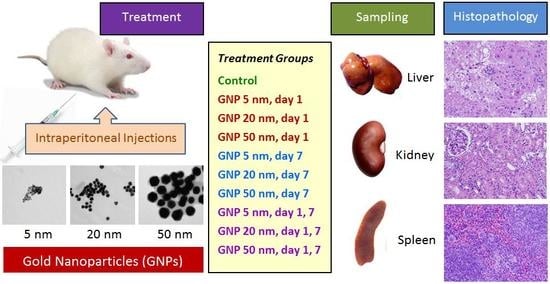
Histopathology of the Liver, Kidney, and Spleen of Mice Exposed to Gold Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatment Groups
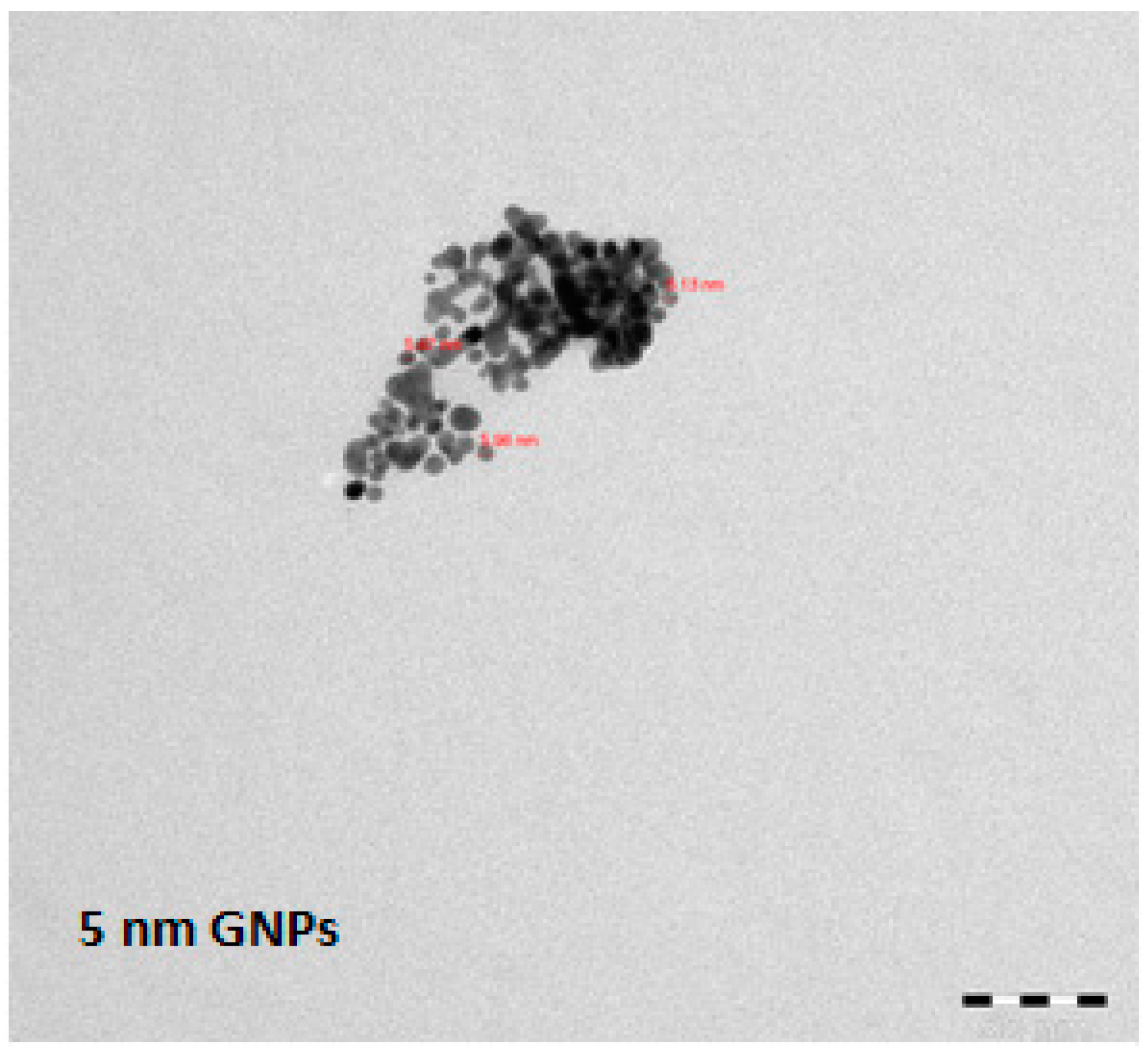
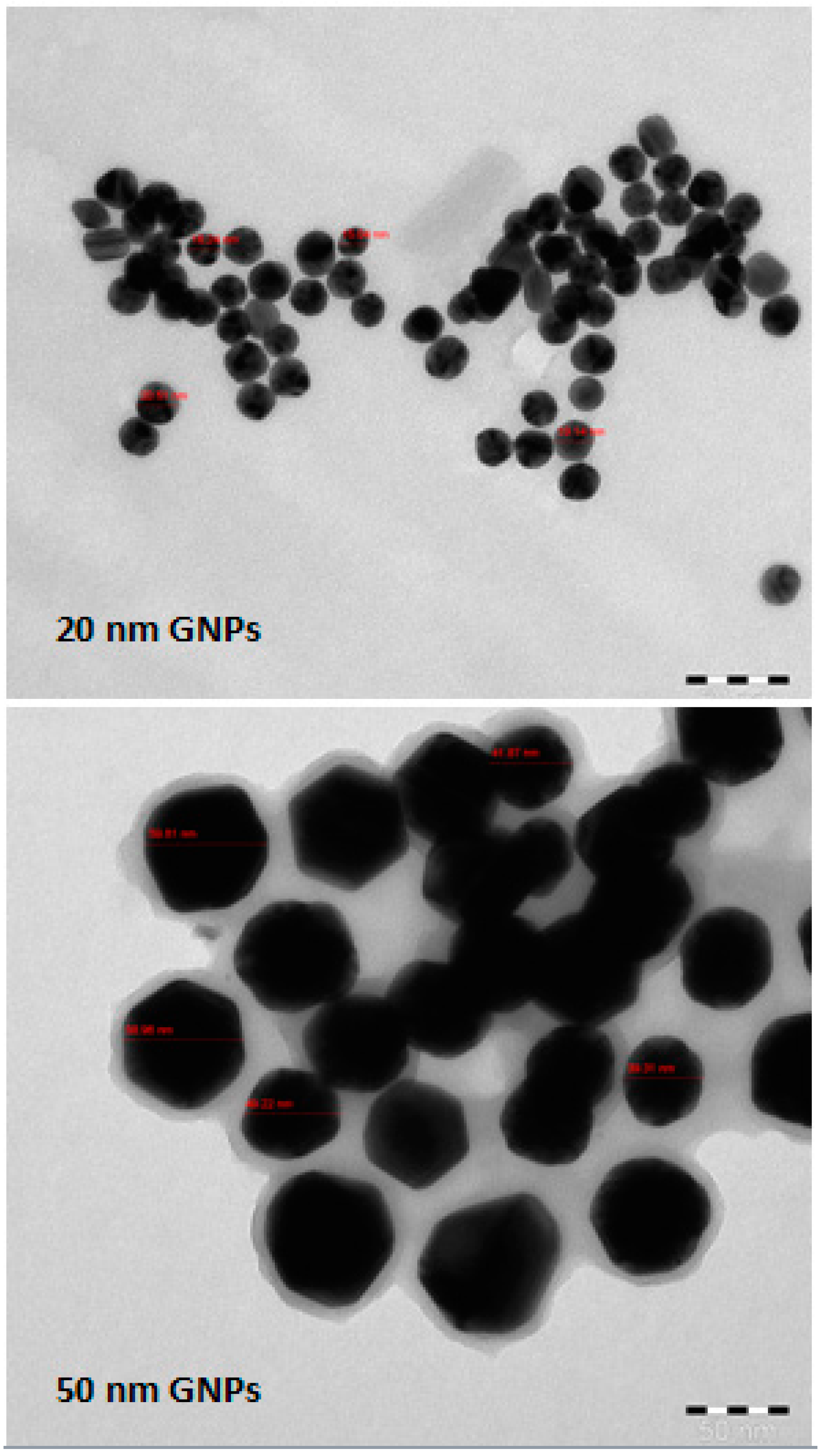
2.2. Gold Nanoparticles
2.3. Animal Dosing
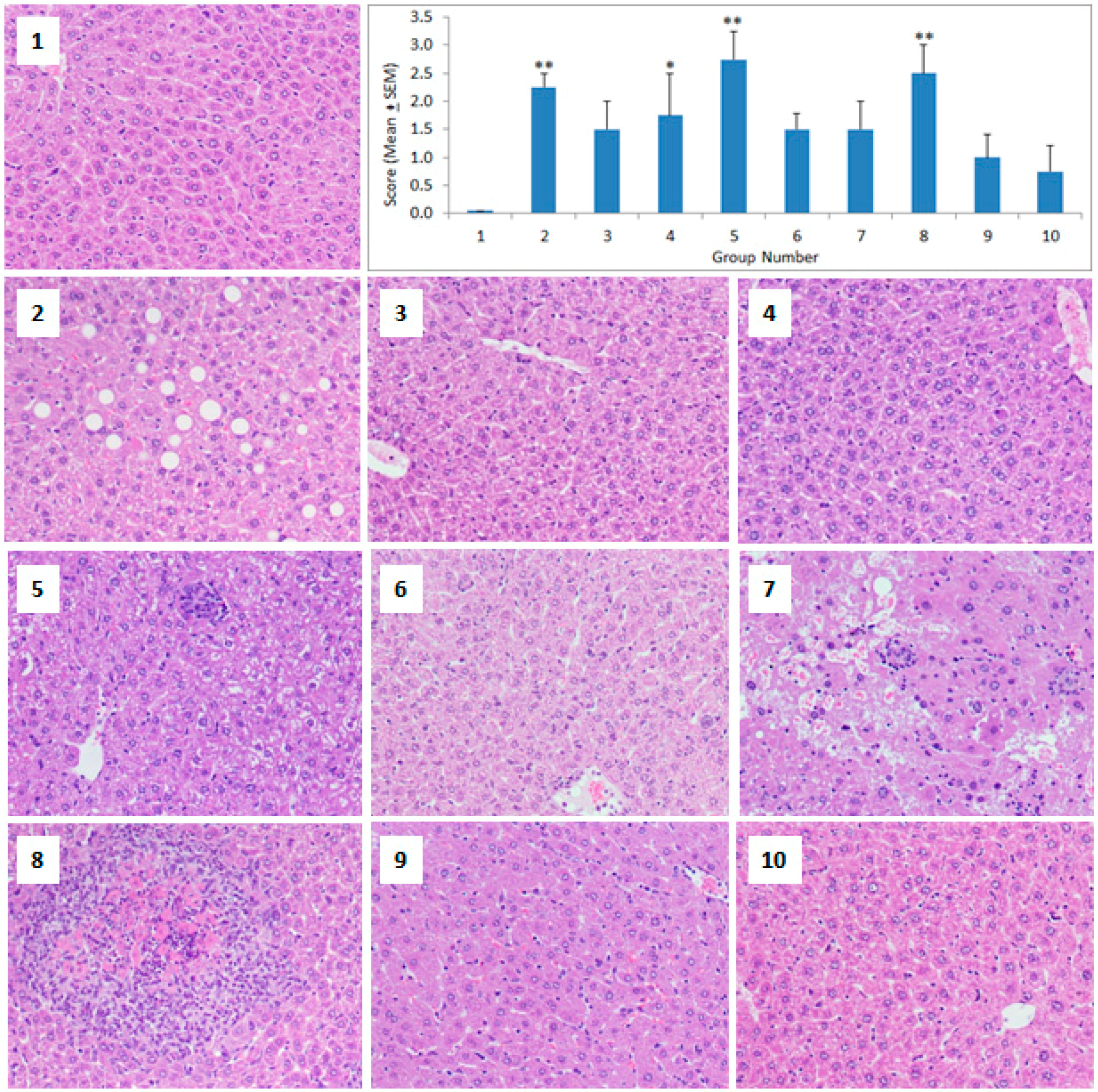
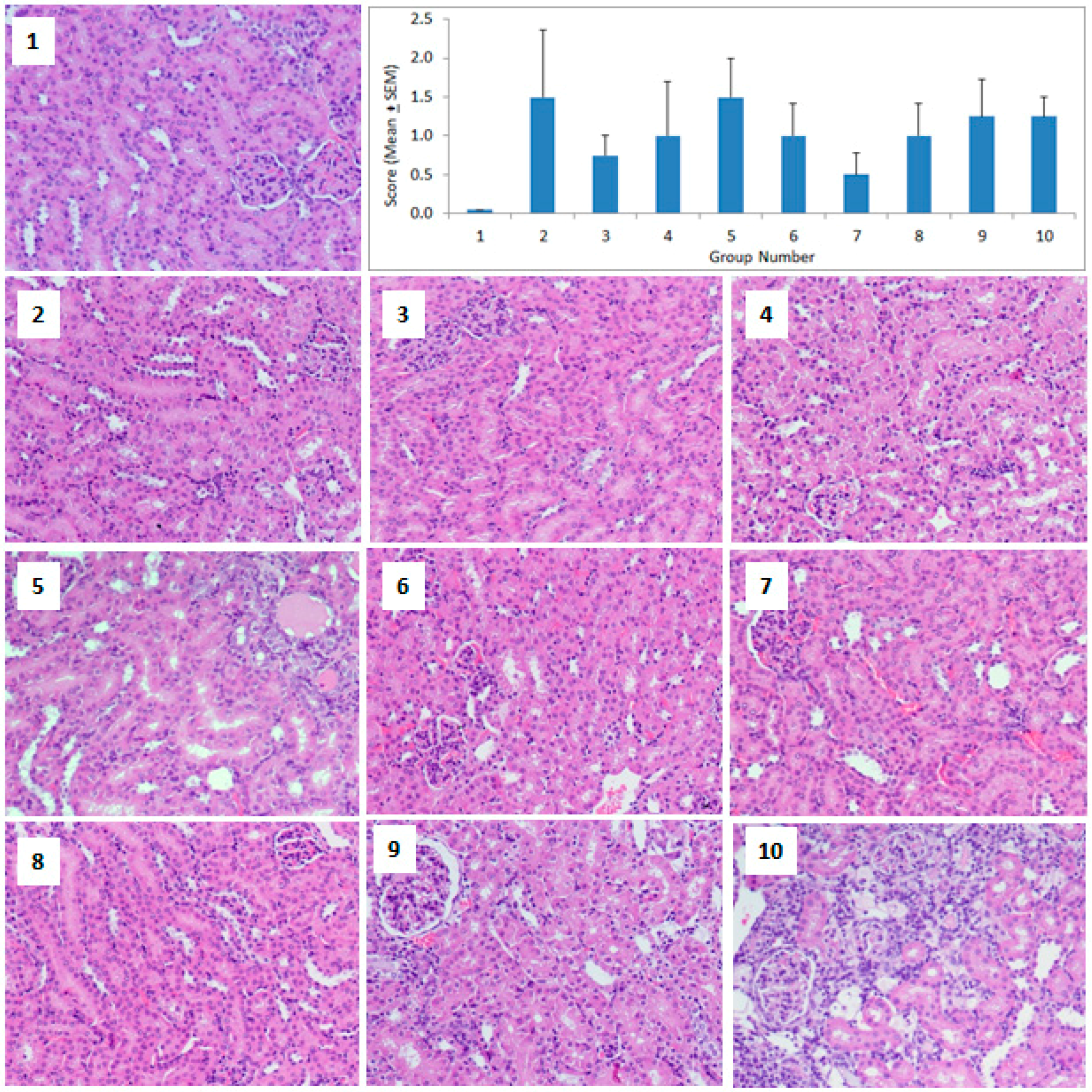
2.4. Histopathology
2.5. Analysis of Glutathione (GSH)
2.6. Analysis of Malondialdehyde (MDA)
2.7. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Nafiujjaman, M.; Nurunnabi, M.; Li, L.; Khan, H.A.; Cho, K.J.; Huh, K.M.; Lee, Y. Hybrid photoactive nanomaterial composed of gold nanoparticles, pheophorbide-A and hyaluronic acid as a targeted bimodal phototherapy. Macromol. Res. 2015, 23, 474–484. [Google Scholar] [CrossRef]
- Eswar, K.A.; Rouhi, J.; Husairi, F.S.; Dalvand, R.; Alrokayan, S.A.; Khan, H.A.; Mahmood, R.; Abdullah, S. Hydrothermal growth of flower-like ZnO nanostructures on porous silicon substrate. J. Mol. Struct. 2014, 1074, 140–143. [Google Scholar] [CrossRef]
- Ibrahim, K.E.; Bakhiet, A.O.; Khan, A.; Khan, H.A. Recent trends in biomedical applications of nanomaterials. Biosci. Biotechnol. Res. Asia 2018, 15, 235–243. [Google Scholar] [CrossRef]
- Khatun, Z.; Nurunnabi, M.; Nafiujjaman, M.; Reeck, G.R.; Khan, H.A.; Cho, K.J.; Lee, Y.K. A hyaluronic acid nanogel for photo-chemo theranostics of lung cancer with simultaneous light-responsive controlled release of doxorubicin. Nanoscale 2015, 7, 10680–10689. [Google Scholar] [CrossRef] [PubMed]
- Nafiujjaman, M.; Khan, H.A.; Lee, Y.K. Peptide-influenced graphene quantum dots on iron oxide nanoparticles for dual imaging of lung cancer cells. J. Nanosci. Nanotechnol. 2017, 17, 1704–1711. [Google Scholar] [CrossRef]
