Structural Moieties Required for Cinnamaldehyde-Related Compounds to Inhibit Canonical IL-1β Secretion
Abstract
1. Introduction
2. Results
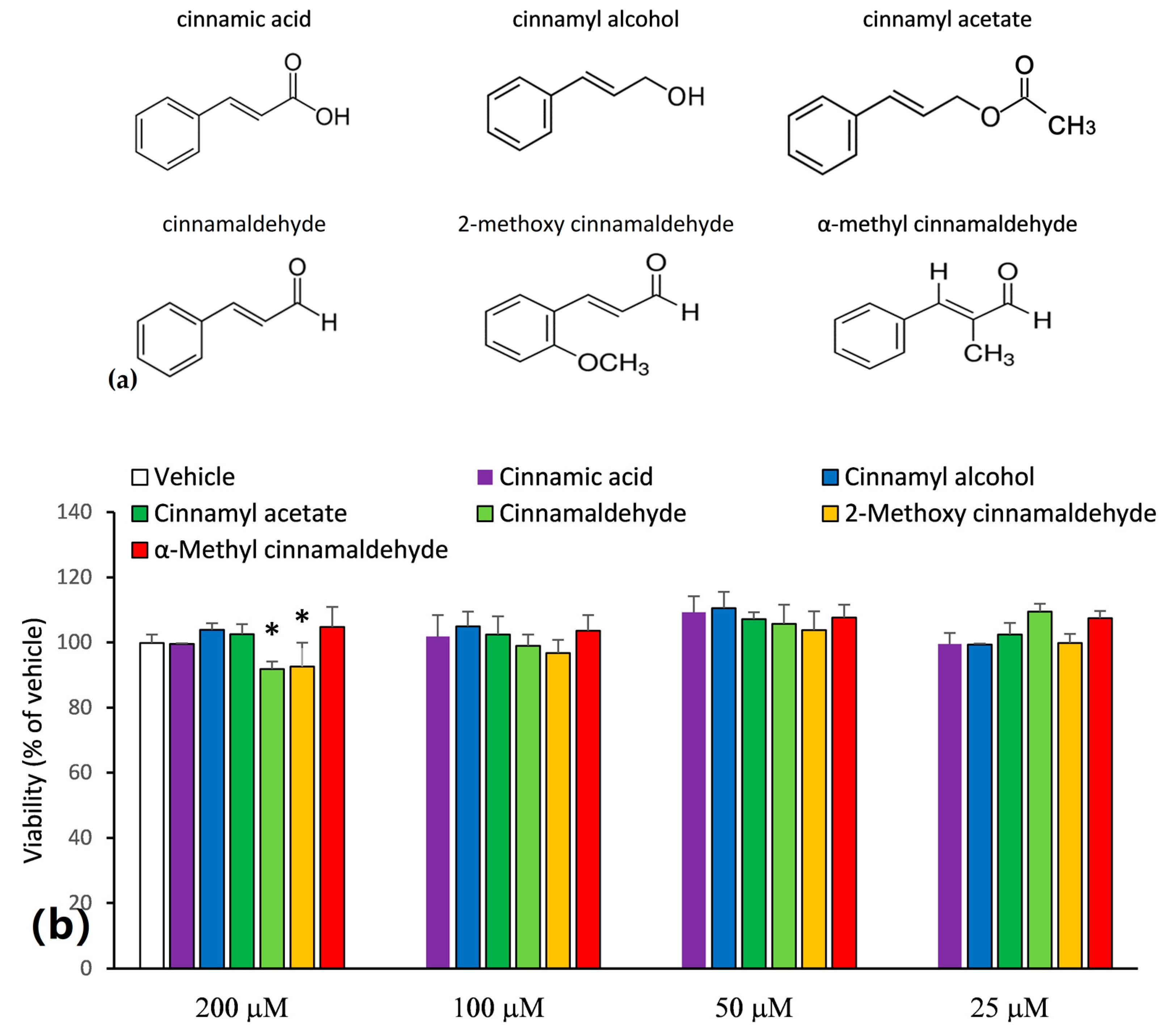
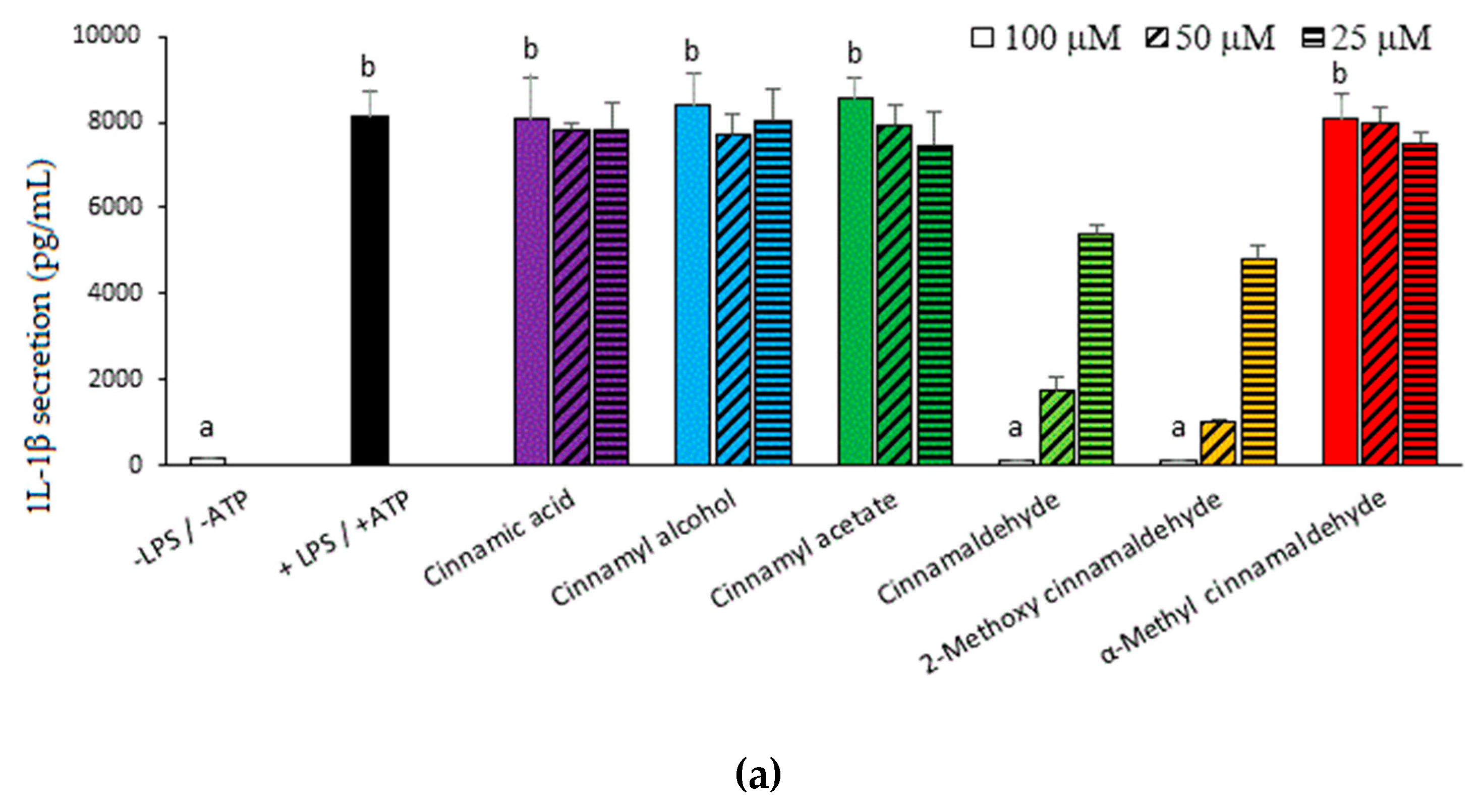
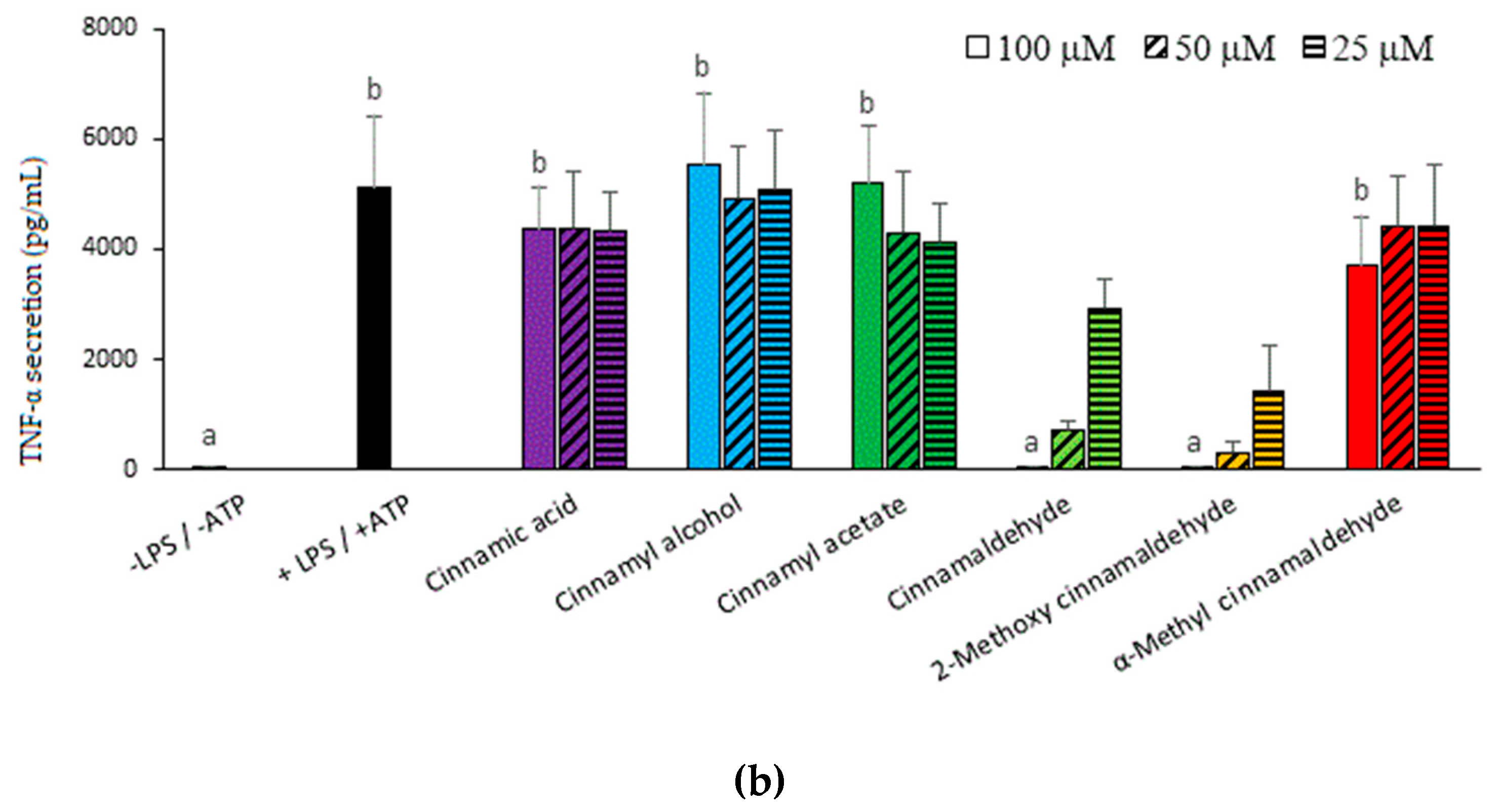
2.1. Inhibitory Capacities of the Cinnamaldehyde-Related Compounds on Canonical IL-1β Secretion
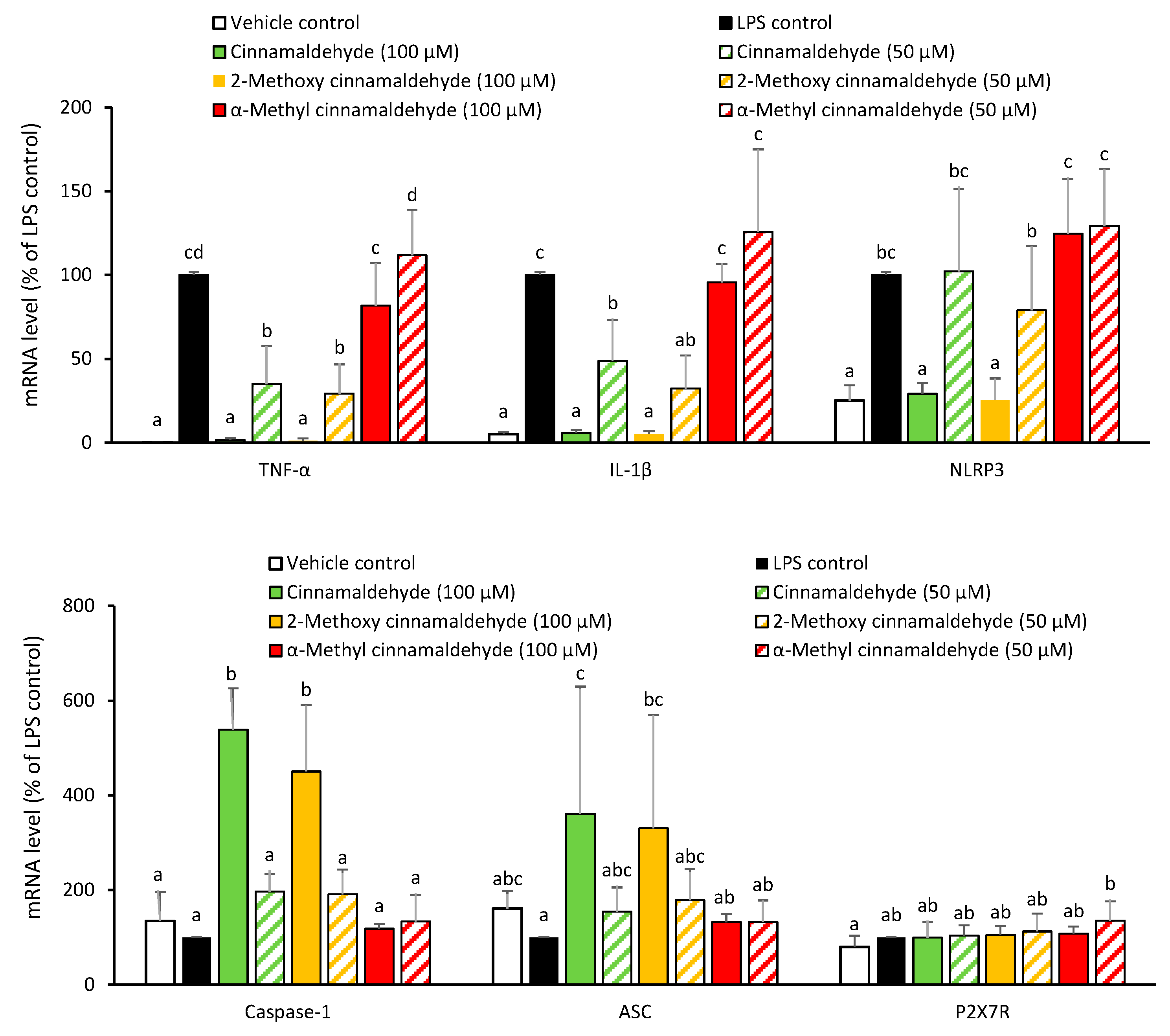
2.2. Influence of Cinnamaldehyde and 2-Methoxy Cinnamaldehyde on the LPS-Primed mRNA Expression of IL-1β and NLRP3 Inflammasome-Related Components
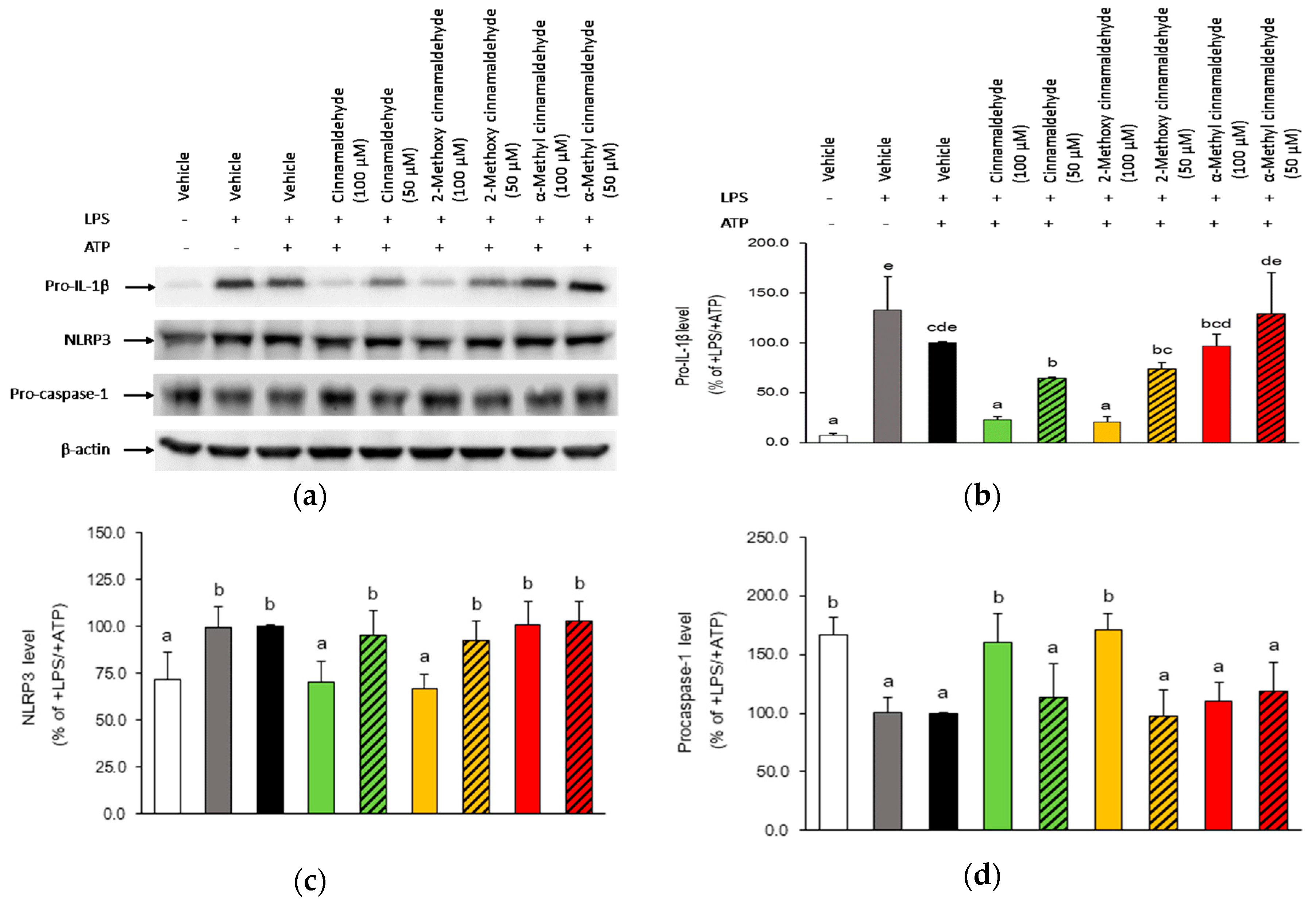
2.3. Influence of Cinnamaldehyde and 2-Methoxy Cinnamaldehyde on Cytosolic Pro-IL-1β, NLRP3 and Pro-Caspase-1 Protein Expression
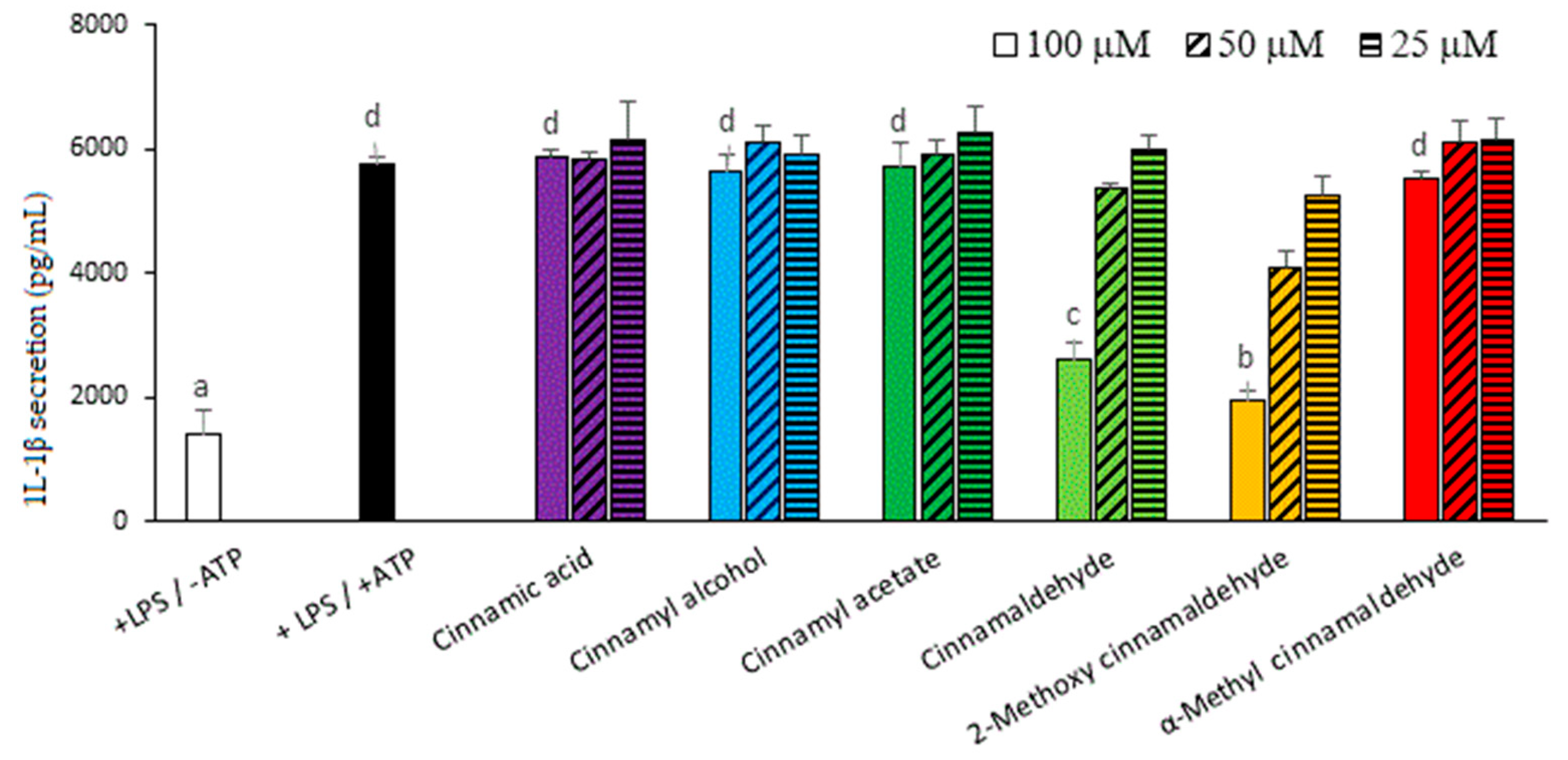
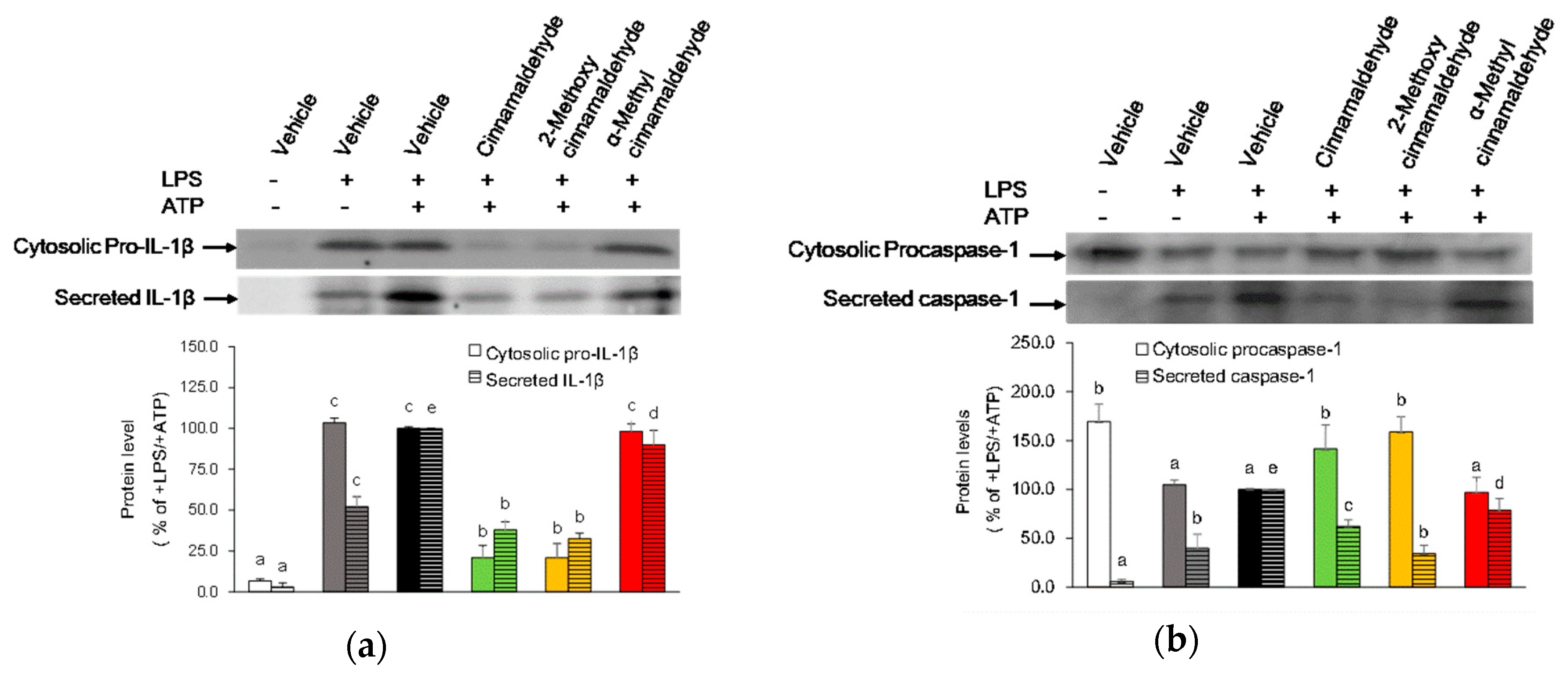
2.4. Effect of Cinnamaldehyde and 2-Methoxy Cinnamaldehyde on Secreted IL-1β and Caspase-1 Protein Levels
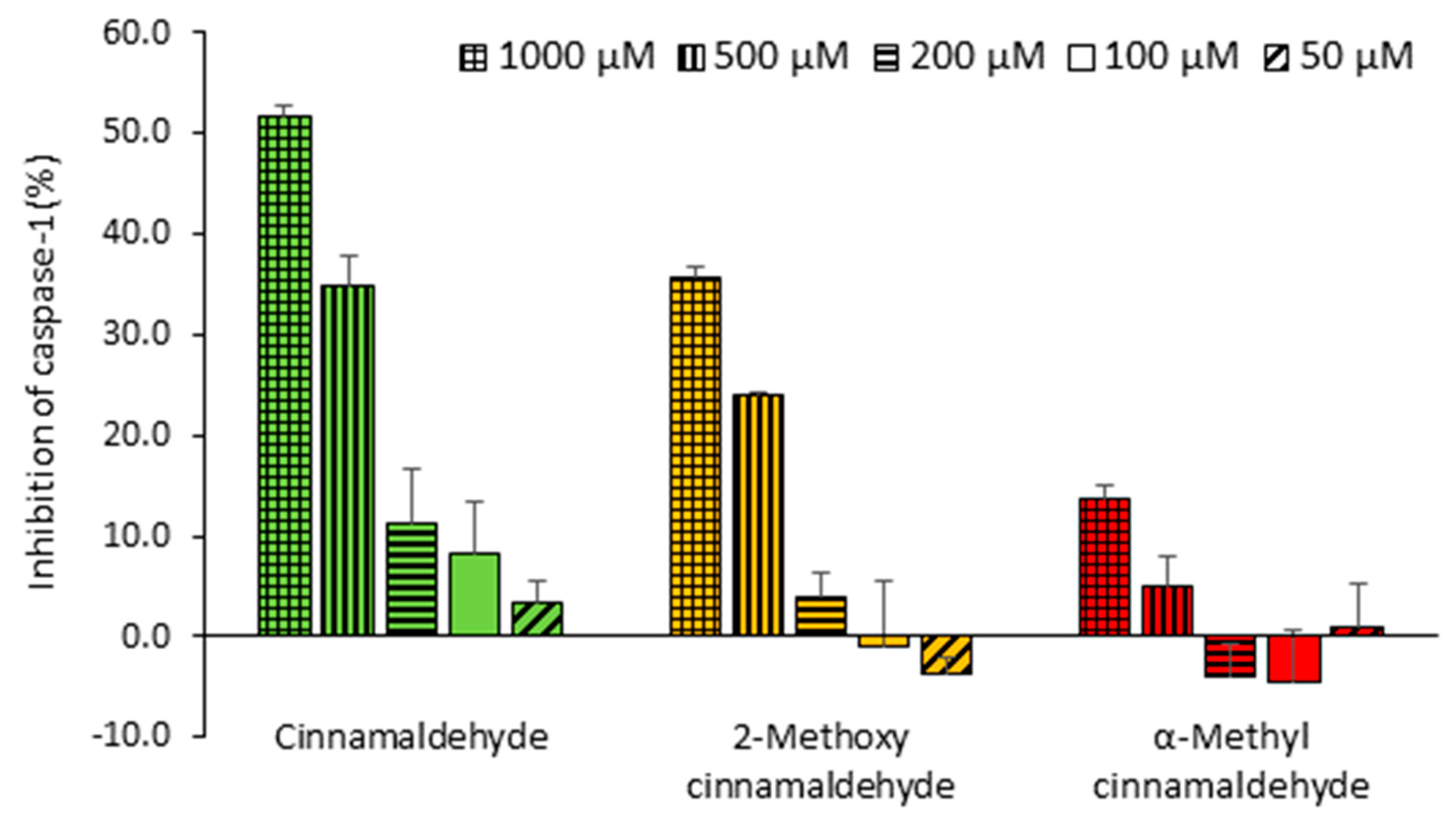
2.5. Direct Inhibitory Effect of Cinnamaldehyde and 2-Methoxy Cinnamaldehyde on Purified Active Caspase-1
