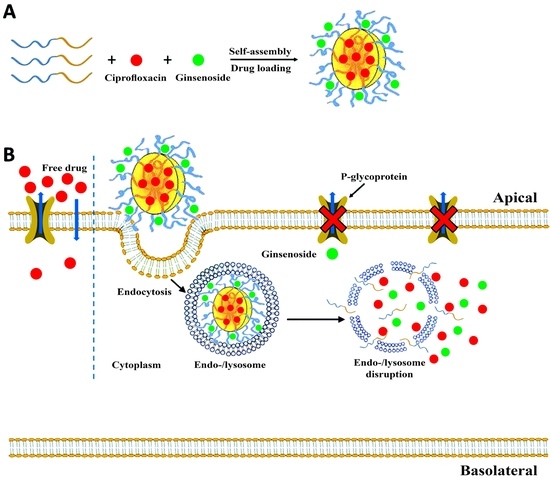
Permeability of Ciprofloxacin-Loaded Polymeric Micelles Including Ginsenoside as P-glycoprotein Inhibitor through a Caco-2 Cells Monolayer as an Intestinal Absorption Model
Abstract
1. Introduction
2. Results and Discussion
2.1. Critical Micelle Concentration (CMC) Results
2.2. Formulation Characterization
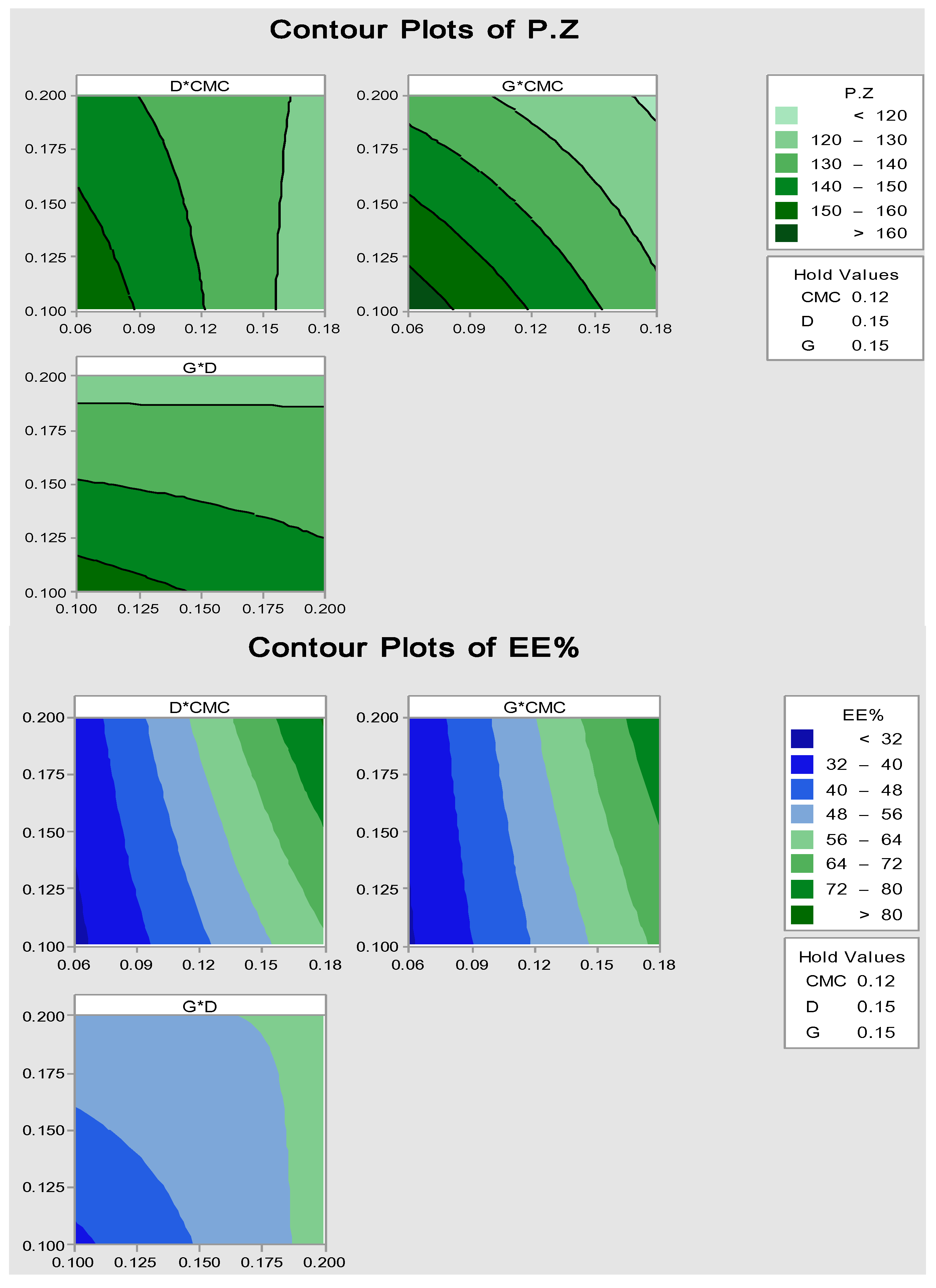
2.2.1. Particle Size and Encapsulation Efficiency (EE%)
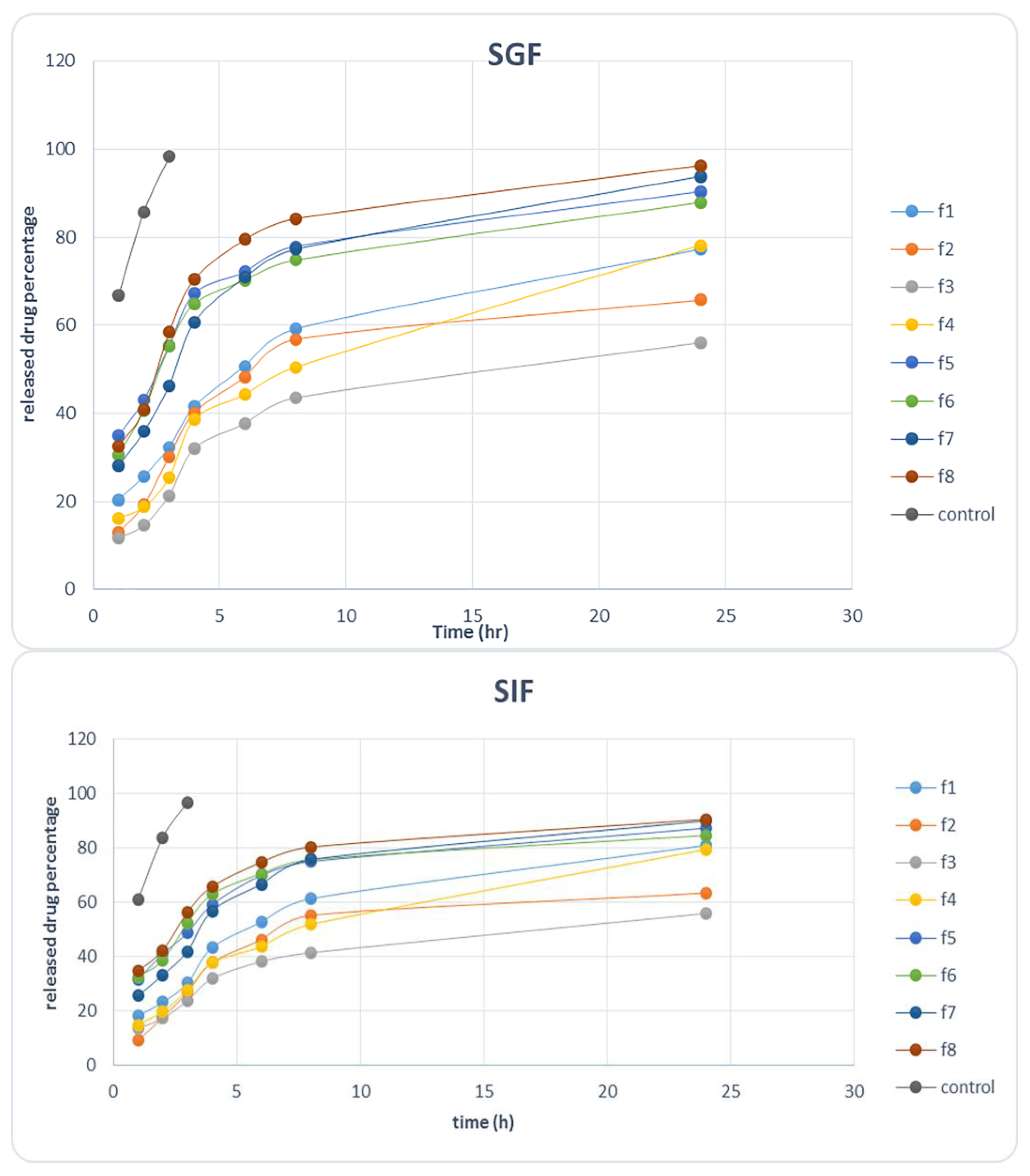
2.2.2. Release Study in Simulated Gastric Fluid (SGF) and Simulated Intestine Fluid (SIF)
2.3. Optimized Formulation
2.3.1. Characterization of Optimized Polymeric Micelle
2.3.2. Optimized Polymeric Micelle Stability
2.3.3. Differential Scanning Calorimetry of Optimized Formulation
2.3.4. Optimized Formulation Permeability through Caco-2 Cells
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Ciprofloxacin Analysis by HPLC
3.2.2. Determination of Critical Micelle Concentration (CMC)
3.2.3. Optimization by Factorial Statistical Design
3.2.4. Micelle Formation and Drug Loading
3.2.5. Characterization of Polymeric Micelles
Determination of Average Particle Size
Encapsulation Efficiency (EE%)
3.2.6. Micelle Stability
3.2.7. In Vitro Release Kinetics of Ciprofloxacin
3.2.8. Differential Scanning Calorimetry (DSC) Analysis
3.2.9. Cell Culture
3.2.10. Permeation Study through Caco-2 Cells Monolayer
Ciprofloxacin Permeability through Caco-2 Cells Pretreatment with Ginsenoside Rg3
3.2.11. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olivera, M.; Manzo, R.; Junginger, H.; Midha, K.; Shah, V.; Stavchansky, S.; Dressman, J.; Barends, D. Biowaiver monographs for immediate release solid oral dosage forms: Ciprofloxacin hydrochloride. J. Pharm. Sci. 2011, 100, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Drusano, G.; Standiford, H.; Plaisance, K.; Forrest, A.; Leslie, J.; Caldwell, J. Absolute oral bioavailability of ciprofloxacin. Antimicrob. Agents Chemother. 1986, 30, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.-H.; Zhang, Q.; Liang, Q.-Y.; Wang, S.-Q.; Zhao, B.-X.; Wang, Y.-T.; Cai, Y.; Li, G.-F. Bioavailability enhancement of paclitaxel via a novel oral drug delivery system: Paclitaxel-loaded glycyrrhizic acid micelles. Molecules 2015, 20, 4337–4356. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, K. The transporters of intestinal tract and techniques applied to evaluate interactions between drugs and transporters. Asian J. Pharm. Sci. 2013, 8, 151–158. [Google Scholar] [CrossRef]
