UHPLC Analysis of Saffron (Crocus sativus L.): Optimization of Separation Using Chemometrics and Detection of Minor Crocetin Esters
Abstract
:1. Introduction
2. Results and Discussion
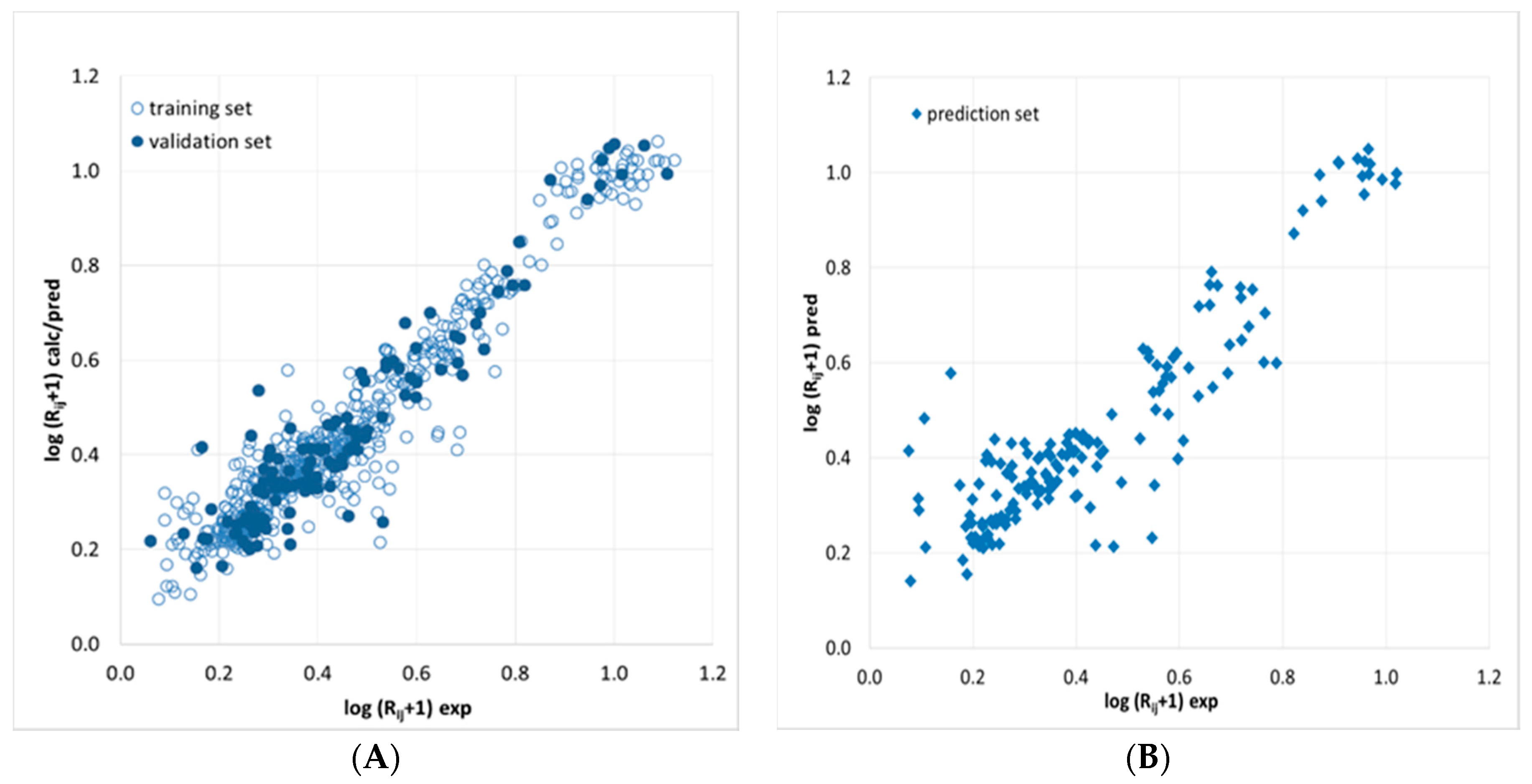
2.1. Artificial Neural Network-Based Modeling of Chromatogram Resolution
2.1.1. Artificial Neural Network Modeling of Peak Pair Resolution
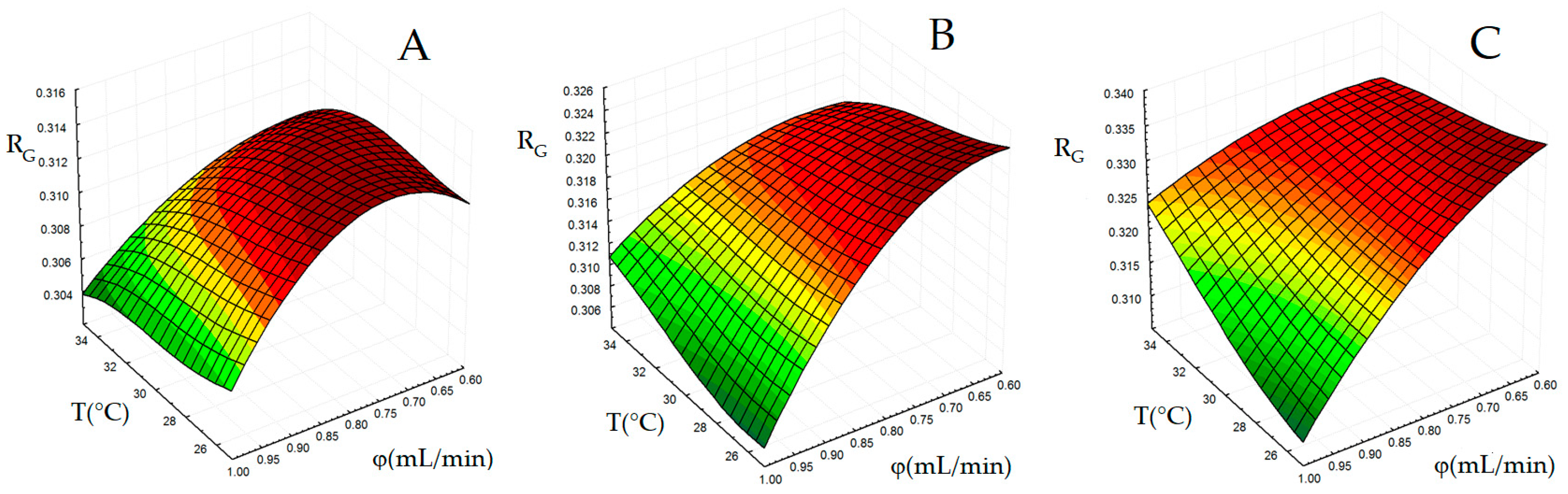
2.1.2. Generation of Surface Response for Global Resolution
2.2. Ultra-High Performance Liquid Chromatography Saffron Analysis Using the Kinetex C18 Column
2.3. Ultra-High Performance Liquid Chromatography Saffron Analysis Using the Luna Omega Polar C18 Column
3. Materials and Methods
3.1. Samples, Chemicals and Solvents
3.2. Saffron Characterization Using Ultraviolet-Vis Spectroscopy
3.3. Ultra-High Performance Liquid Chromatography Sample Preparation
3.4. Ultra-High Performance Liquid Chromatography Analysis
3.5. Quantitative and Qualitative Analysis
3.6. Multivariate Design of Experiments and Statistical Data Treatment
3.6.1. Design of Experiments
3.6.2. Artificial Neural Network Modeling
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Melnyk, J.P.; Wang, S.; Marcone, M.F. Chemical and biological properties of the world’s most expensive spice: Saffron. Food Res. Int. 2010, 43, 1981–1989. [Google Scholar] [CrossRef]
- Licon, C.; Carmona, M.; Llorens, S.; Berruga, M.I.; Alonso, G.L. Potential healthy effects of saffron spice (Crocus sativus L. stigmas) consumption. Funct. Plant Sci. Biotechnol. Saffron 2010, 4, 64–73. [Google Scholar]
- Caballero-Ortega, H.; Pereda-Miranda, R.; Abdullaev, F.I. HPLC quantification of major active components from 11 different saffron (Crocus sativus L.) sources. Food Chem. 2007, 100, 1126–1131. [Google Scholar] [CrossRef]
- Carmona, M.; Zalacain, A.; Sánchez, A.M.; Novella, J.L.; Alonso, G.L. Crocetin esters, picrocrocin and its related compounds present in Crocus sativus stigmas and Gardenia jasminoides fruits. Tentative identification of seven new compounds by LC-ESI-MS. J. Agric. Food Chem. 2006, 54, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Lech, K.; Witowska-Jarosz, J.; Jarosz, M. Saffron yellow: Characterization of carotenoids by high-performance liquid chromatography with electrospray mass spectrometric detection. J. Mass Spectrom. 