- Nafiujjaman, M.; Nurunnabi, M.; Kang, S.H.; Reeck, G.; Khan, H.A.; Lee, Y.K. Ternary graphene quantum dot-polydopamine-Mn3O4 nanoparticles for optical imaging guided photodynamic therapy and T1-weighted magnetic resonance imaging. J. Mater. Chem. B 2015, 3, 5815–5823. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Parvez, K.; Nafiujjaman, M.; Revuri, V.; Khan, H.A.; Feng, X.; Lee, Y. Bioapplication of graphene oxide derivatives: Drug/gene delivery, imaging, polymeric modification, toxicology, therapeutics and challenges. RSC Adv. 2015, 5, 42141–42161. [Google Scholar] [CrossRef]
- Falagan-Lotsch, P.; Grzincic, E.M.; Murphy, C.J. One low-dose exposure of gold nanoparticles induces long-term changes in human cells. Proc. Natl. Acad. Sci. USA 2016, 113, 13318–13323. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, M.; Hardie, J.; Tonga, G.Y.; Ray, M.; Xu, Q.; Rotello, V.M. Chemically engineered nanoparticle-protein interface for real-time cellular oxidative stress monitoring. Small 2016, 12, 3775–3779. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.X.; Wang, M.; Yang, W.W. Gold-quercetin nanoparticles prevent metabolic endotoxemia-induced kidney injury by regulating TLR4/NF-κB signaling and Nrf2 pathway in high fat diet fed mice. Int. J. Nanomed. 2017, 12, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.Y.; Zhang, J.W.; Li, R.F.; Wang, Z.X.; Wang, W.J.; Wang, W. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Cheng, C.Y.; Lin, H.C.; Chen, H.H.; Chen, C.H.; Yang, C.P.; Yang, K.H.; Lin, C.M.; Lin, T.Y.; Shih, C.M.; et al. Multifunctions of excited gold nanoparticles decorated artificial kidney with efficient hemodialysis and therapeutic potential. ACS Appl. Mater. Interfaces 2016, 8, 19691–19700. [Google Scholar] [CrossRef] [PubMed]
- Barreto, E.; Serra, M.F.; Dos Santos, R.V.; Dos Santos, C.E.; Hickmann, J.; Cotias, A.C.; Pão, C.R.; Trindade, S.G.; Schimidt, V.; Giacomelli, C.; et al. Local administration of gold nanoparticles prevents pivotal pathological changes in murine models of atopic asthma. J. Biomed. Nanotechnol. 2015, 11, 1038–1050. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.P.; Ferreira, G.K.; Pires, A.J.; de Bem Silveira, G.; de Souza, D.L.; Brandolfi, J.A.; de Souza, C.T.; Paula, M.M.S.; Silveira, P.C.L. Gold nanoparticles prevent cognitive deficits, oxidative stress and inflammation in a rat model of sporadic dementia of Alzheimer’s type. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Shanker, R. Toxicity of nanomaterials. Biomed. Res. Int. 2015, 2015, 521014. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.R.; Bae, S.H.; Go, M.R.; Kim, H.J.; Hwang, Y.G.; Choi, S.J. Toxicity and biokinetics of colloidal gold nanoparticles. Nanomaterials 2015, 5, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kuang, H.; Zhang, W.; Aguilar, Z.P.; Wei, H.; Xu, H. Comparisons of the biodistribution and toxicological examinations after repeated intravenous administration of silver and gold nanoparticles in mice. Sci. Rep. 2017, 7, 3303. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Roy, P.; Mondal, M.K.; Roy, D.; Gayen, P.; Chowdhury, P.; Babu, S.P. Development of chitosan based gold nanomaterial as an efficient antifilarial agent: A mechanistic approach. Carbohydr. Polym. 2017, 157, 1666–1676. [Google Scholar] [CrossRef] [PubMed]
- Baudrimont, M.; Andrei, J.; Mornet, S.; Gonzalez, P.; Mesmer-Dudons, N.; Gourves, P.Y.; Jaffal, A.; Dedourge-Geffard, O.; Geffard, A.; Geffard, O.; et al. Trophic transfer and effects of gold nanoparticles (AuNPs) in Gammarus fossarum from contaminated periphytic biofilm. Environ. Sci. Pollut. Res. Int. 2018, 25, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Ma, H.; Huang, K.; Liu, J.; Wei, T.; Jin, S.; Zhang, J.; He, S.; Liang, X.J. Superior penetration and retention behavior of 50 nm gold nanoparticles in tumors. Cancer Res. 2013, 73, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Schaeublin, N.M.; Braydich-Stolle, L.K.; Maurer, E.I.; Park, K.; MacCuspie, R.I.; Afrooz, A.R.; Vaia, R.A.; Saleh, N.B.; Hussain, S.M. Does shape matter? Bioeffects of gold nanomaterials in a human skin cell model. Langmuir 2012, 28, 3248–3258. