Ethanolic Extract of Origanum vulgare Suppresses Propionibacterium acnes-Induced Inflammatory Responses in Human Monocyte and Mouse Ear Edema Models
Abstract
1. Introduction
2. Results and Discussion
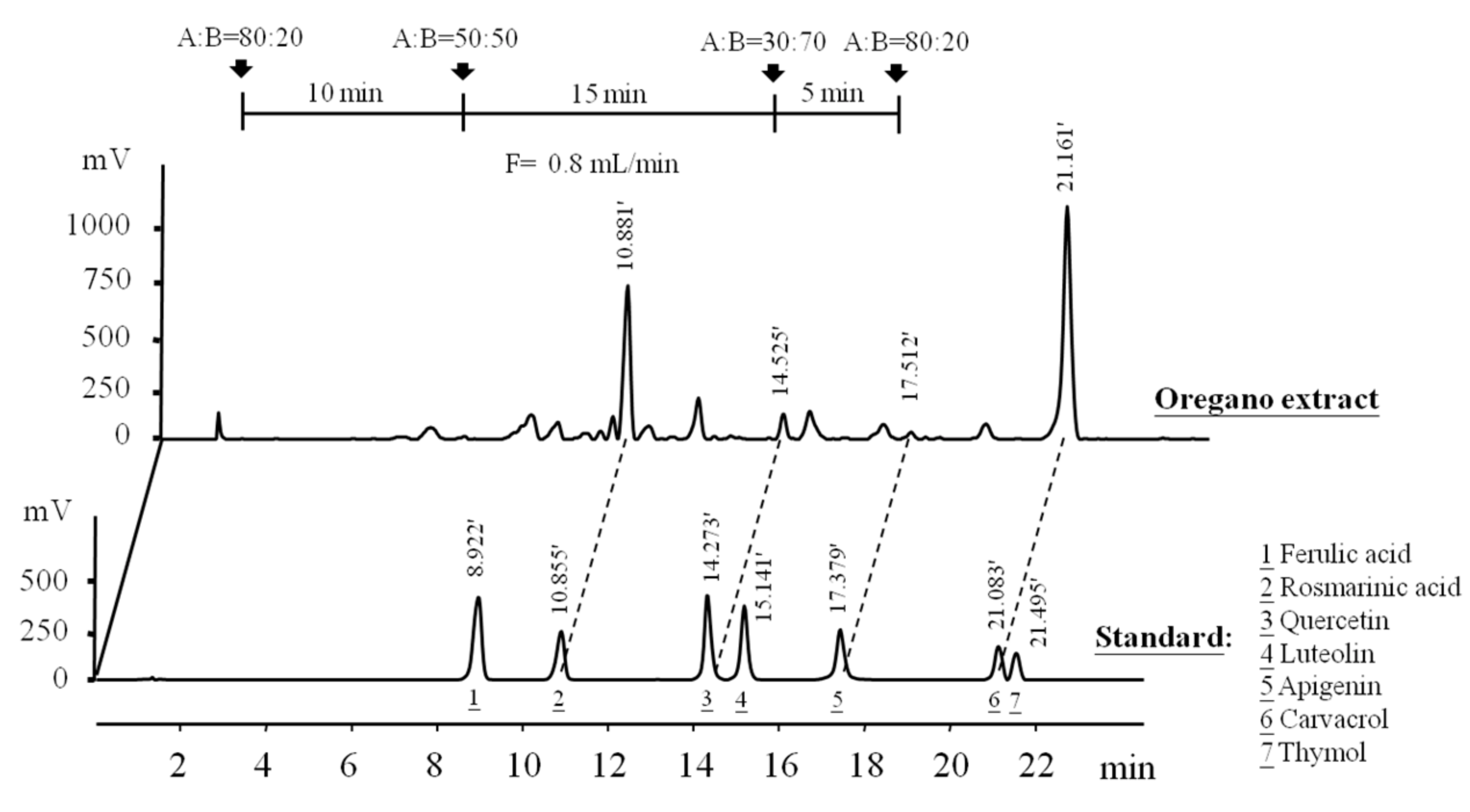
2.1. Determination of Phenolic Compounds in EOE by HPLC Analysis
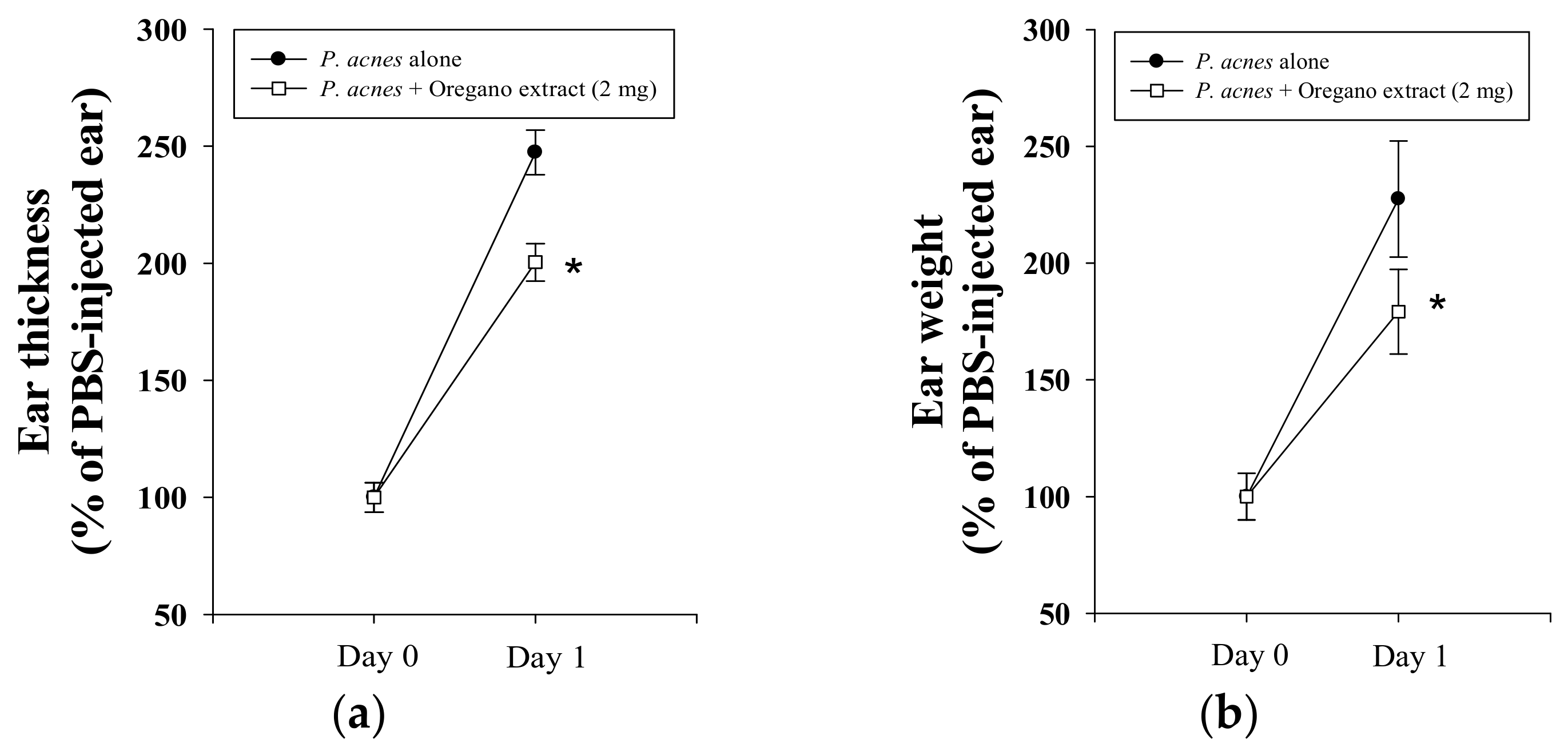
2.2. Effect of EOE on P. acnes-Induced Mouse Ear Edema
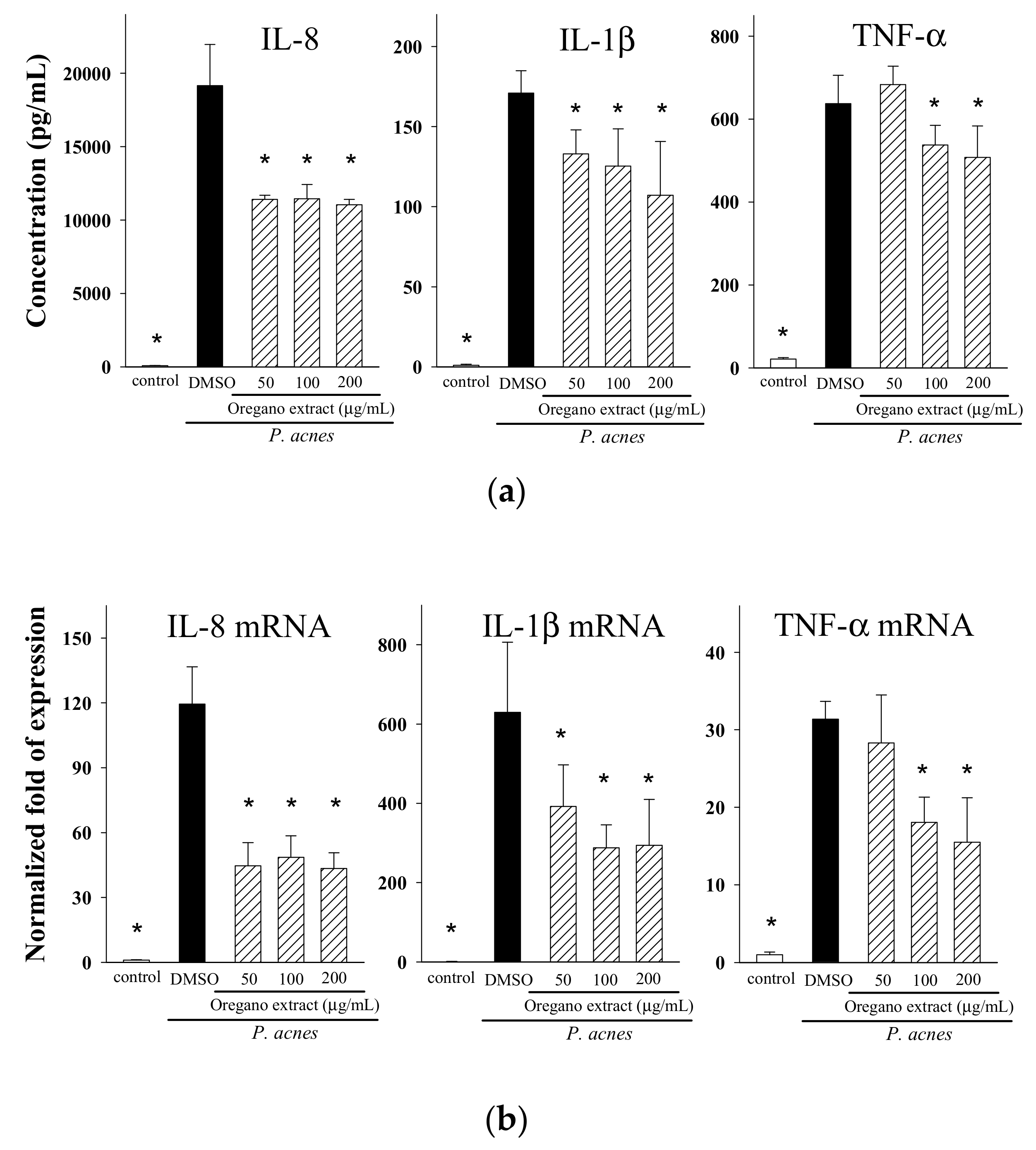
2.3. Effect of EOE on Cytokine Production
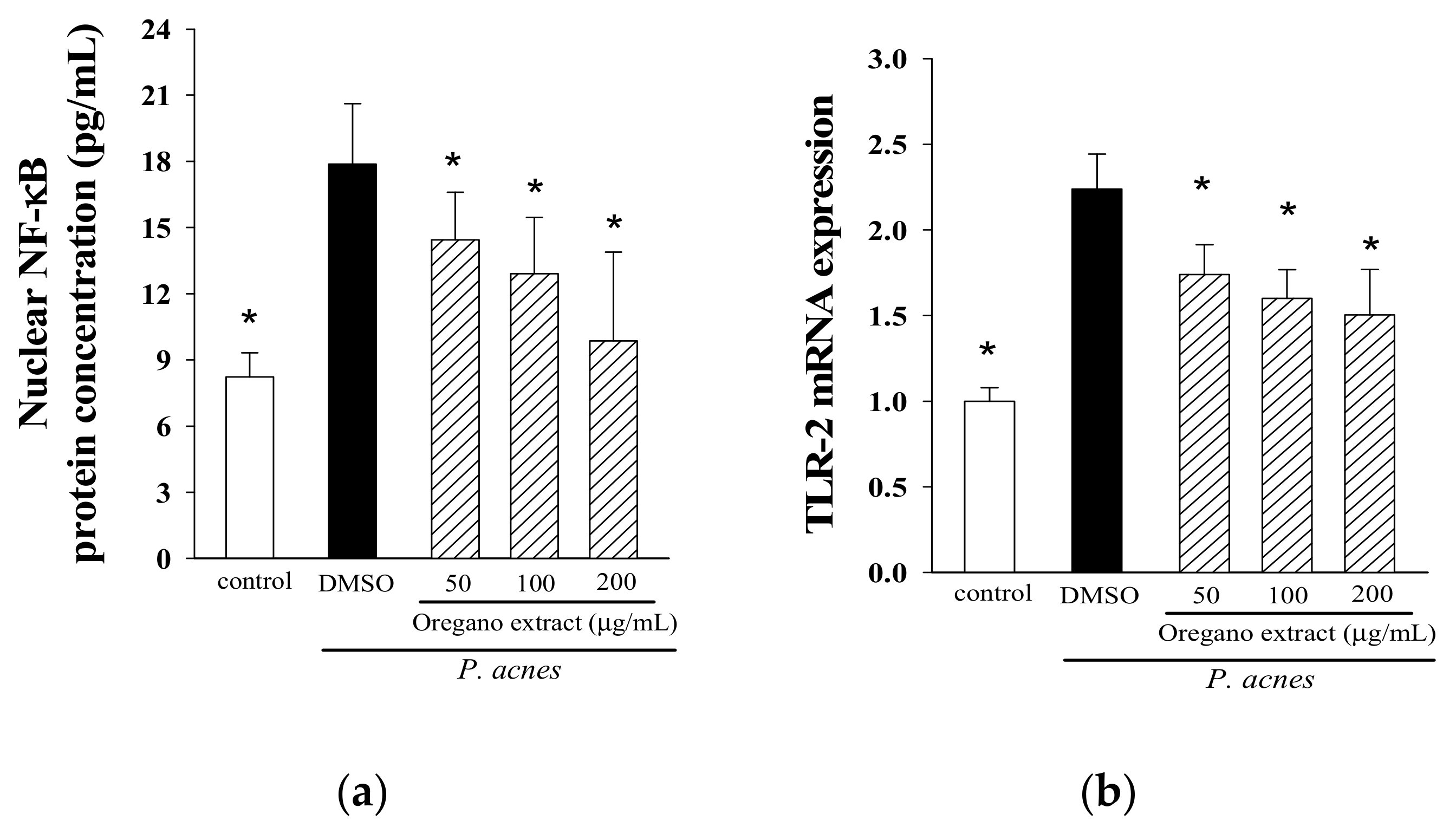
2.4. Effect of EOE on NF-κB Activation and TLR-2 Expression
3. Materials and Methods
3.1. Chemicals
3.2. Ethanol Extraction of Oregano Leaves
3.3. Characterization of Phenolic Compounds in EOE
3.4. Bacterial and Cell Cultures and Growth Conditions
3.5. Effect of EOE on P. acnes-Induced Inflammation In Vivo
3.6. Determination of Cytokine Production by THP-1 Cells
3.7. Analysis of mRNA Levels by Real-Time Reverse Transcription Polymerase Chain Reaction
3.8. Determination of Nuclear Translocation of NF-κB p65
3.9. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tanghetti, E.A. The role of inflammation in the pathology of acne. J. Clin. Aesthet. Dermatol. 2013, 6, 7–35. [Google Scholar]
- Omer, H.; McDowell, A.; Alexeyev, O.A. Understanding the role of Propionibacterium acnes in acne vulgaris: The critical importance of skin sampling methodologies. Clin. Dermatol. 2017, 35, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Pathak, R.; Mary, P.B.; Jha, D.; Sardana, K.; Gautam, H.K. New insights into acne pathogenesis: Exploring the role of acne-associated microbial populations. Dermatol. Sin. 2016, 34, 67–73. [Google Scholar] [CrossRef]