2009, 44, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Koulakiotis, N.S.; Pittenauer, E.; Halabalaki, M.; Tsarbopoulos, A.; Allmaier, G. Comparison of different tandem mass spectrometric techniques (ESI-IT, ESI- and IP-MALDI-QRTOF and vMALDI-TOF/RTOF) for the analysis of crocins and picrocrocin from the stigmas of Crocus sativus L. Rapid Commun. Mass Spectrom. 2012, 26, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Cossignani, L.; Urbani, E.; Simonetti, M.S.; Maurizi, A.; Chiesi, C.; Blasi, F. Characterisation of secondary metabolites in saffron from central Italy (Cascia, Umbria). Food Chem. 2014, 143, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lin, G.; Kwan, Y.W.; Min, Z.D. Simultaneous quantification of five major biologically active ingredients of saffron by high-performance liquid chromatography. J. Chromatogr. A 1999, 849, 349–355. [Google Scholar] [CrossRef]
- Lozano, P.; Castellar, M.R.; Simancas, M.J.; Iborra, J.L. Quantitative high-performance liquid chromatographic method to analyse commercial saffron (Crocus sativus L.) products. J. Chromatogr. A 1999, 830, 477–483. [Google Scholar] [CrossRef]
- Tarantilis, P.A.; Tsoupras, G.; Polissiou, M. Determination of saffron (Crocus sativus L.) components in crude plant extract using high-performance liquid chromatography-UV-visible photodiode-array detection-mass spectrometry. J. Chromatogr. A 1995, 699, 107–118. [Google Scholar] [CrossRef]
- Carmona, M.; Sánchez, A.M.; Ferreres, F.; Zalacain, A.; Tomás-Barberán, F.; Alonso, G.L. Identification of the flavonoid fraction in saffron spice by LC/DAD/MS/MS: Comparative study of samples from different geographical origins. Food Chem. 2007, 100, 445–450. [Google Scholar] [CrossRef]
