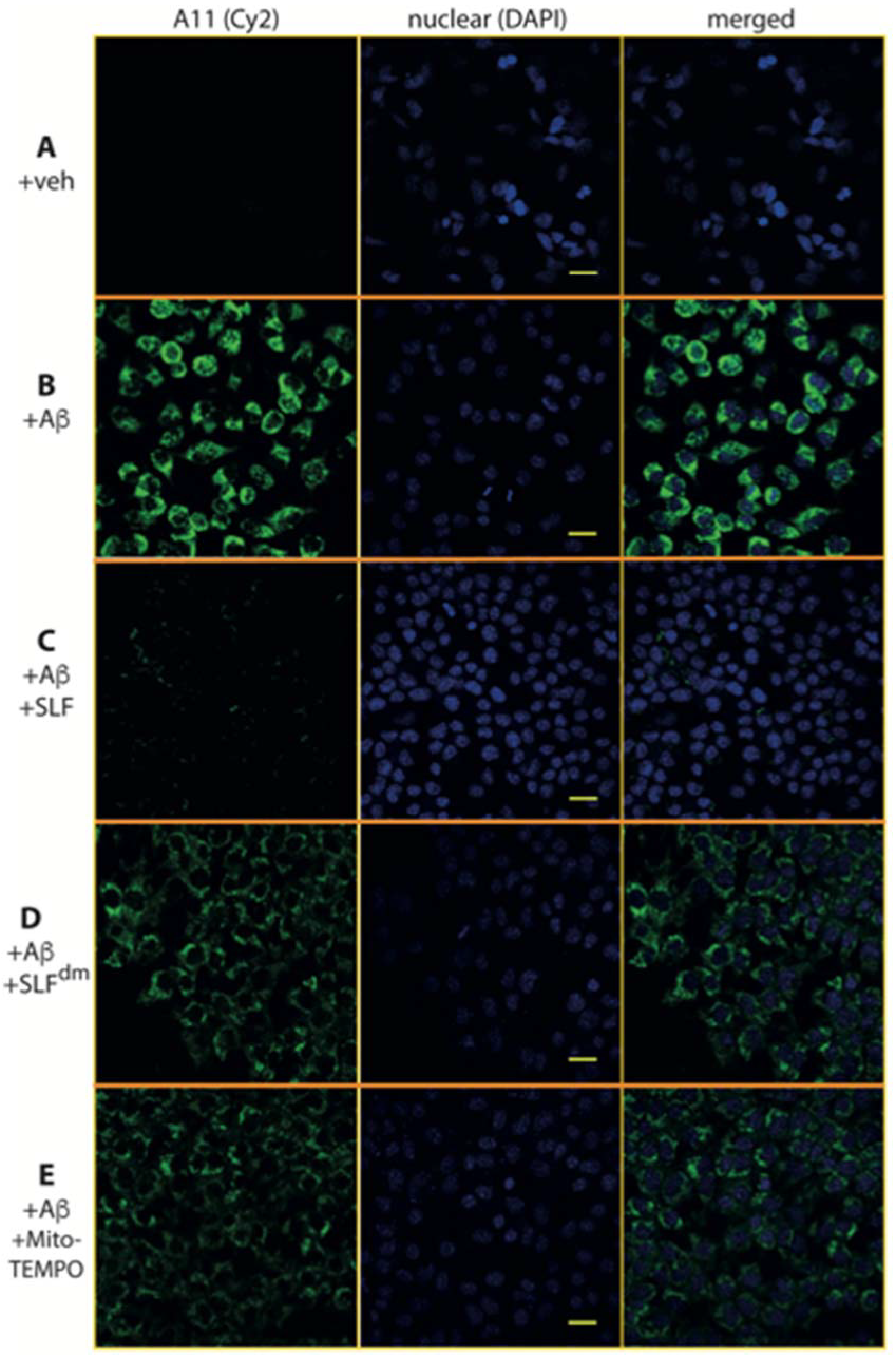
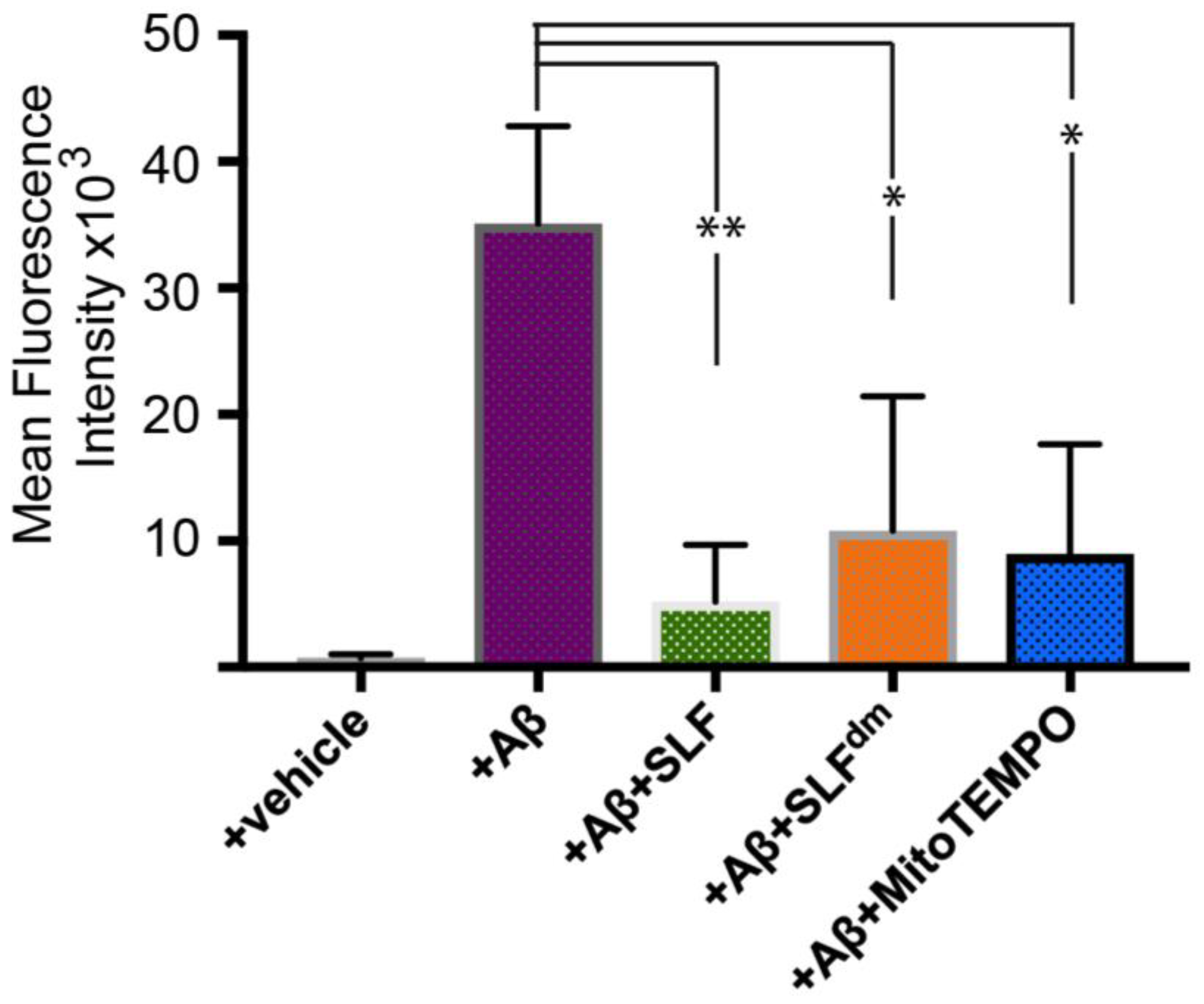
2.5. SLF Decreases the Formation of Intracellular AβO in a Human Neuroblastoma Cell Line Overexpressing the Amyloid Precursor Protein Detected by the Oligomer-Specific Antibody
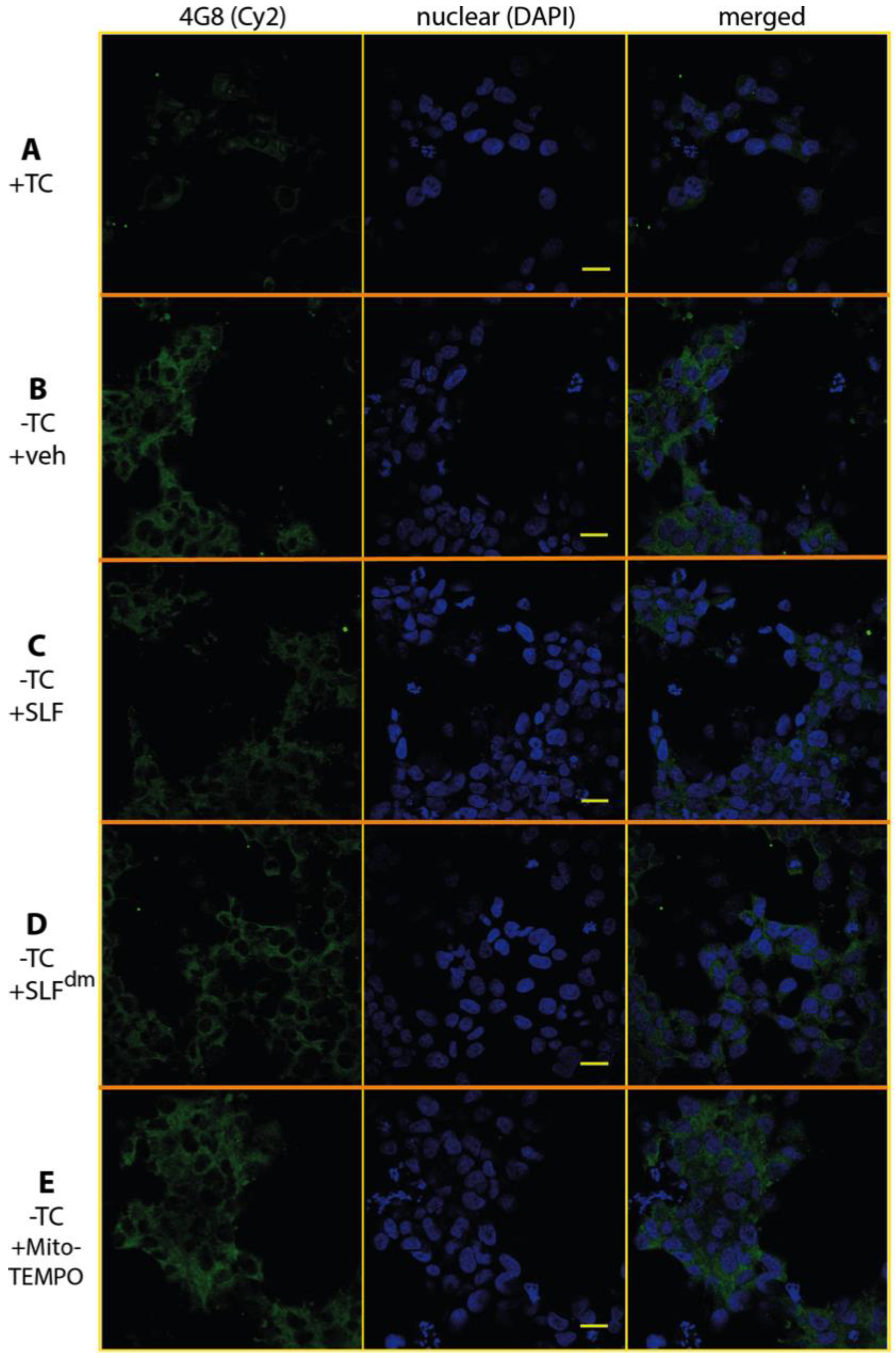
2.6. SLF Decreases Accumulation of Total Intracellular Aβ in a Human Neuroblastoma Cell Line Overexpressing the Amyloid Precursor Protein
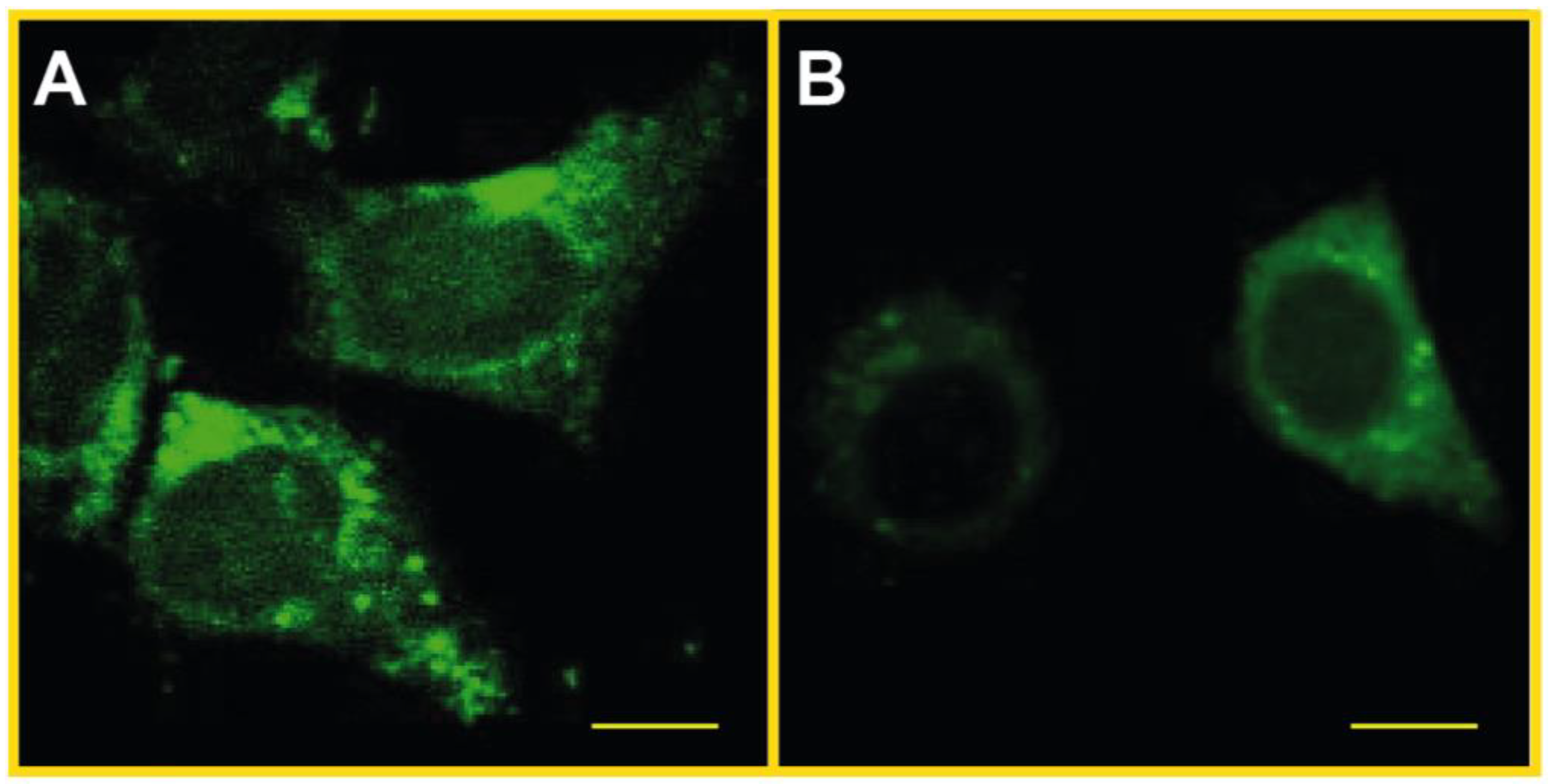
2.7. SLF Decreases Uptake of Aβ in Cultured Neurons Treated with Exogenous Aβ
2.8. SLF Decreases the Presence of AβO in Cultured Neurons Treated with Exogenous Aβ
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Line Model Over-Expressing Intracellular Aβ
4.3. Preparation of Amyloid-Beta Peptide Solution
4.4. Cell Viability Assay
4.5. Cell Culture for the Detection of Intracellular Aβ by Super-Resolution Structured Illumination Imaging
4.6. Super-Resolution Structured Illumination Imaging of FSB-Stained Intracellular Aβ
4.7. Immunofluorescence Staining for the Accumulation of Intracellular Aβ
4.8. Detection of Intracellular Aβ Oligomers, Total Aβ, and Intracellular Uptake of Aβ by Confocal Microscopy
4.9. Detection of the Intracellular Oxidative Stress Signal by Confocal Microscopy
4.10. Statistical Analysis and Quantification of Immunohistochemical Staining
Author Contributions
Funding
Conflicts of Interest
References
- Du, H.; Guo, L.; Yan, S.; Sosunov, A.A.; McKhann, G.M.; Yan, S.S. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proc. Natl. Acad. Sci. USA 2010, 107, 18670–18675. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yan, S.S. Mitochondrial permeability transition pore in Alzheimer’s disease: Cyclophilin D and amyloid beta. Biochim. Biophys. Acta 2010, 1802, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.I.; Carvalho, C.; Zhu, X.; Smith, M.A.; Perry, G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim. Biophys. Acta 2010, 1802, 2–10. [Google Scholar] [CrossRef] [PubMed]
