Cannabinoid CB2 Receptor Gene and Environmental Interaction in the Development of Psychiatric Disorders
Abstract
1. Introduction
2. Results
2.1. Behavioral Analysis
2.2. Gene Expression
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Behavioral Analysis
4.3. Anxiety-Like Behavioral Test
4.4. Locomotor Activity Test
4.5. Gene Expression Analysis
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Onaivi, E.S.; Ishiguro, H.; Gu, S.; Liu, Q.R. CNS effects of CB2 cannabinoid receptors: Beyond neuro-immuno-cannabinoid activity. J. Psychopharmacol. 2012, 26, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, H.; Horiuchi, Y.; Ishikawa, M.; Koga, M.; Imai, K.; Suzuki, Y.; Morikawa, M.; Inada, T.; Watanabe, Y.; Takahashi, M.; et al. Brain cannabinoid CB2 receptor in schizophrenia. Biol. Psychiatry 2010, 67, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, H.; Iwasaki, S.; Teasenfitz, L.; Higuchi, S.; Horiuchi, Y.; Saito, T.; Arinami, T.; Onaivi, E.S. Involvement of cannabinoid CB2 receptor in alcohol preference in mice and alcoholism in humans. Pharmacogenomics J. 2007, 7, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.P.; Patel, S.; Meozzi, P.A.; Myers, L.; Perchuk, A.; Mora, Z.; Tagliaferro, P.A.; Gardner, E.; et al. Functional expression of brain neuronal CB2 cannabinoid receptors are involved in the effects of drugs of abuse and in depression. Ann. N. Y. Acad. Sci. 2008, 1139, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, H.; Onaivi, E.S. Beyond the Kraepelinian Dichotomy of Schizophrenia and Bipolar Disorder. J. Schizophr. Res. 2017, 4, 1032. [Google Scholar]
- Morena, M.; Patel, S.; Bains, J.S.; Hill, M.N. Neurobiological Interactions between Stress and the Endocannabinoid System. Neuropsychopharmacology 2016, 41, 80–102. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, L.; Milaneschi, Y.; Vinkers, C.H.; van Hemert, A.M.; van Velzen, L.; Schmaal, L.; Penninx, B.W. HPA Axis Genes, and Their Interaction with Childhood Maltreatment, are Related to Cortisol Levels and Stress-Related Phenotypes. Neuropsychopharmacology 2017, 42, 2446–2455. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.B. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 2009, 34 (Suppl. 1), S186–S195. [Google Scholar] [CrossRef] [PubMed]
- Denny, W.B.; Valentine, D.L.; Reynolds, P.D.; Smith, D.F.; Scammell, J.G. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology 2000, 141, 4107–4113. [Google Scholar] [CrossRef] [PubMed]