- Guijarro-Díez, M.; Nozal, L.; Marina, M.L.; Crego, A.L. Metabolomic fingerprinting of saffron by LC/MS: Novel authenticity markers. Anal. Bioanal. Chem. 2015, 407, 7197–7213. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, A.A.; Giannitto, A.; Maggi, M.A.; Ruggieri, F. Geographical classification of Italian saffron (Crocus sativus L.) based on chemical constituents determined by high-performance liquid-chromatography and by using linear discriminant analysis. Food Chem. 2016, 212, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Masi, E.; Taiti, C.; Heimler, D.; Vignolini, P.; Romani, A.; Mancuso, S. PTR-TOF-MS and HPLC analysis in the characterization of saffron (Crocus sativus L.) from Italy and Iran. Food Chem. 2016, 192, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Rubert, J.; Lacina, O.; Zachariasova, M.; Hajslova, J. Saffron authentication based on liquid chromatography high resolution tandem mass spectrometry and multivariate data analysis. Food Chem. 2016, 204, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wanrooij, J.; van Bommel, M.; Quye, A. Characterisation of chemical components for identifying historical Chinese textile dyes by ultra high performance liquid chromatography—Photodiode array—Electrospray ionisation mass spectrometer. J. Chromatogr. A 2017, 1479, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Moras, B.; Loffredo, L.; Rey, S. Quality assessment of saffron (Crocus sativus L.) extracts via UHPLC-DAD-MS analysis and detection of adulteration using gardenia fruit extract (Gardenia jasminoides Ellis). Food Chem. 2018, 257, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Novotná, K.; Havliš, J.; Havel, J. Optimisation of high performance liquid chromatography separation of neuroprotective peptides: Fractional experimental designs combined with artificial neural networks. J. Chromatogr. A 2005, 1096, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.T.K.; Hyne, R.V.; Pablo, F.; Day, W.R.; Doble, P. Optimisation of the separation of herbicides by linear gradient high performance liquid chromatography utilising artificial neural networks. Talanta 2007, 71, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, A.A.; Maggi, M.A.; Marinelli, C.; Ruggieri, F.; Stecca, F. Optimisation of temperature-programmed gas chromatographic separation of organochloride pesticides by response surface methodology. J. Chromatogr. A 2015, 1423. [Google Scholar] [CrossRef] [PubMed]