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Hung, Y.C.; Liau, I.; Huang, G.S. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res. Lett. 2009, 4, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.C.; Chan, W.C. Nanotoxicity: The growing need for in vivo study. Curr. Opin. Biotechnol. 2007, 18, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Lasagna-Reeves, C.; Gonzalez-Romero, D.; Barria, M.A.; Olmedo, I.; Clos, A.; Sadagopa Ramanujam, V.M.; Urayama, A.; Vergara, L.; Kogan, M.J.; Soto, C. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem. Biophys. Res. Commun. 2010, 393, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Rambanapasi, C.; Zeevaart, J.R.; Buntting, H.; Bester, C.; Kotze, D.; Hayeshi, R.; Grobler, A. Bioaccumulation and subchronic toxicity of 14 nm gold nanoparticles in rats. Molecules 2016, 21, 763. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, M.A.; Jarrar, B.M. Histological alterations in the liver of rats induced by different gold nanoparticle sizes, doses and exposure duration. J. Nanobiotechnol. 2012, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Abdelhalim, M.A.; Al-Ayed, M.S.; Alhomida, A.S. Effect of gold nanoparticles on glutathione and malondialdehyde levels in liver, lung and heart of rats. Saudi J. Biol. Sci. 2012, 19, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.L.; Tham, P.H.; Yu, H.; Leo, H.L.; Yong Kah, J.C. Aggregation and protein corona formation on gold nanoparticles affect viability and liver functions of primary rat hepatocytes. Nanomedicine 2016, 11, 2275–2287. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.K.; Cardoso, E.; Vuolo, F.S.; Galant, L.S.; Michels, M.; Gonçalves, C.L.; Rezin, G.T.; Dal-Pizzol, F.; Benavides, R.; Alonso-Núñez, G.; et al. Effect of acute and long-term administration of gold nanoparticles on biochemical parameters in rat brain. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Reshi, M.S.; Shrivastava, S.; Jaswal, A.; Sinha, N.; Uthra, C.; Shukla, S. Gold nanoparticles ameliorate acetaminophen induced hepato-renal injury in rats. Exp. Toxicol. Pathol. 2017, 69, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Dkhil, M.A.; Khalil, M.F.; Bauomy, A.A.; Diab, M.S.; Al-Quraishy, S. Efficacy of gold nanoparticles against nephrotoxicity induced by Schistosoma mansoni Infection in mice. Biomed. Environ. Sci. 2016, 29, 773–781. [Google Scholar] [PubMed]
- Volland, M.; Hampel, M.; Martos-Sitcha, J.A.; Trombini, C.; Martínez-Rodríguez, G.; Blasco, J. Citrate gold nanoparticle exposure in the marine bivalve Ruditapes philippinarum: Uptake, elimination and oxidative stress response. Environ. Sci. Pollut. Res. Int. 2015, 22, 17414–17424. [Google Scholar] [CrossRef] [PubMed]
- Opris, R.; Tatomir, C.; Olteanu, D.; Moldovan, R.; Moldovan, B.; David, L.; Nagy, A.; Decea, N.; Kiss, M.L.; Filip, G.A. The effect of Sambucus nigra L. extract and phytosinthesized gold nanoparticles on diabetic rats. Colloids Surf. B Biointerfaces 2017, 150, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Sengani, M. Identification of potential antioxidant indices by biogenic gold nanoparticles in hyperglycemic Wistar rats. Environ. Toxicol. Pharmacol. 2017, 50, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Abdelhalim, M.A.; Alhomida, A.S.; Al-Ayed, M.S. Effects of naked gold nanoparticles on proinflammatory cytokines mRNA expression in rat liver and kidney. Biomed. Res. Int. 2013, 2013, 590730. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Ibrahim, K.E.; Khan, A.; Alrokayan, S.H.; Alhomida, A.S.; Lee, Y.K. Comparative evaluation of immunohistochemistry and real-time PCR for measuring proinflammatory cytokines gene expression in livers of rats treated with gold nanoparticles. Exp. Toxicol. Pathol. 2016, 68, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Ibrahim, K.E.; Khan, A.; Alrokayan, S.H.; Alhomida, A.S. Immunostaining of proinflammatory cytokines in renal cortex and medulla of rats exposed to gold nanoparticles. Histol. Histopathol. 2017, 32, 597–607. [Google Scholar] [PubMed]