- Gassen, N.C.; Hartmann, J.; Schmidt, M.V.; Rein, T. FKBP5/FKBP51 enhances autophagy to synergize with antidepressant action. Autophagy 2015, 11, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, C.; Hosak, L.; Mossner, R.; Giegling, I.; Mandelli, L.; Bellivier, F.; Claes, S.; Collier, D.A.; Corrales, A.; Delisi, L.E.; et al. Consensus paper of the WFSBP Task Force on Genetics: Genetics, epigenetics and gene expression markers of major depressive disorder and antidepressant response. World J. Biol. Psychiatry 2016, 7, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Young, D.A.; Inslicht, S.S.; Metzler, T.J.; Neylan, T.C.; Ross, J.A. The effects of early trauma and the FKBP5 gene on PTSD and the HPA axis in a clinical sample of Gulf War veterans. Psychiatry Res. 2018, 2018, 32068-1. [Google Scholar] [CrossRef] [PubMed]
- Kumsta, R.; Entringer, S.; Koper, J.W.; van Rossum, E.F.; Hellhammer, D.H.; Wust, S. Sex specific associations between common glucocorticoid receptor gene variants and hypothalamus-pituitary-adrenal axis responses to psychosocial stress. Biol. Psychiatry 2007, 62, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Palma-Gudiel, H.; Cordova-Palomera, A.; Tornador, C.; Falcon, C.; Bargallo, N.; Deco, G.; Fananas, L. Increased methylation at an unexplored glucocorticoid responsive element within exon 1D of NR3C1 gene is related to anxious-depressive disorders and decreased hippocampal connectivity. Eur. Neuropsychopharmacol. 2018, 28, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Bae, K.Y.; Kim, S.W.; Shin, I.S.; Kim, H.R.; Shin, M.G.; Yoon, J.S.; Kim, J.M. Longitudinal associations between glucocorticoid receptor methylation and late-life depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 84, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Sarubin, N.; Hilbert, S.; Naumann, F.; Zill, P.; Wimmer, A.M.; Nothdurfter, C.; Rupprecht, R.; Baghai, T.C.; Buhner, M.; Schule, C. The sex-dependent role of the glucocorticoid receptor in depression: Variations in the NR3C1 gene are associated with major depressive disorder in women but not in men. Eur. Arch. Psychiatry Clin. Neurosci. 2017, 267, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Hong, J.P.; Lee, J.K.; Park, Y.M.; Park, Y.; Jeon, J.; Ahn, M.H.; Yoon, S.C. Associations between the neuron-specific glucocorticoid receptor (NR3C1) Bcl-1 polymorphisms and suicide in cancer patients within the first year of diagnosis. Behav. Brain Funct. 2016, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Galfalvy, H.; Pantazatos, S.P.; Huang, Y.Y.; Rosoklija, G.B.; Dwork, A.J.; Burke, A.; Arango, V.; Oquendo, M.A.; Mann, J.J. Glucocorticoid Receptor-Related Genes: Genotype and Brain Gene Expression Relationships to Suicide and Major Depressive Disorder. Depress. Anxiety 2016, 33, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Schatzberg, A.F.; Keller, J.; Tennakoon, L.; Lembke, A.; Williams, G.; Kraemer, F.B.; Sarginson, J.E.; Lazzeroni, L.C.; Murphy, G.M. HPA