- Cirera-Domènech, E.; Estrada-Tejedor, R.; Broto-Puig, F.; Teixidó, J.; Gassiot-Matas, M.; Comellas, L.; Lliberia, J.L.; Méndez, A.; Paz-Estivill, S.; Delgado-Ortiz, M.R. Quantitative structure-retention relationships applied to liquid chromatography gradient elution method for the determination of carbonyl-2,4-dinitrophenylhydrazone compounds. J. Chromatogr. A 2013, 1276, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Golubović, J.; Protić, A.; Zečević, M.; Otašević, B.; Mikić, M.; Živanović, L. Quantitative structure-retention relationships of azole antifungal agents in reversed-phase high performance liquid chromatography. Talanta 2012, 100, 329–337. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, A.A.; Maggi, M.A.; Mazzeo, P.; Ruggieri, F. Quantitative structure-retention relationships of pesticides in reversed-phase high-performance liquid chromatography based on WHIM and GETAWAY molecular descriptors. Anal. Chim. Acta 2008, 628, 162–172. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, A.A.; Maggi, M.A.; Ruggieri, F. Multiple-column RP-HPLC retention modelling based on solvatochromic or theoretical solute descriptors. J. Sep. Sci. 2010, 33, 155–166. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, A.A.; Incani, A.; Ruggieri, F. Cross-column prediction of gas-chromatographic retention of polychlorinated biphenyls by artificial neural networks. J. Chromatogr. A 2011, 1218, 8679–8690. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, A.A.; Maggi, M.A.; Ruggieri, F. Artificial neural network prediction of multilinear gradient retention in reversed-phase HPLC: Comprehensive QSRR-based models combining categorical or structural solute descriptors and gradient profile parameters. Anal. Bioanal. Chem. 2015, 407, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Kennard, R.W.; Stone, L.A. Computer Aided Design of Experiments. Technometrics 1969, 11, 137–148. [Google Scholar] [CrossRef]
- Žuvela, P.; David, J.; Wong, M.W. Interpretation of ANN-based QSAR models for prediction of antioxidant activity of flavonoids. J. Comput. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Héberger, K. Sum of ranking differences compares methods or models fairly. TrAC—Trends Anal. Chem. 2010, 29, 101–109. [Google Scholar] [CrossRef]