- Sui, B.; Xu, H.; Jin, J.; Gou, J.; Liu, J.; Tang, X.; Zhang, Y.; Xu, J.; Zhang, H.; Jin, X. Self-assembled micelles composed of doxorubicin conjugated Y-shaped PEG-poly (glutamic acid) 2 copolymers via hydrazone linkers. Molecules 2014, 19, 11915–11932. [Google Scholar] [CrossRef] [PubMed]
- Luis Vázquez, J.; Berlanga, M.; Merino, S.; Domènech, Ò.; Viñas, M.; Teresa Montero, M.; Hernández-Borrell, J. Determination by fluorimetric titration of the ionization constants of ciprofloxacin in solution and in the presence of liposomes. J. Photochem. Photobiol. 2001, 73, 14–19. [Google Scholar] [CrossRef]
- Park, M.S.; Okochi, H.; Benet, L.Z. Is ciprofloxacin a substrate of P-glycoproteins? Arch. Drug Inf. Banner 2011, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, N.M.; Hirst, B.H.; Simmons, N.L. Active intestinal secretion of the fluoroquinolone ciprofloxacin, norfloxacin and pefloxacin; A common secretory pathway? J. Pharmacol. Exp. Ther. 1994, 269, 469–502. [Google Scholar]
- Dale, K.Y. The contribution of P-glycoprotein to pharmacokinetic drug-drug interactions. J. Clin. Pharmacol. 1999, 39, 1203–1211. [Google Scholar]
- Rodrıguez-Ibánez, M.; Nalda-Molina, R.; Montalar-Montero, M.; Bermejo, M.; Merino, V.; Garrigues, T. Transintestinal secretion of ciprofloxacin, grepafloxacin and sparfloxacin: In vitro and in situ inhibition studies. Eur. J. Pharm. Biopharm. 2003, 55, 241–246. [Google Scholar] [CrossRef]
- Kruijtzer, C.; Beijnen, J.; Rosing, H.; ten Bokkel Huinink, W.; Schot, M.; Jewell, R.; Paul, E.; Schellens, J. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF. J. Clin. Oncol. 2002, 20, 2943–2950. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, U.I.; Gramatté, T.; Krappweis, J.; Oertel, R.; Kirch, W. P-glycoprotein inhibitor erythromycin increases oral bioavailability of talinolol in humans. Int. J. Clin. Pharmacol. Ther. 2000, 38, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, D.; Zhang, Q.; Chen, Y.; Zheng, D.; Hao, L.; Duan, C.; Jia, L.; Liu, G.; Liu, Y. Synergistic effect of folate-mediated targeting and verapamil-mediated P-gp inhibition with paclitaxel-polymer micelles to overcome multi-drug resistance. Biomaterials 2011, 32, 9444–9456. [Google Scholar] [CrossRef] [PubMed]