- Kim, J.; Ochoa, M.T.; Krutzik, S.R.; Takeuchi, O.; Uematsu, S.; Legaspi, A.J.; Brightbill, H.D.; Holland, D.; Cunliffe, W.J.; Akira, S.; et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J. Immunol. 2002, 169, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Review of the innate immune response in acne vulgaris: Activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology 2005, 211, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Brennan, K.; Zheng, J. Interleukin 8. In xPharm: The Comprehensive Pharmacology Reference; Elsevier Inc.: New York, NY, USA, 2011; pp. 1–4. [Google Scholar]
- Demina, O.M.; Kartelishev, A.V.; Karpova, E.I.; Olga, I.; Danischuk, O.I. Role of cytokines in the pathogenesis of acne. Int. J. Biomed. 2017, 7, 37–40. [Google Scholar] [CrossRef]
- Goze, I.; Cetin, A.; Goze, A. Investigation of effects of essential oils of Origanum minutiflorum O Schwarz PH Davis and Cyclotrichium niveum (Labiatae) plants on angiogenesis in shell-less chick embryo culture. Afr. J. Biotechnol. 2010, 9, 2156–2160. [Google Scholar]
- Sheibani, V.; Hajializadeh, Z.; Afarinesh, M. Evaluation of Origanum vulgare L. ssp. viridis leaves extract effect on discrimination learning and LTP induction in the CA1 region of the rat hippocampus. Iran. J. Basic Med. Sci. 2010, 14, 161–169. [Google Scholar]
- Targhi, R.G.; Changizi, V.; Haddad, F.; Homayoun, M.; Soleymanifard, S. Origanum vulgare leaf extract protects mice bone marrow cells against ionizing radiation. Avicenna J. Phytomed. 2016, 6, 678–685. [Google Scholar]
- Habibi, E.; Shokrzadeh, M.; Chabra, A.; Naghshvar, F.; Keshavarz-Maleki, R.; Ahmadi, A. Protective effects of Origanum vulgare ethanol extract against cyclophosphamide-induced liver toxicity in mice. Pharm. Biol. 2015, 53, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Nassier, O.A. The antihyperglycaemic effect of the aqueous extract of Origanium vulgare leaves in streptozotocin-induced diabetic rats. Jordan J. Biol. Sci. 2013, 6, 31–38. [Google Scholar] [CrossRef]