- Vera Candioti, L.; De Zan, M.M.; Cámara, M.S.; Goicoechea, H.C. Experimental design and multiple response optimization. Using the desirability function in analytical methods development. Talanta 2014, 124, 123–138. [Google Scholar] [CrossRef] [PubMed]
- ISO 3632-2. Saffron (Crocus sativus L.); Part 2 (Test methods); International Organization for Standardization: Genève, Switzerland, 2010. [Google Scholar]
- Sánchez, A.M.; Carmona, M.; Zalacain, A.; Carot, J.M.; Jabaloyes, J.M.; Alonso, G.L. Rapid determination of crocetin esters and picrocrocin from saffron spice (Crocus sativus L.) using UV-visible spectrophotometry for quality control. J. Agric. Food Chem. 2008, 56, 3167–3175. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.M.; Carmona, M.; Ordoudi, S.A.; Tsimidou, M.Z.; Alonso, G.L. Kinetics of individual crocetin ester degradation in aqueous extracts of saffron (Crocus sativus L.) upon thermal treatment in the dark. J. Agric. Food Chem. 2008, 56, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, A.A.; Maggi, M.A. Geographical identification of saffron (Crocus sativus L.) by linear discriminant analysis applied to the UV–visible spectra of aqueous extracts. Food Chem. 2017, 219, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Zupan, J.; Gasteiger, J. Neural networks: A new method for solving chemical problems or just a passing phase? Anal. Chim. Acta 1991, 248, 1–30. [Google Scholar] [CrossRef]
- Svozil, D.; Kvasnička, V.; Pospíchal, J. Introduction to multi-layer feed-forward neural networks. Chemom. Intell. Lab. Syst. 1997, 39, 43–62. [Google Scholar] [CrossRef]
- Lopez, R. Open NN: An Open Source Neural Networks C++ Library; Artificial Intelligence Techniques, Ltd.: Salamanca, Spain, 2014. [Google Scholar]
Sample Availability: Saffron samples investigated in this work are available from the authors. |
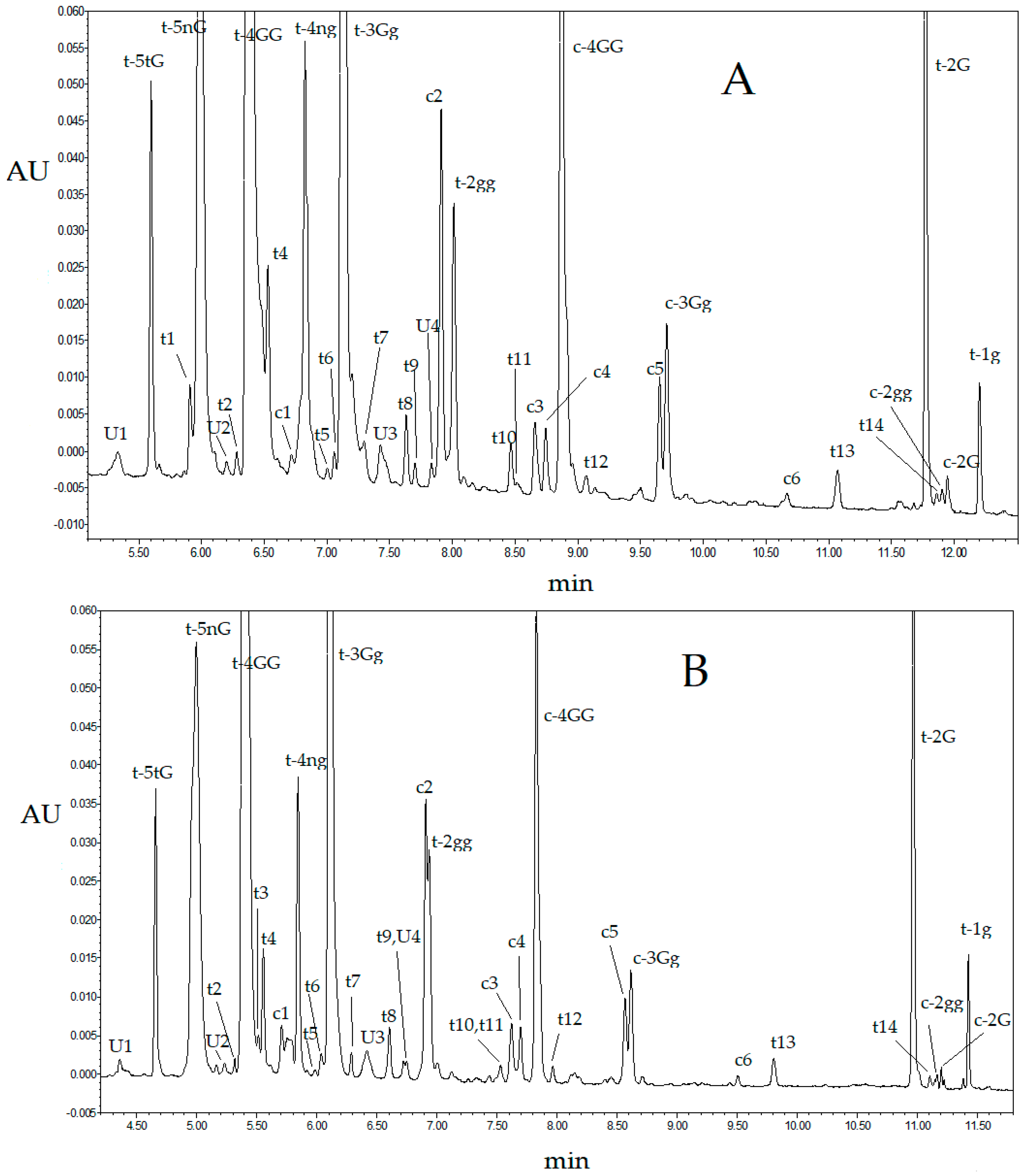
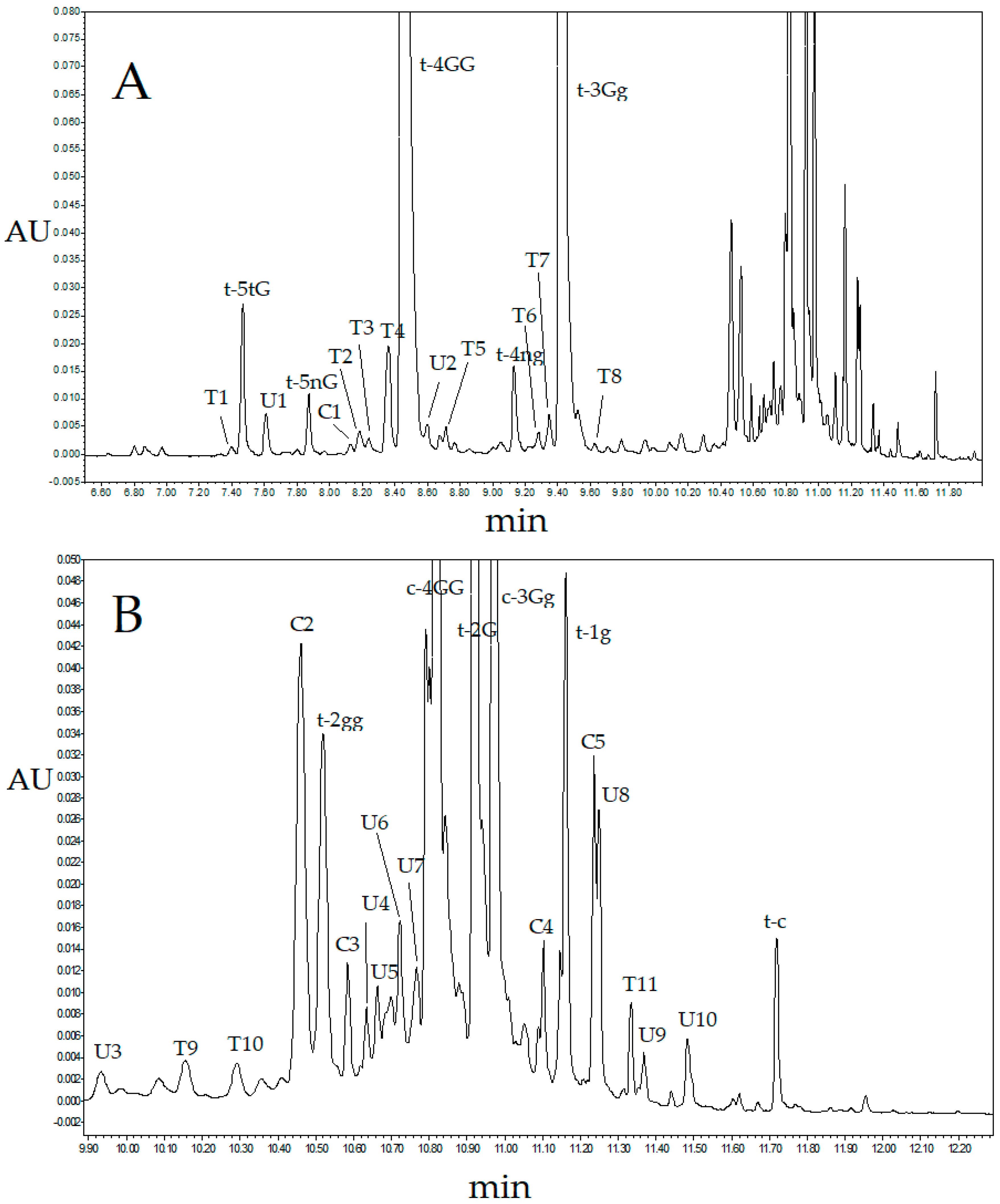
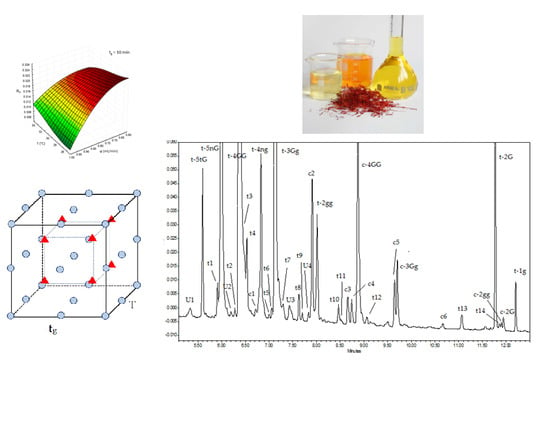
| RT (min) | Compound/Chemical Class | Abbreviation a | λmax |
|---|---|---|---|
| 5.34 | unknown | U1 | 230, 423 |