- LaFerla, F.M.; Green, K.N.; Oddo, S. Intracellular amyloid-beta in Alzheimer’s disease. Nat. Rev. Neurosci. 2007, 8, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Kienlen-Campard, P.; Miolet, S.; Tasiaux, B.; Octave, J.N. Intracellular amyloid-β1-42, but not extracellular soluble amyloid-β peptides, induces neuronal apoptosis. J. Biol. Chem. 2002, 277, 15666–15670. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, M.; Konietzko, U.; Krebs, D.C.; Nitsch, R.M. Intracellular Aβ and cognitive deficits precede β-amyloid deposition in transgenic arcAβ mice. Neurobiol. Aging 2007, 28, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.H.; Nagao, T.; Gouras, G.K. Plaque formation and the intraneuronal accumulation of β-amyloid in Alzheimer’s disease. Pathol. Int. 2017, 67, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; McLaughlin, R.; Goodyer, C.; LeBlanc, A. Selective cytotoxicity of intracellular amyloid β peptide(1–42) through p53 and Bax in cultured primary human neurons. J. Cell Biol. 2002, 156, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S.; Caccamo, A.; Smith, I.F.; Green, K.N.; LaFerla, F.M. A dynamic relationship between intracellular and extracellular pools of Abeta. Am. J. Pathol. 2006, 168, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Mao, P. Oxidative Stress and Its Clinical Applications in Dementia. J. Neurodegener. Dis. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Persson, T.; Popescu, B.O.; Cedazo-Minguez, A. Oxidative stress in Alzheimer’s disease: Why did antioxidant therapy fail? Oxid. Med. Cell. Longev. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rosini, M.; Simoni, E.; Milelli, A.; Minarini, A.; Melchiorre, C. Oxidative stress in Alzheimer’s disease: Are we connecting the dots? J. Med. Chem. 2014, 57, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.A.; Xiong-Fister, S.; Markesbery, W.R.; Lovell, M.A. Elevated 4-hydroxyhexenal in Alzheimer’s disease (AD) progression. Neurobiol. Aging 2012, 33, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Scheff, S.W. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J. Neuropathol. Exp. Neurol. 2010, 69, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Kuhla, B.; Haase, C.; Flach, K.; Luth, H.J.; Arendt, T.; Munch, G. Effect of pseudophosphorylation and cross-linking by lipid peroxidation and advanced glycation end product precursors on tau aggregation and filament formation. J. Biol. Chem. 2007, 282, 6984–6991. [Google Scholar] [CrossRef] [PubMed]
- Tabner, B.J.; El-Agnaf, O.M.; German, M.J.; Fullwood, N.J.; Allsop, D. Protein aggregation, metals and oxidative stress in neurodegenerative diseases. Biochem. Soc. Trans. 2005, 33, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Alzheimer s disease and oxidative stress: A review. Curr. Med. Chem. 2014, 21, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Zimbron, L.F.; Luna-Munoz, J.; Mena, R.; Vazquez-Ramirez, R.; Kubli-Garfias, C.; Cribbs, D.H.; Manoutcharian, K.; Gevorkian, G. Amyloid-β peptide binds to cytochrome C oxidase subunit 1. PLoS ONE 2012, 7, e42344. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H. Mitochondrial oxidative damage in aging and Alzheimer’s disease: Implications for mitochondrially targeted antioxidant therapeutics. J. Biomed. Biotechnol. 2006, 2006, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Ly, S.; Hilt, S.; Petrlova, J.; Maezawa, I.; Kalai, T.; Hideg, K.; Jin, L.W.; Laurence, T.A.; Voss, J.C. Protective spin-labeled fluorenes maintain amyloid beta peptide in small oligomers and limit transitions in secondary structure. Biochim. Biophys. Acta 2015, 1854, 1860–1870. [Google Scholar] [CrossRef] [PubMed]