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Culture and Differentiation
4.3. Stimulation and Analysis of IL-1β and TNF-α
4.4. RNA Isolation and Quantitative RT-PCR
4.5. Secreted and Cytosolic Protein Preparation, and Western Blotting
4.6. Measurement of Inhibitory Capacities on the Caspase-1 Catalytic Activity
4.7. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Keyel, P.A. How is inflammation initiated? Individual influences of IL-1, IL-18 and HMGB1. Cytokine 2014, 69, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.K.; Kim, J.K.; Shin, D.M.; Sasakawa, C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 2016, 13, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Tyzsér, J.; Benky, S. Natural compounds as regulators of NLRP3 inflammasome-mediated IL-1β production. Mediat. Inflamm. 2016, 2016. [Google Scholar] [CrossRef]
- Dinarello, C.A. Biologic basis of interleukin-1 in disease. Blood 1996, 87, 2095–2147. [Google Scholar] [PubMed]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [PubMed]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The Interleukin-1 family: Back to the future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.C.; Deng, J.S.; Chiu, C.S.; Hou, W.C.; Huang, S.S.; Shie, P.H.; Huang, G.J. Anti-inflammatory activities of Cinnamomum cassia constituents in vitro and in vivo. Evid. Based Complement. Alternat. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.S.; Lee, J.K.; Choi, Y.J.; Saitoh, S.I.; Miyake, K.; Hwang, D.H.; Lee, J.Y. Cinnamaldehyde suppresses toll like receptor 4 activation mediated through the inhibition of receptor oligomerization. Biochem. Pharmacol. 2008, 2, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, C.H.; Kim, M.S.; Kim, J.Y.; Jung, K.J.; Chung, J.H.; An, W.G.; Lee, J.W.; Yu, B.P.; Chung, H.Y. Suppression of age-related inflammatory NF-κB activation by cinnamaldehyde. Biogerontology 2007, 5, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Ka, S.M.; Chao, L.K.; Lin, J.C.; Chen, S.T.; Li, W.T.; Lin, C.N.; Cheng, J.C.; Jheng, H.L.; Chen, A.; Hua, K.F. A low toxicity synthetic cinnamaldehyde derivative ameliorates renal inflammation