- Vujicic, M.; Nikolic, I.; Kontogianni, V.G.; Saksida, T.; Charisiadis, P.; Orescanin-Dusic, Z.; Blagojevic, D.; Stosic-Grujicic, S.; Tzakos, A.G.; Stojanovic, I. Methanolic extract of Origanum vulgare ameliorates type 1 diabetes through antioxidant, anti-inflammatory and anti-apoptotic activity. Br. J. Nutr. 2015, 113, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Brđanin, S.; Bogdanović, N.; Kolundžić, M.; Milenković, M.; Golić, N.; Kojić, M.; Kundaković, T. Antimicrobial activity of oregano (Origanum vulgare L.) and basil (Ocimum basilicum L.) extracts. Adv. Technol. 2015, 4, 5–10. [Google Scholar] [CrossRef]
- Yang, R.; Shetty, K. Stimulation of rosmarinic acid in shoot cultures of oregano (Origanum vulgare) clonal line in response to proline, proline analogue, and proline precursors. J. Agric. Food Chem. 1998, 46, 2888–2893. [Google Scholar] [CrossRef]
- Lambert, R.J.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Coccimiglio, J.; Alipour, M.; Jiang, Z.H.; Gottardo, C.; Suntres, Z. Antioxidant, antibacterial, and cytotoxic activities of the ethanolic Origanum vulgare extract and its major constituents. Oxid. Med. Cell Longev. 2016, 2016, 1404505. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Marrelli, M.; Menichini, F.; Tundis, R.; Statti, G.A.; Solimene, U.; Menichini, F. Chemical composition and protective effect of oregano (Origanum heracleoticum L.) ethanolic extract on oxidative damage and on inhibition of NO in LPS-stimulated RAW 264.7 macrophages. J. Enzym. Inhib. Med. Chem. 2011, 26, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B. Identification and quantification of a