- Petrlova, J.; Kalai, T.; Maezawa, I.; Altman, R.; Harishchandra, G.; Hong, H.S.; Bricarello, D.A.; Parikh, A.N.; Lorigan, G.A.; Jin, L.W.; et al. The influence of spin-labeled fluorene compounds on the assembly and toxicity of the abeta peptide. PLoS ONE 2012, 7, e35443. [Google Scholar] [CrossRef] [PubMed]
- Hilt, S.; Rojalin, T.; Viitala, T.; Koivuniemi, A.; Bunker, A.; Wachsmann-Hogiu, S.; Kálai, T.; Hideg, K.; Yliperttula, M.; Voss, J.C. Oligomerization Alters Binding Affinity between Amyloid Beta and a Modulator of Peptide Aggregation. J. Phys. Chem. C 2017, 121, 23974–23987. [Google Scholar] [CrossRef]
- Hong, H.S.; Maezawa, I.; Budamagunta, M.; Rana, S.; Shi, A.; Vassar, R.; Liu, R.; Lam, K.S.; Cheng, R.H.; Hua, D.H.; et al. Candidate anti-Aβ fluorene compounds selected from analogs of amyloid imaging agents. Neurobiol. Aging 2010, 31, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Kalai, T.; Kuppusamy, M.L.; Balog, M.; Selvendiran, K.; Rivera, B.K.; Kuppusamy, P.; Hideg, K. Synthesis of N-substituted 3,5-bis(arylidene)-4-piperidones with high antitumor and antioxidant activity. J. Med. Chem. 2011, 54, 5414–5421. [Google Scholar] [CrossRef] [PubMed]
- Venditti, E.; Scire, A.; Tanfani, F.; Greci, L.; Damiani, E. Nitroxides are more efficient inhibitors of oxidative damage to calf skin collagen than antioxidant vitamins. Biochim. Biophys. Acta 2008, 1780, 58–68. [Google Scholar] [CrossRef] [PubMed]
- ElNaggar, A.C.; Saini, U.; Naidu, S.; Wanner, R.; Sudhakar, M.; Fowler, J.; Nagane, M.; Kuppusamy, P.; Cohn, D.E.; Selvendiran, K. Anticancer potential of diarylidenyl piperidone derivatives, HO-4200 and H-4318, in cisplatin resistant primary ovarian cancer. Cancer Biol. Ther. 2016, 17, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Coble, V.; Vasalatiy, O.; Swenson, R.E.; Krishna, M.C.; Mitchell, J.B. An efficient synthesis of 3-(N-piperidinemethyl)-2,2,5,5-tetramethyl-1-oxy-3-pyrroline, a promising radioprotector for cancer radiotherapy. Tetrahedron Lett. 2014, 55, 5570–5571. [Google Scholar] [CrossRef] [PubMed]