in mice by inhibiting NLRP3 inflammasome and its related signaling pathways. Free Radical Biol. Med. 2016, 91, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Bossaller, L.; Chiang, P.I.; Schmidt-Lauber, C.; Ganesan, S.; Kaiser, W.J.; Rathinam, V.A.K.; Mocarski, E.S.; Subramanian, D.; Green, D.R.; Silverman, N.; et al. FAS mediates non-canonical IL-1β and IL-18 maturation via caspase-8 in a Rip3-independent manner. J. Immunol. 2012, 189, 5508–5512. [Google Scholar] [CrossRef] [PubMed]
- Cullen, S.P.; Kearney, C.J.; Clancy, D.M.; Martin, S.J. Diverse activators of the NLRP3 inflammasome promote IL-1β secretion by triggering necrosis. Cell Rep. 2015, 11, 1535–1548. [Google Scholar] [CrossRef]
- Kwon, B.M.; Lee, S.H.; Choi, S.U.; Park, S.H.; Lee, C.O.; Cho, Y.K.; Sung, N.D.; Bok, S.H. Synthesis and in vitro cytotoxicity of cinnamaldehydes to human solid tumor cells. Arch. Pharm. Res. 1998, 21, 147–152. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, B.S.; Kim, M.K. Suppression effect of Cinnamomum cassia derived component on nitric oxide synthase. J. Agric. Food Chem. 2002, 50, 7700–7703. [Google Scholar] [CrossRef]