- Ibrahim, K.E.; Bakhiet, A.O.; Awadalla, M.E.; Khan, H.A. A priming dose protects against gold nanoparticles-induced proinflammatory cytokines mRNA expression in mice. Nanomedicine 2018, 13, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.K.; Cardoso, E.; Vuolo, F.S.; Michels, M.; Zanoni, E.T.; Carvalho-Silva, M.; Gomes, L.M.; Dal-Pizzol, F.; Rezin, G.T.; Streck, E.L.; et al. Gold nanoparticles alter parameters of oxidative stress and energy metabolism in organs of adult rats. Biochem. Cell Biol. 2015, 93, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, R.; Kushwaha, P.; Bhutia, Y.C.; Flora, S. Oxidative stress following exposure to silver and gold nanoparticles in mice. Toxicol. Ind. Health 2016, 32, 1391–1404. [Google Scholar] [CrossRef]
- Negahdary, M.; Chelongar, R.; Zadeh, S.K.; Ajdary, M. The antioxidant effects of silver, gold, and zinc oxide nanoparticles on male mice in in vivo condition. Adv. Biomed. Res. 2015, 4, 69. [Google Scholar]
- Ferchichi, S.; Trabelsi, H.; Azzouz, I.; Hanini, A.; Rejeb, A.; Tebourbi, O.; Sakly, M.; Abdelmelek, H. Evaluation of oxidative response and tissular damage in rat lungs exposed to silica-coated gold nanoparticles under static magnetic fields. Int. J. Nanomed. 2016, 11, 2711–2719. [Google Scholar] [CrossRef] [PubMed]
- Schlinkert, P.; Casals, E.; Boyles, M.; Tischler, U.; Hornig, E.; Tran, N.; Zhao, J.; Himly, M.; Riediker, M.; Oostingh, G.J.; et al. The oxidative potential of differently charged silver and gold nanoparticles on three human lung epithelial cell types. J. Nanobiotechnol. 2015, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Iswarya, V.; Manivannan, J.; De, A.; Paul, S.; Roy, R.; Johnson, J.B.; Kundu, R.; Chandrasekaran, N.; Mukherjee, A.; Mukherjee, A. Surface capping and size-dependent toxicity of gold nanoparticles on different trophic levels. Environ. Sci. Pollut. Res. Int. 2016, 23, 4844–4858. [Google Scholar] [CrossRef]