- Zarling, J.A.; Brunt, V.E.; Vallerga, A.K.; Li, W.; Tao, A.; Zarling, D.A.; Minson, C.T. Nitroxide pharmaceutical development for age-related degeneration and disease. Front. Genet. 2015, 6, 325. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.S.; Maezawa, I.; Yao, N.; Xu, B.; Diaz-Avalos, R.; Rana, S.; Hua, D.H.; Cheng, R.H.; Lam, K.S.; Jin, L.W. Combining the rapid MTT formazan exocytosis assay and the MC65 protection assay led to the discovery of carbazole analogs as small molecule inhibitors of Abeta oligomer-induced cytotoxicity. Brain Res. 2007, 1130, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Hureau, C.; Faller, P. Aβ-mediated ROS production by Cu ions: Structural insights, mechanisms and relevance to Alzheimer’s disease. Biochimie 2009, 91, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.G.; Cappai, R.; Barnham, K.J. The redox chemistry of the Alzheimer’s disease amyloid beta peptide. Biochim. Biophys. Acta 2007, 1768, 1976–1990. [Google Scholar] [CrossRef] [PubMed]
- Varadarajan, S.; Yatin, S.; Aksenova, M.; Butterfield, D.A. Review: Alzheimer’s amyloid β-peptide-associated free radical oxidative stress and neurotoxicity. J. Struct. Biol. 2000, 130, 184–208. [Google Scholar] [CrossRef] [PubMed]
- Maezawa, I.; Hong, H.S.; Wu, H.C.; Battina, S.K.; Rana, S.; Iwamoto, T.; Radke, G.A.; Pettersson, E.; Martin, G.M.; Hua, D.H.; et al. A novel tricyclic pyrone compound ameliorates cell death associated with intracellular amyloid-β oligomeric complexes. J. Neurochem. 2006, 98, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Higuchi, M.; Iwata, N.; Saido, T.C.; Sasamoto, K. Fluoro-substituted and 13C-labeled styrylbenzene derivatives for detecting brain amyloid plaques. Eur. J. Med. Chem. 2004, 39, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, D.P.; Walsh, S.M.; Kiyota, T.; Dong, Y.; Ikezu, T.; Vennerstrom, J.L. Polyfluorinated Bis-styrylbenzene β-Amyloid Plaque Binding Ligands. J. Med. Chem. 2007, 50, 4986–4992. [Google Scholar] [CrossRef] [PubMed]