| 5.59 | trans-crocetin (tri-β-d-glucosyl)(β-d-gentiobiosyl) ester | t-5tG b | 262, 443, 466 |
| 5.91 | trans-crocin | t1 b | 262, 441, 465 |
| 5.99 | trans-crocetin (β-d-neapolitanosyl)(β-d-gentiobiosyl) ester | t-5nG b | 241, 418, 440 |
| 6.20 | unknown | U2 | 301, 443, 471 |
| 6.28 | trans-crocin | t2 | 262, 442, 466 |
| 6.37 | trans-crocetin bis(β-d-gentiobiosyl) ester | t-4GG b | 262, 441, 465 |
| 6.53 | trans-crocin | t3 b | 259, 438, 465 |
| 6.60 | trans-crocin | t4 b | 260, 440, 465 |
| 6.72 | cis-crocin | c1 b | 256, 325, 431, 440 sh |
| 6.83 | trans-crocetin (β-d-neapolitanosyl)(β-d-glucosyl) ester | t-4ng b | 261, 441, 464 |
| 7.00 | trans-crocin | t5 | 261, 438, 464 |
| 7.05 | trans-crocin | t6 | 260, 441, 463 |
| 7.13 | trans-crocetin (β-d-gentiobiosyl)(β-d-glucosyl) ester | t-3Gg b | 262, 441, 465 |
| 7.30 | trans-crocin | t7 b | 260, 440, 463 |
| 7.43 | unknown | U3 b | 317, 426, 444 sh |
| 7.63 | trans-crocin | t8 b | 260, 441, 466 |
| 7.70 | trans-crocin | t9 b | 253, 440, 463 |
| 7.83 | unknown | U4 b | 243, 308, 413, 438sh |
| 7.91 | cis-crocin | c2 b | 263, 329, 440, 465 |
| 8.04 | trans-crocetin bis(β-d-glucosyl) ester | t-2gg b | 260, 440, 465 |
| 8.46 | trans-crocin | t10 b | 258, 435, 460 sh |
| 8.56 | trans-crocin | t11 b | 252, 432, 462 sh |
| 8.66 | cis-crocin | c3 b | 223, 265, 327, 439 |
| 8.75 | cis-crocin | c4 | 262, 329, 440, 463 |
| 8.87 | cis-crocetin bis(β-d-gentiobiosyl) ester | c-4GG b | 262, 326, 435, 458 sh |
| 9.06 | trans-crocin | t12 | 264, 442, 467 |
| 9.65 | cis-crocin | c5 b | 262, 327, 434, 457 |
| 9.70 | cis-crocetin (β-d-gentiobiosyl)(β-d-glucosyl) ester | c-3Gg b | 263, 326, 433, 460 |
| 10.66 | cis-crocin | c6 | 263, 326, 432, 456 sh |
| 11.06 | trans-crocin | t13 | 263, 443, 469 |
| 11.76 | trans-crocetin mono(β-d-gentiobiosyl) ester | t-2G | 258, 434, 459 |
| 11.85 | trans-crocin | t14 | 259, 431, 455 |
| 11.90 | cis-crocetin bis(β-d-glucosyl) ester | c-2gg | 321, 426, 451 |
| 11.94 | cis-crocetin mono(β-d-gentiobiosyl) ester | c-2G | 258, 313, 426, 450 sh |
| 12.22 | trans-crocetin mono(β-d-glucosyl) ester | t-1g | 257, 432, 458 |
| RT (min) | Compound/Chemical Class | Abbreviation a | λmax |
|---|---|---|---|