- Muller, A.P.; Ferreira, G.K.; da Silva, S.; Nesi, R.T.; de Bem Silveira, G.; Mendes, C.; Pinho, R.A.; da Silva Paula, M.M.; Silveira, P.C.L. Safety protocol for the gold nanoparticles administration in rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1145–1150. [Google Scholar] [CrossRef]
- Fraga, S.; Brandao, A.; Soares, M.; Morais, T.; Duarte, J.A.; Pereira, L.; Soares, L.; Neves, C.; Pereira, E.; Bastos Mde, L.; et al. Short- and long-term distribution and toxicity of gold nanoparticles in the rat after a single-dose intravenous administration. Nanomed. Nanotechnol. 2014, 10, 1757–1766. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Group No. | Group Name | Dosage | Number of Injections | Day of Injections | Day of Sacrifice |
|---|---|---|---|---|---|
| (5 µg Au/Animal) | |||||
| 1 | Control | 100 µL saline | 1 | 1 | 2 |
| 2 | GNP 5 (day 1) | 100 µL GNP 5 nm | 1 | 1 | 2 |
| 3 | GNP 20 (day 1) | 100 µL GNP 20 nm | 1 | 1 | 2 |
| 4 | GNP 50 (day 1) | 100 µL GNP 50 nm | 1 | 1 | 2 |
| 5 | GNP 5 (day 7) | 100 µL GNP 5 nm | 1 | 1 | 8 |
| 6 | GNP 20 (day 7) | 100 µL GNP 20 nm | 1 | 1 | 8 |
| 7 | GNP 50 (day 7) | 100 µL GNP 50 nm | 1 | 1 | 8 |
| 8 | GNP 5 (day 1,7) | 100 µL GNP 5 nm | 2 | 1 and 7 | 8 |
| 9 | GNP 20 (day 1,7) | 100 µL GNP 20 nm | 2 | 1 and 7 | 8 |
| 10 | GNP 50 (day 1,7) | 100 µL GNP 50 nm | 2 | 1 and 7 | 8 |
| Treatment Group | Liver | Kidney | Spleen |
|---|---|---|---|
| Control | 4256.50 ± 482.58 | 1553.75 ± 245.16 | 3120.58 ± 381.88 |
| GNP 5 (day 1) | 4567.75 ± 368.64 | 1630.42 ± 181.99 | 2917.25 ± 100.46 |
| GNP 20 (day 1) | 4580.50 ± 263.99 | 1162.00 ± 210.81 | 2920.50 ± 185.36 |
| GNP 50 (day 1) | 4654.75 ± 269.26 | 1358.75 ± 147.53 | 2818.50 ± 076.92 |
| GNP 5 (day 7) | 4479.00 ± 481.80 | 1311.50 ± 163.33 | 3291.75 ± 188.92 |
| GNP 20 (day 7) | 4052.50 ± 226.11 | 1455.00 ± 357.16 | 3215.50 ± 150.63 |