- Kayed, R.; Head, E.; Thompson, J.L.; McIntire, T.M.; Milton, S.C.; Cotman, C.W.; Glabe, C.G. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 2003, 300, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Goodman, J.L.; Mukhopadhyay, S.; Pacheco, C.D.; Lemke, E.A.; Deniz, A.A.; Lindquist, S. Conserved features of intermediates in amyloid assembly determine their benign or toxic states. Proc. Natl. Acad. Sci. USA 2012, 109, 11172–11177. [Google Scholar] [CrossRef] [PubMed]
- Hubin, E.; van Nuland, N.A.; Broersen, K.; Pauwels, K. Transient dynamics of Aβ contribute to toxicity in Alzheimer’s disease. Cell. Mol. Life Sci. 2014, 71, 3507–3521. [Google Scholar] [CrossRef] [PubMed]
- Cleary, J.P.; Walsh, D.M.; Hofmeister, J.J.; Shankar, G.M.; Kuskowski, M.A.; Selkoe, D.J.; Ashe, K.H. Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nat. Neurosci. 2005, 8, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Lesne, S.; Koh, M.T.; Kotilinek, L.; Kayed, R.; Glabe, C.G.; Yang, A.; Gallagher, M.; Ashe, K.H. A specific amyloid-β protein assembly in the brain impairs memory. Nature 2006, 440, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.M.; Klyubin, I.; Fadeeva, J.V.; Rowan, M.J.; Selkoe, D.J. Amyloid-β oligomers: Their production, toxicity and therapeutic inhibition. Biochem. Soc. Trans. 2002, 30, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Parihar, M.S.; Brewer, G.J. Amyloid-β as a modulator of synaptic plasticity. J. Alzheimers Dis. 2010, 22, 741–763. [Google Scholar] [CrossRef] [PubMed]
- Pearson, H.A.; Peers, C. Physiological roles for amyloid β peptides. J. Physiol. 2006, 575, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.M.; Tseng, B.P.; Rydel, R.E.; Podlisny, M.B.; Selkoe, D.J. The oligomerization of amyloid β-protein begins intracellularly in cells derived from human brain. Biochemistry 2000, 39, 10831–10839. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Maezawa, I.; Zou, B.; Di Lucente, J.; Cao, W.S.; Pascual, C.; Weerasekara, S.; Zhang, M.; Xie, X.S.; Hua, D.H.; Jin, L.W. The Anti-Amyloid-β and Neuroprotective Properties of a Novel Tricyclic Pyrone Molecule. J. Alzheimers Dis. 2017, 58, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, B.; Yew, D.T.; Kusiak, J.W.; Roth, G.S. Processing of Alzheimer’s amyloid precursor protein during H2O2-induced apoptosis in human neuronal cells. Biochem. Biophys. Res. Commun. 1997, 235, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, G.; Hess, C.; Savaskan, E.; Ly, C.; Meier, F.; Baysang, G.; Brockhaus, M.; Muller-Spahn, F. Melatonin protects SHSY5Y neuroblastoma cells from cobalt-induced oxidative stress, neurotoxicity and increased β-amyloid secretion. J. Pineal Res. 2001, 31, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Misonou, H.; Morishima-Kawashima, M.; Ihara, Y. Oxidative stress induces intracellular accumulation of amyloid β-protein (Aβ) in human neuroblastoma cells. Biochemistry 2000, 39, 6951–6959. [Google Scholar] [CrossRef] [PubMed]
- Paola, D.; Domenicotti, C.; Nitti, M.; Vitali, A.; Borghi, R.; Cottalasso, D.; Zaccheo, D.; Odetti, P.; Strocchi, P.; Marinari, U.M.; et al. Oxidative stress induces increase in intracellular amyloid β-protein production and selective activation of βI and βII PKCs in NT2 cells. Biochem. Biophys. Res. Commun. 2000, 268, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Chen, Y.; Liu, H.; Zhang, K.; Zhang, T.; Lin, A.; Jing, N. Hydrogen peroxide promotes Aβ production through JNK-dependent activation of gamma-secretase. J. Biol. Chem. 2008, 283, 17721–17730. [Google Scholar] [CrossRef] [PubMed]