- Srivalli, K.M.R.; Lakshmi, P. Overview of P-glycoprotein inhibitors: A rational outlook. Braz. J. Pharm. Sci. 2012, 48, 353–367. [Google Scholar] [CrossRef]
- Robert, J.; Jarry, C. Multidrug resistance reversal agents. J. Med. Chem. 2003, 46, 4805–4817. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.L. P-glycoprotein inhibition for optimal drug delivery. Drug Target Insights 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Sharom, F.J. The P-glycoprotein multidrug transporter. Essays Biochem. 2011, 50, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Lomovskaya, O.; Bostian, K.A. Practical applications and feasibility of efflux pump inhibitors in the clinic—A vision for applied use. Biochem. Pharmacol. 2006, 71, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Pusztai, L.; Wagner, P.; Ibrahim, N.; Rivera, E.; Theriault, R.; Booser, D.; Symmans, F.W.; Wong, F.; Blumenschein, G.; Fleming, D.R. Phase II study of tariquidar, a selective P-glycoprotein inhibitor, in patients with chemotherapy-resistant, advanced breast carcinoma. Cancer 2005, 104, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, J.; Wang, B.; Sheng, L.; Liu, Z.; Yang, S.; Li, Y. Inhibitory effects of herbal constituents on P-glycoprotein in vitro and in vivo: Herbal-drug interactions mediated via P-gp. Toxicol. Appl. Pharmacol. 2014, 2, 163–175. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Collins, X.; Huang, Y.; Edeki, T. Herbal componets inhibit P-GP mediated digoxin transport in transwell cultured Caco-2 cell model. Clin. Pharmacol. Ther. 2005, 77. [Google Scholar] [CrossRef]
- Kwon, H.-Y.; Kim, E.-H.; Kim, S.-W.; Kim, S.-N.; Park, J.-D.; Rhee, D.-K. Selective toxicity of ginsenoside Rg3 on multidrug resistant cells by membrane fluidity modulation. Arch. Pharm. Res. 2008, 31, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Kwon, H.-Y.; Chi, D.-W.; Shim, J.-H.; Park, J.-D.; Lee, Y.-H.; Pyo, S.; Rhee, D.-K. Reversal of P-glycoprotein-mediated multi drug resistance by ginsenoside Rg3. Biochem. Pharmacol. 2003, 65, 75–82. [Google Scholar] [CrossRef]