| GNP 50 (day 7) | 4417.20 ± 089.86 | 1189.80 ± 103.53 | 2983.80 ± 179.55 |
| GNP 5 (day 1,7) | 4510.50 ± 418.60 | 1422.75 ± 138.21 | 3254.75 ± 267.70 |
| GNP 20 (day 1,7) | 4175.00 ± 394.74 | 1248.75 ± 105.21 | 3098.00 ± 176.51 |
| GNP 50 (day 1,7) | 4321.80 ± 269.27 | 1628.00 ± 261.17 | 3595.00 ± 163.97 |
| Treatment Group | Liver | Kidney | Spleen |
|---|---|---|---|
| Control | 5.11 ± 0.44 | 7.11 ± 0.60 | 6.44 ± 0.23 |
| GNP 5 (day 1) | 5.08 ± 0.71 | 7.53 ± 0.96 | 6.28 ± 0.34 |
| GNP 20 (day 1) | 5.74 ± 0.59 | 7.62 ± 0.78 | 6.02 ± 0.35 |
| GNP 50 (day 1) | 7.12 ± 0.83 | 8.26 ± 1.31 | 5.81 ± 0.29 |
| GNP 5 (day 7) | 6.49 ± 0.91 | 7.50 ± 1.39 | 5.65 ± 0.28 |
| GNP 20 (day 7) | 6.30 ± 0.47 | 7.10 ± 1.10 | 5.58 ± 0.36 |
| GNP 50 (day 7) | 5.16 ± 0.72 | 6.72 ± 1.62 | 6.59 ± 0.37 |
| GNP 5 (day 1,7) | 7.00 ±1.19 | 5.84 ± 0.93 | 6.08 ± 0.28 |
| GNP 20 (day 1,7) | 6.97 ± 0.58 | 6.11 ± 1.46 | 6.04 ± 0.35 |
| GNP 50 (day 1,7) | 6.68 ± 0.92 | 6.84 ± 1.04 | 6.59 ± 0.51 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, K.E.; Al-Mutary, M.G.; Bakhiet, A.O.; Khan, H.A. Histopathology of the Liver, Kidney, and Spleen of Mice Exposed to Gold Nanoparticles. Molecules 2018, 23, 1848. https://doi.org/10.3390/molecules23081848
Ibrahim KE, Al-Mutary MG, Bakhiet AO, Khan HA. Histopathology of the Liver, Kidney, and Spleen of Mice Exposed to Gold Nanoparticles. Molecules. 2018; 23(8):1848. https://doi.org/10.3390/molecules23081848
Chicago/Turabian StyleIbrahim, Khalid Elfaki, Mohsen Ghaleb Al-Mutary, Amel Omer Bakhiet, and Haseeb Ahmad Khan. 2018. "Histopathology of the Liver, Kidney, and Spleen of Mice Exposed to Gold Nanoparticles" Molecules 23, no. 8: 1848. https://doi.org/10.3390/molecules23081848
APA StyleIbrahim, K. E., Al-Mutary, M. G., Bakhiet, A. O., & Khan, H. A. (2018). Histopathology of the Liver, Kidney, and Spleen of Mice Exposed to Gold Nanoparticles. Molecules, 23(8), 1848. https://doi.org/10.3390/molecules23081848