major anti-oxidant and anti-inflammatory phenolic compound found in basil, lemon thyme, mint, oregano, rosemary, sage, and thyme. Int. J. Food Sci. Nutr. 2011, 62, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Guo, Y.S.; Wang, C.H.; Li, G.Q.; Xu, J.J.; Chung, H.Y.; Ye, W.C.; Li, Y.L.; Wang, G.C. Phenolic compounds from Origanum vulgare and their antioxidant and antiviral activities. Food Chem. 2014, 152, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Gschwendt, M.; Kittstein, W.; Fürstenberger, G.; Marks, F. The mouse ear edema: A quantitatively evaluable assay for tumor promoting compounds and for inhibitors of tumor promotion. Cancer Lett. 1984, 25, 177–185. [Google Scholar] [CrossRef]
- Sánchez-Campillo, M.; Gabaldon, J.A.; Castillo, J.; Benavente-García, O.; Del Baño, M.J.; Alcaraz, M.; Vicente, V.; Alvarez, N.; Lozano, J.A. Rosmarinic acid, a photo-protective agent against UV and other ionizing radiations. Food Chem. Toxicol. 2009, 47, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.H.; Huang, W.C.; Lien, T.J.; Huang, Y.H.; Chang, H.; Yu, C.H.; Tsai, P.J. Clove extract and eugenol suppress inflammatory responses elicited by Propionibacterium acnes in vitro and in vivo. Food Agric. Immunol. 2017, 28, 916–931. [Google Scholar] [CrossRef]
- Tsai, T.H.; Chuang, L.T.; Lien, T.J.; Liing, Y.R.; Chen, W.Y.; Tsai, P.J. Rosmarinus officinalis extract suppresses Propionibacterium acnes-induced inflammatory responses. J. Med. Food 2013, 16, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Tsai, T.H.; Huang, C.J.; Li, Y.Y.; Chyuan, J.H.; Chuang, L.T.; Tsai, P.J. Inhibitory effects of wild bitter melon leaf extract on Propionibacterium acnes-induced skin inflammation in mice and cytokine production in vitro. Food Funct. 2015, 6, 2550–2560. [Google Scholar] [CrossRef] [PubMed]