- Breda, S.A.; Jimenez-Kairuz, A.F.; Manzo, R.H.; Olivera, M.E. Solubility behavior and biopharmaceutical classification of novel high-solubility ciprofloxacin and norfloxacin pharmaceutical derivatives. Int. J. Pharm. 2009, 371, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, P.X. Host-guest interaction mediated polymeric core-shell assemblies: Versatile nanocarriers for drug delivery. Angew. Chem. Int. Ed. Engl. 2009, 48, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Smeets, N.M.B. Amphiphilic hyperbranched polymers from the copolymerization of vinyl and divinyl monomers: The potential of catalytic chain transfer polymerization. Eur. Polym. J. 2013, 49, 2528–2544. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Pérez, Y.A.; Urista, C.M.; Martínez, J.I.; Nava, M.D.C.D.; Rodríguez, F.A.R. Functionalized polymers for enhance oral bioavailability of sensitive molecules. Polymers 2016, 8, 214. [Google Scholar] [CrossRef]
- Des Rieux, A.; Fievez, V.; Garinot, M.; Schneider, Y.-J.; Préat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release 2006, 116, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, G. Polymeric Micelle as a New Carrier in Oral Drug Delivery Systems. Asian J. Pharm. 2018, 11. [Google Scholar] [CrossRef]
- Xu, W.; Ling, P.; Zhang, T. Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J. Drug Deliv. 2013, 2013, 340315. [Google Scholar] [CrossRef] [PubMed]
- Rege, B.D.; Kao, J.P.; Polli, J.E. Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur. J. Pharm. Sci. 2002, 16, 237–246. [Google Scholar] [CrossRef]
- Wei, Z.; Yuan, S.; Hao, J.; Fang, X. Mechanism of inhibition of P-glycoprotein mediated efflux by Pluronic P123/F127 block copolymers: Relationship between copolymer concentration and inhibitory activity. Eur. J. Pharm. Biopharm. 2013, 83, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Taymouri, S.; Hassanzadeh, F.; Haghjooy Javanmard, S. Self-assembly micelles with lipid core of cholesterol for decetaxel delivery to B16F10 melanoma and HepG2 cells. J. Liposome Res. 2015, 25, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Su, C.Y.; Liu, J.J.; Ho, Y.S.; Huang, Y.Y; Chang, V.H.S; Liu, D.Z; Chen, L.C; Ho, H.O; Sheu, M.T. Development and characterization of decetaxel-loaded lecithin-stabilized micellar drug delivery system for improving the therapeutic efficacy and reducing systemic toxicity. Eur. J. Pharm. Biopharm. 2018, 123, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Francis, M.F.; Cristea, M.; Winnik, F.M. Polymeric micelles for oral drug delivery: Why and how. Pure Appl. Chem. 2004, 76, 1321–1335. [Google Scholar] [CrossRef]