- Kalai, T.; Petrlova, J.; Balog, M.; Aung, H.H.; Voss, J.C.; Hideg, K. Synthesis and study of 2-amino-7-bromofluorenes modified with nitroxides and their precursors as dual anti-amyloid and antioxidant active compounds. Eur. J. Med. Chem. 2011, 46, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.W.; Shie, F.S.; Maezawa, I.; Vincent, I.; Bird, T. Intracellular accumulation of amyloidogenic fragments of amyloid-β precursor protein in neurons with Niemann-Pick type C defects is associated with endosomal abnormalities. Am. J. Pathol. 2004, 164, 975–985. [Google Scholar] [CrossRef]
- Maezawa, I.; Hong, H.S.; Liu, R.; Wu, C.Y.; Cheng, R.H.; Kung, M.P.; Kung, H.F.; Lam, K.S.; Oddo, S.; Laferla, F.M.; et al. Congo red and thioflavin-T analogs detect Aβ oligomers. J. Neurochem. 2008, 104, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds SLF and SLFdm are available from the authors. |



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilt, S.; Altman, R.; Kálai, T.; Maezawa, I.; Gong, Q.; Wachsmann-Hogiu, S.; Jin, L.-W.; Voss, J.C. A Bifunctional Anti-Amyloid Blocks Oxidative Stress and the Accumulation of Intraneuronal Amyloid-Beta. Molecules 2018, 23, 2010. https://doi.org/10.3390/molecules23082010
Hilt S, Altman R, Kálai T, Maezawa I, Gong Q, Wachsmann-Hogiu S, Jin L-W, Voss JC. A Bifunctional Anti-Amyloid Blocks Oxidative Stress and the Accumulation of Intraneuronal Amyloid-Beta. Molecules. 2018; 23(8):2010. https://doi.org/10.3390/molecules23082010
Chicago/Turabian StyleHilt, Silvia, Robin Altman, Tamás Kálai, Izumi Maezawa, Qizhi Gong, Sebastian Wachsmann-Hogiu, Lee-Way Jin, and John C. Voss. 2018. "A Bifunctional Anti-Amyloid Blocks Oxidative Stress and the Accumulation of Intraneuronal Amyloid-Beta" Molecules 23, no. 8: 2010. https://doi.org/10.3390/molecules23082010
APA StyleHilt, S., Altman, R., Kálai, T., Maezawa, I., Gong, Q., Wachsmann-Hogiu, S., Jin, L.-W., & Voss, J. C. (2018). A Bifunctional Anti-Amyloid Blocks Oxidative Stress and the Accumulation of Intraneuronal Amyloid-Beta. Molecules, 23(8), 2010. https://doi.org/10.3390/molecules23082010