- Ho, S.C.; Chang, K.S.; Chang, P.W. Inhibition of neuroinflammation by cinnamon and its main components. Food Chem. 2013, 138, 2275–2282. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, S.Y.; Son, D.J.; Lee, H.; Yoo, H.S.; Song, S.; Oh, K.W.; Han, D.C.; Kwon, B.M.; Hong, J.T. Inhibitiory effect of 2’-hydroxycinnamaldehyde on nitric oxide production through inhibition of NF-κB activation in RAW 264.7 cells. Biochem. Pharmacol. 2005, 69, 791–799. [Google Scholar] [CrossRef]
- Kwon, B.M.; Lee, S.H.; Cho, Y.K.; Bok, S.H.; So, S.H.; Youn, M.R.; Chang, S.I. Synthesis and biological activity of cinnamaldehydes as angiogenesis inhibitors. Bioorg. Med. Chem. Lett. 1997, 7, 2473–2476. [Google Scholar] [CrossRef]
- Rossi, A.; Kapahi, P.; Natoli, G.; Takahashi, T.; Chen, Y.; Karin, M.; Santoro, M.G. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature 2000, 403, 103–108. [Google Scholar] [CrossRef]
- García-Piñeres, A.J.; Castro, V.; Mora, G.; Schmidt, T.J.; Strunck, E.; Pahl, H.L.; Merfort, I. Cysteine 38 in p65/NF-κB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J. Biol. Chem. 2001, 276, 39713–39720. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lee, J.Y.; Hwang, D.H. Inhibition of pattern recognition receptor-mediated inflammation by bioactive phytochemicals. Nutr. Rev. 2011, 69, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Lee, Y.G.; Lee, J.; Lee, J.Y.; Cho, J.Y. Regulatory effect of cinnamaldehyde on monocyte/macrophage-mediated inflammatory responses. Mediators Inflamm. 2010, 2010, 529359. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, L.J.; Dubin, A.E.; Evans, M.J.; Marr, F.; Schultz, P.G.; Cravatt, B.F.; Patapoutian, A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 2007, 445, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.G.; Brough, D.; Freeman, S. Inhibiting the inflammasome: A chemical perspective. J. Med. Chem. 2016, 59, 1691–1710. [Google Scholar] [CrossRef] [PubMed]
- Kisselev, A.F.; Goldberg, A.L. Proteasome inhibitors: From research tools to drug candidates. Chem. Biol. 2001, 8, 739–758. [Google Scholar] [CrossRef]