- Jain, D.; Banerjee, R. Comparison of ciprofloxacin hydrochloride-loaded protein, lipid, and chitosan nanoparticles for drug delivery. J. Biomed. Mater. Res. B 2008, 86, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Taha, E.I.; Badran, M.M.; El-Anazi, M.H.; Bayomi, M.A.; El-Bagory, I.M. Role of Pluronic F127 micelles in enhancing ocular delivery of ciprofloxacin. J. Mol. Liq. 2014, 199, 251–256. [Google Scholar] [CrossRef]
- Camilleri, M.; Colemont, L.; Phillips, S.F.; Brown, M.L.; Thomforde, G.M.; Chapman, N.; Zinsmeister, A.R. Human gastric emptying and colonic filling of solids characterized by a new method. Am. J. Physiol. Gastrointest. Liver Physiol. 1989, 257, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Sagiri, S.S.; Behera, B.; Sudheep, T.; Pal, K. Effect of composition on the properties of tween-80-span-80-based organogels. Des. Monomers Polym. 2012, 15, 253–273. [Google Scholar] [CrossRef]
- Shen, J.; Burgess, D.J. Accelerated in-vitro release testing methods for extended-release parenteral dosage forms. J. Pharm. Pharmacol. 2012, 64, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Shazly, G.A. Ciprofloxacin controlled-solid lipid nanoparticles: Characterization, in vitro release, and antibacterial activity assessment. BioMed Res. Int. 2017, 2017, 2120734. [Google Scholar] [PubMed]
- Cavet, M.; West, M.; Simmons, N. Fluoroquinolone (ciprofloxacin) secretion by human intestinal epithelial (Caco-2) cells. Br. J. Pharmacol. 1997, 121, 1567–1578. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Chang, S.-F.; Lee, D.; Yang, L.-Y.; Lee, Y.-H.; Hsu, C.Y.; Lin, S.-J.; Liaw, J. Bioavailability effect of methylprednisolone by polymeric micelles. Pharm. Res. 2008, 25, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Batrakova, E.V.; Li, S.; Vinogradov, S.V.; Alakhov, V.Y.; Miller, D.W.; Kabanov, A.V. Mechanism of pluronic effect on P-glycoprotein efflux system in blood-brain barrier: Contributions of energy depletion and membrane fluidization. J. Pharmacol. Exp. Ther. 2001, 299, 483–493. [Google Scholar] [PubMed]