axis genetic variation, cortisol and psychosis in major depression. Mol. Psychiatry 2014, 19, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, S.; Cai, J.; Wei, T.J.; Liu, L.Y.; Zhao, H.Y.; Liu, B.H.; Jing, H.B.; Jin, Z.R.; Liu, M.; et al. Activation of CRF/CRFR1 signaling in the basolateral nucleus of the amygdala contributes to chronic forced swim-induced depressive-like behaviors in rats. Behav. Brain Res. 2018, 338, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, Y.; Kasahara, T.; Kato, M.; Sakai, S.; Deguchi, Y.; Tani, M.; Kuroda, K.; Hattori, K.; Yoshida, S.; Goto, Y.; et al. The relationship between circulating mitochondrial DNA and inflammatory cytokines in patients with major depression. J. Affect. Disord. 2017, 0327, 30661–30664. [Google Scholar] [CrossRef] [PubMed]
- Misiak, B.; Stanczykiewicz, B.; Kotowicz, K.; Rybakowski, J.K.; Samochowiec, J.; Frydecka, D. Cytokines and C-reactive protein alterations with respect to cognitive impairment in schizophrenia and bipolar disorder: A systematic review. Schizophr. Res. 2017, 9964, 30202–30205. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Q.; Chen, D.C.; Tan, Y.L.; Tan, S.P.; Xiu, M.H.; Wang, Z.R.; Yang, F.D.; Soares, J.C.; Zhang, X.Y. Altered interleukin-18 levels are associated with cognitive impairment in chronic schizophrenia. J. Psychiatr. Res. 2016, 76, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakeim, H.K.; Al-Rammahi, D.A.; Al-Dujaili, A.H. IL-6, IL-18, sIL-2R, and TNFalpha proinflammatory markers in depression and schizophrenia patients who are free of overt inflammation. J. Affect. Disord. 2015, 182, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Shirts, B.H.; Wood, J.; Yolken, R.H.; Nimgaonkar, V.L. Comprehensive evaluation of positional candidates in the IL-18 pathway reveals suggestive associations with schizophrenia and herpes virus seropositivity. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008, 147, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Xiu, M.H.; Chen, D.C.; Wang, D.; Zhang, K.; Dong, A.; Tang, W.; Zhang, F.; Liu, L.J.; Liu, J.H.; Liu, H.B.; et al. Elevated interleukin-18 serum levels in chronic schizophrenia: Association with psychopathology. J. Psychiatr. Res. 2012, 46, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Tang, W.; Xiu, M.H.; Chen, D.C.; Yang, F.D.; Tan, Y.L.; Wang, Z.R.; Zhang, F.; Liu, J.; Liu, L.; et al. Interleukin 18 and cognitive impairment in first episode and drug naive schizophrenia versus healthy controls. Brain Behav. Immun. 2013, 32, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Kohler, C.A.; Freitas, T.H.; Stubbs, B.; Maes, M.; Solmi, M.; Veronese, N.; de Andrade, N.Q.; Morris, G.; Fernandes, B.S.; Brunoni, A.R.; et al. Peripheral Alterations in Cytokine and Chemokine Levels after Antidepressant Drug Treatment for Major Depressive Disorder: Systematic Review and Meta-Analysis. Mol. Neurobiol. 2018, 55, 4195–4206. [Google Scholar] [CrossRef] [PubMed]