| 7.40 | trans-crocin | T1 | 259, 444, 466 |
| 7.46 | trans-crocetin (tri-β-d-glucosyl)(β-d-gentiobiosyl) ester | t-5tG | 262, 442, 465 |
| 7.60 | unknown | U1 | 243, 418, 440 |
| 7.86 | trans-crocetin (β-d-neapolitanosyl) (β-d-gentiobiosyl) ester | t-5nG | 262, 440, 464 |
| 8.16 | cis-crocin | C1 | 260, 327, 441, 464 |
| 8.20 | trans-crocin | T2 | 262, 441, 462 sh |
| 8.26 | trans-crocin | T3 | 259, 442, 464 |
| 8.37 | trans-crocin | T4 | 264, 437, 466 sh |
| 8.48 | trans-crocetin bis(β-d-gentiobiosyl) ester | t-4GG | 262, 443, 466 |
| 8.60 | unknown | U2 | 244, 415, 440 |
| 8.71 | trans-crocin | T5 | 262, 437, 464 |
| 9.14 | trans-crocetin (β-d-neapolitanosyl)(β-d-glucosyl) ester | t-4ng | 262, 441, 465 |
| 9.28 | trans-crocin | T6 | 261, 438, 464 |
| 9.35 | trans-crocin | T7 | 260, 441, 463 |
| 9.44 | trans-crocetin (β-d-gentiobiosyl)(β-d-glucosyl) ester | t-3Gg | 262, 441, 465 |
| 9.52 | trans-crocin | T8 | 260, 440, 464 |
| 9.94 | unknown | U3 | 308, 413, 434 |
| 10.16 | trans-crocin | T9 | 262, 442, 464 |
| 10.30 | trans-crocin | T10 | 262, 440, 461 sh |
| 10.46 | cis-crocin | C2 | 263, 329, 440, 465 sh |
| 10.53 | trans-crocetin bis(β-d-glucosyl) ester | t-2gg | 262, 327, 440, 465 |
| 10.58 | cis-crocin | C3 | 262, 315, 442, 466 |
| 10.67 | unknown | U4 | 239, 409, 434 |
| 10.71 | unknown | U5 | 245, 321, 440, 465 |
| 10.77 | unknown | U6 | 254, 301, 434, 466 sh |
| 10.80 | unknown | U7 | 329, 487 |
| 10.85 | cis-crocetin bis(β-d-gentiobiosyl) ester | c-4GG | 262, 326, 434, 458 |
| 10.92 | trans-crocetin mono(β-d-gentiobiosyl) ester | t-2G | 258, 435, 459 |
| 10.90 | cis-crocetin (β-d-gentiobiosyl)(β-d-glucosyl) ester | c-3Gg | 262, 326, 434, 460 |
| 11.09 | cis-crocin | C4 | 258, 323, 432, 458 |
| 11.15 | trans-crocetin mono(β-d-gentiobiosyl) ester | t-2G | 258, 435, 459 |
| 11.23 | cis-crocin | C5 | 320, 428, 451 |
| 11.25 | unknown | U8 | 428, 451 |
| 11.32 | trans-crocin | T11 | 257, 434, 458 |
| 11.36 | unknown | U9 | 234, 402, 427 |
| 11.47 | unknown | U10 | 264, 442, 467 |
| 11.71 | trans-crocetin | t-c | 258, 433, 458 |
| Crocin | Column | Ref [33] | Ref [32] | |||
|---|---|---|---|---|---|---|
| Kinetex C18 | Luna Omega Polar C18 | |||||