- Taylor, K.M.; Morris, R.M. Thermal analysis of phase transition behaviour in liposomes. Thermochim. Acta 1995, 248, 289–301. [Google Scholar] [CrossRef]
- Müller, M.; Müller-Goymann, C. Influence of temperature on the manufacturing of liposomes. Arch. Pharm. Med. Chem. 2001, 334, 79. [Google Scholar]
- Dabholkar, R.D.; Sawant, R.M.; Mongayt, D.A.; Devarajan, P.V.; Torchilin, V.P. Polyethylene glycol–phosphatidylethanolamine conjugate (PEG–PE)-based mixed micelles: Some properties, loading with paclitaxel, and modulation of P-glycoprotein-mediated efflux. Int. J. Pharm. 2006, 315, 148–157. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all compounds are available from the authors. |

| Batch No. | Chol | Leci | O.A | Polox | Lab + las (CMC) | PG | PEG | Cipro | R-g3 | EE% | Particle Size (nm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.135 | 0.45 | 0.06 | 0.2 | (3 CMC) | 2 | 0.7 | 0.01 | 0.01 | 43.7 ± 5.7 | 137 ± 4.5 |
| 2 | 0.135 | 0.45 | 0.06 | 0.2 | (3 CMC) | 2 | 0.7 | 0.025 | 0.02 | 73.5 ± 6.6 | 121 ± 8.2 |
| 3 | 0.135 | 0.45 | 0.06 | 0.2 | (3 CMC) | 2 | 0.7 | 0.01 | 0.02 | 82.8 ± 5.8 | 120 ± 10.9 |
| 4 | 0.135 | 0.45 | 0.06 | 0.2 | (3 CMC) | 2 | 0.7 | 0.025 | 0.01 | 87.9 ± 7.3 | 127 ± 14.3 |
| 5 | 0.135 | 0.45 | 0.06 | 0.2 | (1 CMC) | 2 | 0.7 | 0.01 | 0.01 | 33.7 ± 3.7 | 183 ± 12.5 |
| 6 | 0.135 | 0.45 | 0.06 | 0.2 | (1 CMC) | 2 | 0.7 | 0.025 | 0.02 | 39.5 ± 4.2 | 126 ± 13.1 |
| 7 | 0.135 | 0.45 | 0.06 | 0.2 | (1 CMC) | 2 | 0.7 | 0.01 | 0.02 | 27.9 ± 3.1 | 109 ± 7.2 |
| 8 | 0.135 | 0.45 | 0.06 | 0.2 | (1 CMC) | 2 | 0.7 | 0.025 | 0.01 | 30.4 ± 2.4 | 153 ± 9.9 |
| Surfactant + Co-Surfactant Concentration (mg/mL) | Ciprofloxacin Concentration (mg/mL) | Ginsenoside Concentration (mg/mL) | |
|---|---|---|---|
| Batch Components | 0.139 | 0.153 | 0.2 |
| Batch characterization | EE% | Actual value | 66.5 |
| Predicted value | 63.3 | ||
| p value | 0.461 | ||
| Particle Size (nm) | Actual value | 135.3 | |
| Predicted value | 133.7 | ||
| p value | 0.348 | ||
| D4% | Actual value | 48.7 | |
| Predicted value | 46.2 | ||
| p value | 0.394 |
| Formulation | Phase Transition Temperature (C°) | Phase Transition Enthalpy (mJ/mg) |
|---|---|---|
| Optimized formulation | −20 ± 3 | 742 ± 73 |