- Ellul, P.; Boyer, L.; Groc, L.; Leboyer, M.; Fond, G. Interleukin-1 beta-targeted treatment strategies in inflammatory depression: Toward personalized care. Acta Psychiatr. Scand. 2016, 134, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Xu, Y.Q.; Li, Y.Y.; Lu, M.F.; Shi, S.X.; Ji, J.L.; Wang, L.W. Difference in proinflammatory cytokines produced by monocytes between patients with major depressive disorder and healthy controls. J. Affect. Disord. 2018, 234, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Norman, G.J.; Karelina, K.; Zhang, N.; Walton, J.C.; Morris, J.S.; Devries, A.C. Stress and IL-1beta contribute to the development of depressive-like behavior following peripheral nerve injury. Mol. Psychiatry 2010, 15, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Wang, H.Y.; Zhang, C.; Liu, B.P.; Peng, Z.L.; Li, Y.Y.; Liu, F.M.; Song, C. Mifepristone attenuates depression-like changes induced by chronic central administration of interleukin-1beta in rats. Behav. Brain Res. 2018, 347, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, R.; Kishi, T.; Atake, K.; Katsuki, A.; Iwata, N. Serum Brain-Derived Neurotrophic Factor, and Plasma Catecholamine Metabolites in People with Major Depression: Preliminary Cross-Sectional Study. Front. Psychiatry 2018, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.P.; Poddighe, L.; Boi, M.; Sanna, F.; Piludu, M.A.; Corda, M.G.; Giorgi, O.; Quartu, M. Expression of BDNF and trkB in the hippocampus of a rat genetic model of vulnerability (Roman low-avoidance) and resistance (Roman high-avoidance) to stress-induced depression. Brain Behav. 2017, 7, e00861. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Shi, R.; Wang, J.; Wang, J.F.; Li, X.M. Unpredictable chronic mild stress not chronic restraint stress induces depressive behaviours in mice. Neuroreport 2014, 25, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, S.; Khan, D.; Kong, E.; Berger, A.; Pollak, A.; Pollak, D.D. The poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol. Ther. 2015, 149, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Levy-Gigi, E.; Szabo, C.; Kelemen, O.; Keri, S. Association among clinical response, hippocampal volume, and FKBP5 gene expression in individuals with posttraumatic stress disorder receiving cognitive behavioral therapy. Biol. Psychiatry 2013, 74, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C.; Kelemen, O.; Keri, S. Changes in FKBP5 expression and memory functions during cognitive-behavioral therapy in posttraumatic stress disorder: A preliminary study. Neurosci. Lett. 2014, 569, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Z.Z.; Zuo, W.; Zhang, S.; Chu, S.F.; Chen, N.H. Effects of chronic mild stress on behavioral and neurobiological parameters—Role of glucocorticoid. Horm. Behav. 