- Tax, G.; Urbán, E.; Palotás, Z.; Puskás, R.; Kónya, Z.; Bíró, T.; Kemény, L.; Szabó, K. Propionic acid produced by Propionibacterium acnes strains contributes to their pathogenicity. Acta Derm. Venereol. 2016, 96, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Pivarcsi, A.; Bodai, L.; Réthi, B.; Kenderessy-Szabó, A.; Koreck, A.; Széll, M.; Beer, Z.; Bata-Csörgoo, Z.; Magócsi, M.; Rajnavölgyi, E.; et al. Expression and function of toll-like receptors 2 and 4 in human keratinocytes. Int. Immunol. 2003, 15, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Arican, O.; Kurutas, E.B.; Sasmaz, S. Oxidative stress in patients with acne vulgaris. Mediators Inflamm. 2005, 2005, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.H.; Lee, W.R.; Wang, P.H.; Cheng, K.T.; Chen, Y.C.; Shen, S.C. Propionibacterium acnes-induced iNOS and COX-2 protein expression via ROS-dependent NF-κB and AP-1 activation in macrophages. J. Dermatol. Sci. 2013, 69, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Pastore, S.; Potapovich, A.; Kostyuk, V.; Mariani, V.; Lulli, D.; De Luca, C.; Korkina, L. Plant polyphenols effectively protect HaCaT cells from ultraviolet C-triggered necrosis and suppress inflammatory chemokine expression. Ann. N. Y. Acad. Sci. 2009, 1171, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Adomako-Bonsu, A.G.; Chan, S.L.F.; Pratten, M.; Fry, J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. In Vitro 2017, 40, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, P. In vitro antioxidant assays. In Pharmacological Assays of Plant-Based Natural Products. Progress in Drug Research; Springer Nature Switzerland AG: Basel, Switzerland, 2016; Volume 71, pp. 57–72. [Google Scholar]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of