| 0 ± 0.4 | −100 ± 12 | |
| 140 ± 12 | −723 ± 57 | |
| Optimized formulation without ginsenoside | −20 ± 3 | 189 ± 18 |
| 0 ± 0.6 | −185 ± 22 | |
| 140 ± 10 | −160 ± 20 | |
| Blank formulation excluding ginsenoside | −20 ± 2 | 229 ± 25 |
| 0 ± 0.3 | −221 ± 13 | |
| 140 ± 9 | −336 ± 44 | |
| Ciprofloxacin powder | 260 ± 22 | 812 ± 69 |
| Formulation | AP-BL Direction | BL-AP Direction | ||
|---|---|---|---|---|
| Q4% | P (cm/s) × 104 | Q4% | P (cm/s) × 104 | |
| Optimized formulation | 72 ± 1.9 | 2.40 ± 0.19 | 84.6 ± 5.1 | 2.89 ± 0.22 |
| Optimized formulation without ginsenoside | 58.3 ± 1.3 | 1.9 ± 0.16 | 79.2 ± 5.6 | 2.77 ± 0.28 |
| Ciprofloxacin 0.14 mg/mL + ginsenoside 0.02 mg/mL (control 1) | 47.2 ± 4.2 | 1.27 ± 0.11 | 65.5 ± 7.1 | 2.13 ± 0.15 |
| Ciprofloxacin 0.14 mg/mL + ginsenoside 0.08 mg/mL (control 2) | 60.7 ± 2.8 | 2.05 ± 0.14 | 67.7 ± 8.1 | 2.22 ± 0.32 |
| Ciprofloxacin 0.14 mg/mL + ginsenoside 0.23 mg/mL (control 3) | 77.3 ± 2.5 | 2.67 ± 014 | 64.7 ± 10.2 | 2.28 ± 0.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharif Makhmal Zadeh, B.; Esfahani, G.; Salimi, A. Permeability of Ciprofloxacin-Loaded Polymeric Micelles Including Ginsenoside as P-glycoprotein Inhibitor through a Caco-2 Cells Monolayer as an Intestinal Absorption Model. Molecules 2018, 23, 1904. https://doi.org/10.3390/molecules23081904
Sharif Makhmal Zadeh B, Esfahani G, Salimi A. Permeability of Ciprofloxacin-Loaded Polymeric Micelles Including Ginsenoside as P-glycoprotein Inhibitor through a Caco-2 Cells Monolayer as an Intestinal Absorption Model. Molecules. 2018; 23(8):1904. https://doi.org/10.3390/molecules23081904
Chicago/Turabian StyleSharif Makhmal Zadeh, Behzad, Golbarg Esfahani, and Anayatollah Salimi. 2018. "Permeability of Ciprofloxacin-Loaded Polymeric Micelles Including Ginsenoside as P-glycoprotein Inhibitor through a Caco-2 Cells Monolayer as an Intestinal Absorption Model" Molecules 23, no. 8: 1904. https://doi.org/10.3390/molecules23081904
APA StyleSharif Makhmal Zadeh, B., Esfahani, G., & Salimi, A. (2018). Permeability of Ciprofloxacin-Loaded Polymeric Micelles Including Ginsenoside as P-glycoprotein Inhibitor through a Caco-2 Cells Monolayer as an Intestinal Absorption Model. Molecules, 23(8), 1904. https://doi.org/10.3390/molecules23081904