- Kang, L.L.; Zhang, D.M.; Ma, C.H.; Zhang, J.H.; Jia, K.K.; Liu, J.H.; Wang, R.; Kong, L.D. Cinnamaldehyde and allopurinol reduce fructose-induced cardiac inflammation and fibrosis by attenuating CD36-mediated TLR4/6-IRAK4/1 signaling to suppress NLRP3 inflammasome activation. Sci. Rep. 2016, 6, 27460. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, F.; Wen, T.; Sang, W.; Wang, D.; Zeng, N. Inhibition of NLRP3 inflammasome: A new protective mechanism of cinnamaldehyde in endotoxin poisoning of mice. Immunopharmacol. Immunotoxicol. 2017, 39, 296–304. [Google Scholar] [CrossRef]
- Lee, S.C.; Wang, S.Y.; Li, C.C.; Liu, C.T. Anti-inflammatory effect of cinnamaldehyde and linalool from the leaf essential oil of Cinnamomum osmophloeum Kanehira in endotoxin-induced mice. J. Food Drug Anal. 2018, 26, 211–220. [Google Scholar] [CrossRef]
- Ho, S.C.; Chang, Y.H. Comparison of inhibitory capacities of 6-, 8- and 10-gingerols/shogaols on the canonical NLRP3 inflammasome-mediated IL-1β secretion. Molecules 2018, 23, 446. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real time quantitative PCR andthe 22DDCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, S.-C.; Chang, Y.-H.; Chang, K.-S. Structural Moieties Required for Cinnamaldehyde-Related Compounds to Inhibit Canonical IL-1β Secretion. Molecules 2018, 23, 3241. https://doi.org/10.3390/molecules23123241
Ho S-C, Chang Y-H, Chang K-S. Structural Moieties Required for Cinnamaldehyde-Related Compounds to Inhibit Canonical IL-1β Secretion. Molecules. 2018; 23(12):3241. https://doi.org/10.3390/molecules23123241
Chicago/Turabian StyleHo, Su-Chen, Yi-Huang Chang, and Ku-Shang Chang. 2018. "Structural Moieties Required for Cinnamaldehyde-Related Compounds to Inhibit Canonical IL-1β Secretion" Molecules 23, no. 12: 3241. https://doi.org/10.3390/molecules23123241
APA StyleHo, S.-C., Chang, Y.-H., & Chang, K.-S. (2018). Structural Moieties Required for Cinnamaldehyde-Related Compounds to Inhibit Canonical IL-1β Secretion. Molecules, 23(12), 3241. https://doi.org/10.3390/molecules23123241