antioxidants and natural products in inflammation. Oxid. Med. Cell Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, A.; Sánchez-Marzo, N.; Barrajón-Catalán, E.; Castillo, J.; Herranz-López, M.; Micol, V. Antioxidant activity and intestinal absorption of apigenin and its potassium salt derivative in Caco-2 cell monolayers. Free Radic. Biol. Med. 2017, 108, S79. [Google Scholar] [CrossRef]
- Quiroga, P.R.; Asensio, C.M.; Nepote, V. Antioxidant effects of the monoterpenes carvacrol, thymol and sabinene hydrate on chemical and sensory stability of roasted sunflower seeds. J. Sci. Food Agric. 2015, 95, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Tang, D.-C.C.; Zhang, L.; Gallo, R.L.; Huang, C.-M. Propionibacterium acnes CAMP factor and host acid sphingomyelinase contribute to bacterial virulence: Potential targets for inflammatory acne treatment. PLoS ONE 2011, 6, e14797. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Kao, M.C.; Fang, J.Y.; Zouboulis, C.C.; Zhang, L.; Gallo, R.L.; Huang, C.M. Antimicrobial property of lauric acid against Propionibacterium acnes: Its therapeutic potential for inflammatory acne vulgaris. J. Investig. Dermatol. 2009, 129, 2480–2488. [Google Scholar] [CrossRef] [PubMed]
- Kistowska, M.; Gehrke, S.; Jankovic, D.; Kerl, K.; Fettelschoss, A.; Feldmeyer, L.; Fenini, G.; Kolios, A.; Navarini, A.; Ganceviciene, R.; et al. IL-1β drives inflammatory responses to Propionibacterium acnes in vitro and in vivo. J. Investig. Dermatol. 2014, 134, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Aubin, G.G.; Baud’huin, M.; Lavigne, J.P.; Régis, B.; François, G.; Didier, L.; Cédric, J.; Dominique, H.; Karim, A.; Stéphane, C. Interaction of Cutibacterium (formerly Propionibacterium) acnes with bone cells: A step toward understanding bone and joint infection development. Sci. Rep. 2017, 7, 42918. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Tsai, T.H.; Chuang, L.T.; Li, Y.Y.; Zouboulis, C.C.; Tsai, P.J. Anti-bacterial and anti-inflammatory properties of capric acid against Propionibacterium acnes: A comparative study with lauric acid. J. Dermatol. Sci. 2014, 73, 232–240. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, L.-T.; Tsai, T.-H.; Lien, T.-J.; Huang, W.-C.; Liu, J.-J.; Chang, H.; Chang, M.-L.; Tsai, P.-J. Ethanolic Extract of Origanum vulgare Suppresses Propionibacterium acnes-Induced Inflammatory Responses in Human Monocyte and Mouse Ear Edema Models. Molecules 2018, 23, 1987. https://doi.org/10.3390/molecules23081987
Chuang L-T, Tsai T-H, Lien T-J, Huang W-C, Liu J-J, Chang H, Chang M-L, Tsai P-J. Ethanolic Extract of Origanum vulgare Suppresses Propionibacterium acnes-Induced Inflammatory Responses in Human Monocyte and Mouse Ear Edema Models. Molecules. 2018; 23(8):1987. https://doi.org/10.3390/molecules23081987
Chicago/Turabian StyleChuang, Lu-Te, Tsung-Hsien Tsai, Tsung-Jung Lien, Wen-Cheng Huang, Jun-Jen Liu, Hsiang Chang, Mei-Ling Chang, and Po-Jung Tsai. 2018. "Ethanolic Extract of Origanum vulgare Suppresses Propionibacterium acnes-Induced Inflammatory Responses in Human Monocyte and Mouse Ear Edema Models" Molecules 23, no. 8: 1987. https://doi.org/10.3390/molecules23081987
APA StyleChuang, L.-T., Tsai, T.-H., Lien, T.-J., Huang, W.-C., Liu, J.-J., Chang, H., Chang, M.-L., & Tsai, P.-J. (2018). Ethanolic Extract of Origanum vulgare Suppresses Propionibacterium acnes-Induced Inflammatory Responses in Human Monocyte and Mouse Ear Edema Models. Molecules, 23(8), 1987. https://doi.org/10.3390/molecules23081987