| Sample | ||||||
| AQ | AQ | IR | MO | |||
| t-5tG | 3.32 ± 0.06 | 2.8 ± 0.6 | 3.1 ± 0.2 | 3.1 ± 0.4 | 3.6 ± 0.1 | |
| t-5nG | 13.6 ± 0.1 | 0.8 ± 0.1 | 1.09 ± 0.09 | 0.9 ± 0.1 | 3.8 ± 0.1 | |
| t-4GG | 159.8 ± 0.4 | 153 ± 1 | 146.9 ± 0.8 | 169 ± 1 | 157.2 ± 0.3 | 145 ± 3 |
| t-3Gg | 54.5 ± 0.4 | 52.3 ± 0.1 | 50.8 ± 0.1 | 60.4 ± 0.5 | 76.4 ± 0.4 | 70 ± 2 |
| t-2gg | 2.46 ± 0.06 | 1.84 ± 0.08 | 1.76 ± 0.08 | 2.12 ± 0.09 | 6.0 ± 0.2 | |
| c-4GG | 11.5 ± 0.2 | 19.6 ± 0.4 | 19.2 ± 0.8 | 26 ± 1 | 4.8 ± 0.3 | 12 ± 1 |
| c-3Gg | 2.13 ± 0.08 | 8.0 ± 0.6 | 5.0 ± 0.3 | 7.2 ± 0.6 | 2.4 ± 0.1 | 5.2 ± 0.4 |
| t-2G | 4.0 ± 0.1 | 9.6 ± 0.8 | 12.2 ± 0.2 | 11.4 ± 0.6 | 9.8 ± 0.2 | 4.8 ± 0.2 |
| t-1g | 0.47 ± 0.02 | 0.77 ± 0.09 | 1.37 ± 0.04 | 0.83 ± 0.09 | 0.9 ± 0.1 | |
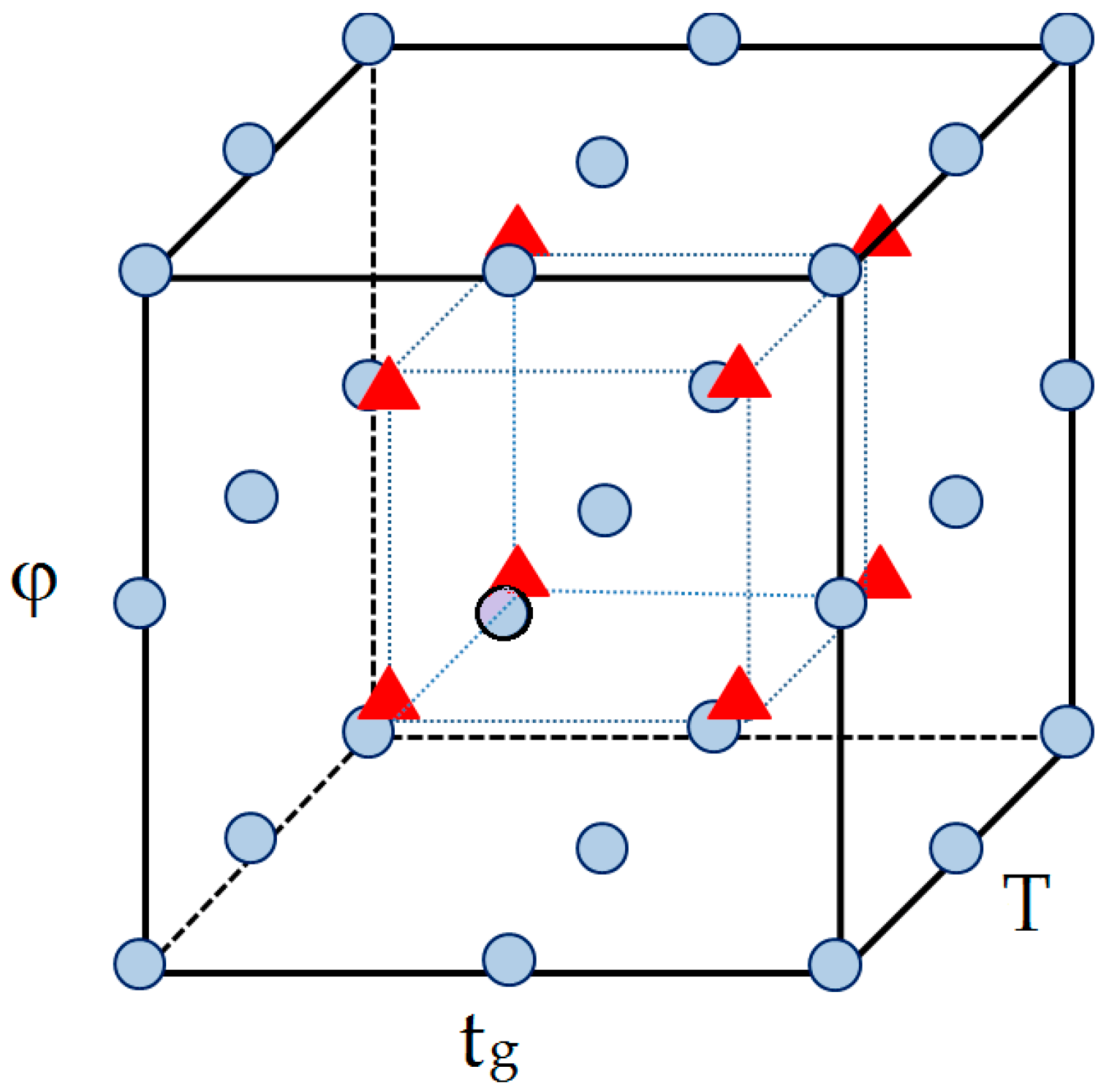
| Factors | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| T (°C) | 25 | 30 | 35 |
| tg (min) | 8 | 10 | 12 |
| φ (mL/min) | 0.6 | 0.8 | 1.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Archivio, A.A.; Di Donato, F.; Foschi, M.; Maggi, M.A.; Ruggieri, F. UHPLC Analysis of Saffron (Crocus sativus L.): Optimization of Separation Using Chemometrics and Detection of Minor Crocetin Esters. Molecules 2018, 23, 1851. https://doi.org/10.3390/molecules23081851
D’Archivio AA, Di Donato F, Foschi M, Maggi MA, Ruggieri F. UHPLC Analysis of Saffron (Crocus sativus L.): Optimization of Separation Using Chemometrics and Detection of Minor Crocetin Esters. Molecules. 2018; 23(8):1851. https://doi.org/10.3390/molecules23081851
Chicago/Turabian StyleD’Archivio, Angelo Antonio, Francesca Di Donato, Martina Foschi, Maria Anna Maggi, and Fabrizio Ruggieri. 2018. "UHPLC Analysis of Saffron (Crocus sativus L.): Optimization of Separation Using Chemometrics and Detection of Minor Crocetin Esters" Molecules 23, no. 8: 1851. https://doi.org/10.3390/molecules23081851
APA StyleD’Archivio, A. A., Di Donato, F., Foschi, M., Maggi, M. A., & Ruggieri, F. (2018). UHPLC Analysis of Saffron (Crocus sativus L.): Optimization of Separation Using Chemometrics and Detection of Minor Crocetin Esters. Molecules, 23(8), 1851. https://doi.org/10.3390/molecules23081851