2016, 78, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U. Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol. Psychiatry 2014, 75, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Cabral, G.A.; Griffin-Thomas, L. Emerging role of the cannabinoid receptor CB2 in immune regulation: Therapeutic prospects for neuroinflammation. Expert Rev. Mol. Med. 2009, 11, e3. [Google Scholar] [CrossRef] [PubMed]
- Palazuelos, J.; Aguado, T.; Egia, A.; Mechoulam, R.; Guzman, M.; Galve-Roperh, I. Non-psychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation. FASEB J. 2006, 20, 2405–2407. [Google Scholar] [CrossRef] [PubMed]
- Hollins, S.L.; Zavitsanou, K.; Walker, F.R.; Cairns, M.J. Alteration of transcriptional networks in the entorhinal cortex after maternal immune activation and adolescent cannabinoid exposure. Brain Behav. Immun. 2016, 56, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, B.; Miller, M.L.; Hurd, Y.L. Cannabis Use during Adolescent Development: Susceptibility to Psychiatric Illness. Front. Psychiatry 2013, 4, 129. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.V.; Rincon-Cortes, M.; Grace, A.A. Adolescence as a period of vulnerability and intervention in schizophrenia: Insights from the MAM model. Neurosci. Biobehav. Rev. 2016, 70, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, P.; Wozniak, A.; Nowakowska, E. Animal models of schizophrenia: Developmental preparation in rats. Acta Neurobiol. Exp. 2013, 73, 472–484. [Google Scholar]
- Bailey, K.A.; Baker, A.L.; McElduff, P.; Kavanagh, D.J. The Influence of Parental Emotional Neglect on Assault Victims Seeking Treatment for Depressed Mood and Alcohol Misuse: A Pilot Study. J. Clin. Med. 2016, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Dong, X.; Wang, Y.; Liu, M.; Sun, A.; Li, N.; Lin, Y.; Geng, Z.; Jin, Y.; Li, X. Adolescent escitalopram prevents the effects of maternal separation on depression- and anxiety-like behaviours and regulates the levels of inflammatory cytokines in adult male mice. Int. J. Dev. Neurosci. 2017, 62, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Malkesman, O.; Lavi-Avnon, Y.; Maayan, R.; Weizman, A. A cross-fostering study in a genetic animal model of depression: Maternal behavior and depression-like symptoms. Pharmacol. Biochem. Behav. 2008, 91, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Llorente, R.; Llorente-Berzal, A.; Petrosino, S.; Marco, E.M.; Guaza, C.; Prada, C.; Lopez-Gallardo, M.; Di Marzo, V.; Viveros, M.P. Gender-dependent cellular and biochemical effects of maternal deprivation on the hippocampus of neonatal rats: A possible role for the endocannabinoid system. Dev. Neurobiol. 2008, 68, 1334–1347. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gallardo, M.; Llorente, R.; Llorente-Berzal, A.; Marco, E.M.; Prada, C.; Di Marzo, V.; Viveros, M.P. Neuronal and glial alterations in the cerebellar cortex of maternally deprived rats: Gender differences and modulatory effects of two inhibitors of endocannabinoid inactivation. Dev. Neurobiol. 2008, 68, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Recamier-Carballo, S.; Estrada-Camarena, E.; Lopez-Rubalcava, C. Maternal separation induces long-term effects on monoamines and brain-derived neurotrophic factor levels on the frontal cortex, amygdala, and hippocampus: Differential effects after a stress challenge. Behav. Pharmacol. 2017, 28, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Van Bodegom, M.; Homberg, J.R.; Henckens, M. Modulation of the Hypothalamic-Pituitary-Adrenal Axis by Early Life Stress Exposure. Front. Cell. Neurosci. 2017, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Page, G.G.; Corwin, E.J.; Dorsey, S.G.; Redeker, N.S.; McCloskey, D.J.; Austin, J.K.; Guthrie, B.J.; Moore, S.M.; Barton, D.; Kim, M.T.; et al. Biomarkers as Common Data Elements for Symptom and Self-Management Science. J. Nurs. Scholarsh. 2018, 50, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Buckley, N.E. The peripheral cannabinoid receptor knockout mice: An update. Br. J. Pharmacol. 2008, 153, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Commons, K.G.; Cholanians, A.B.; Babb, J.A.; Ehlinger, D.G. The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS Chem. Neurosci. 2017, 8, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Maejima, Y.; Rita, R.S.; Santoso, P.; Aoyama, M.; Hiraoka, Y.; Nishimori, K.; Gantulga, D.; Shimomura, K.; Yada, T. Nasal oxytocin administration reduces food intake without affecting locomotor activity and glycemia with c-Fos induction in limited brain areas. Neuroendocrinology 2015, 101, 35–44. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Behavioral Test | Treatment/Strain | Statics ANOVA | Noted by Post-Hoc Analysis |
|---|---|---|---|
| Behavior | |||
| Zero maze test | Saline/Cnr2 ko mice (no Figure) | F1,34 = 0.48, p = 0.49 | |
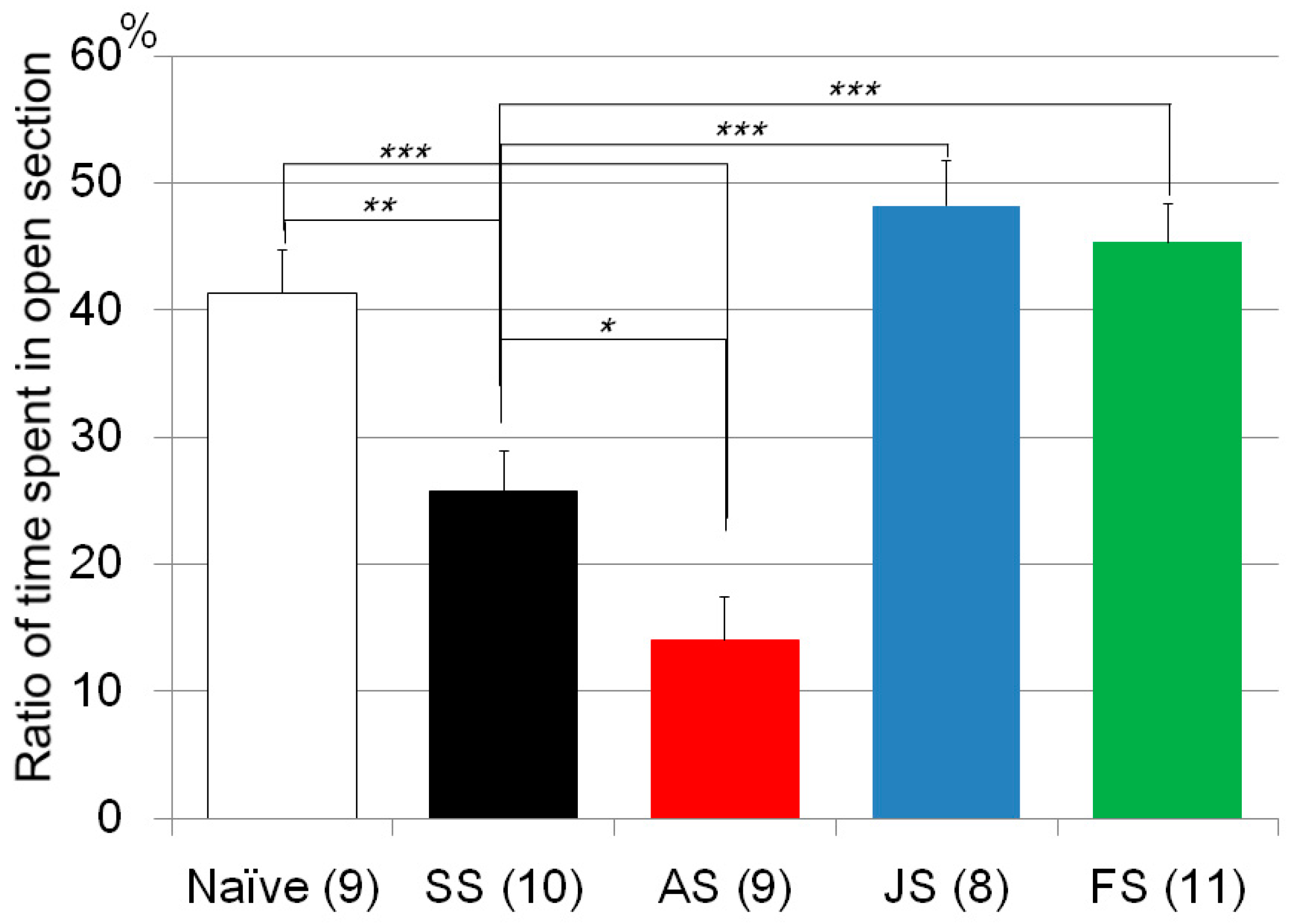
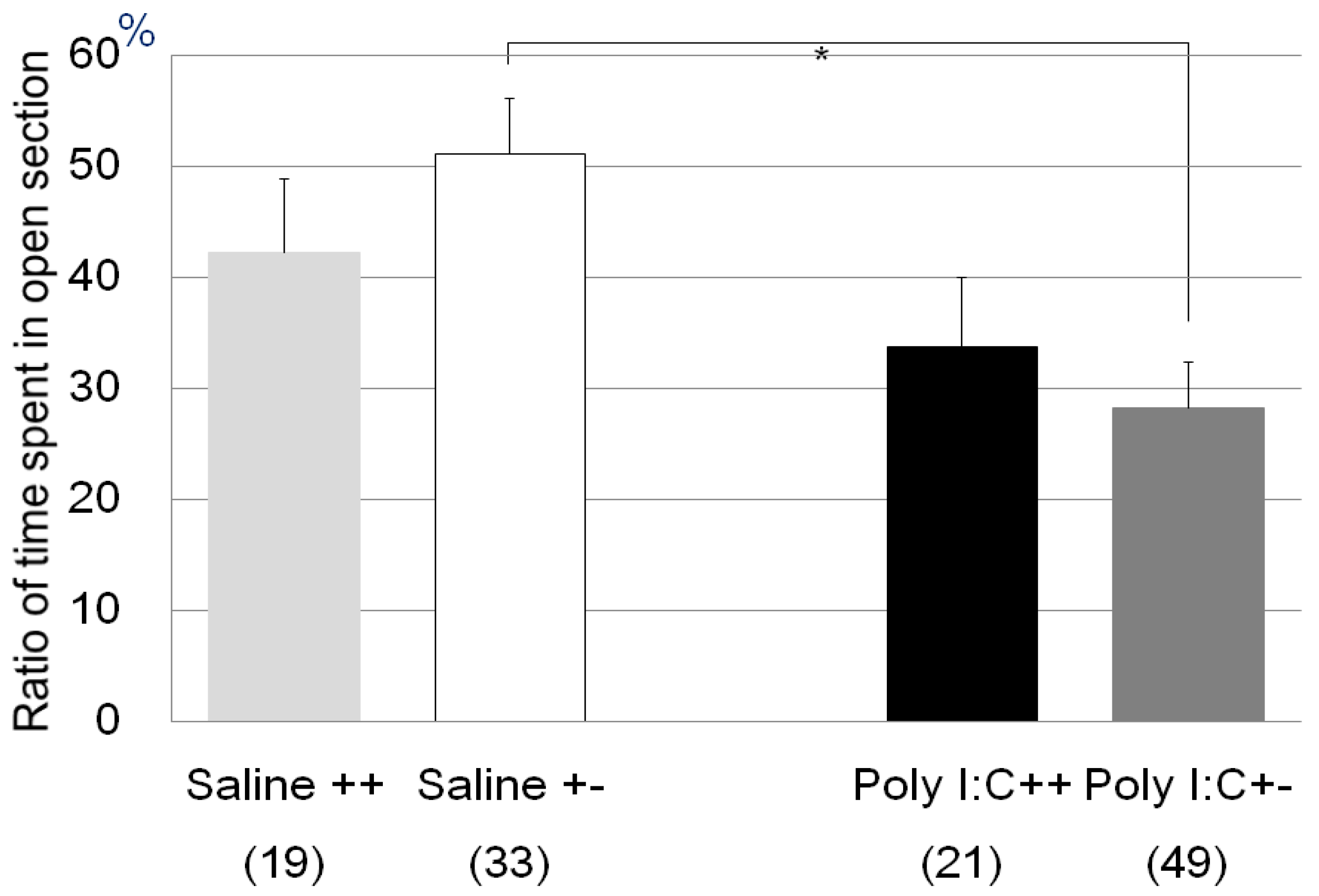
| Zero maze test | Poly I:C/Cnr2 ko mice (Figure 1) | F3,118 = 4.4, p = 0.0055 | Poly I:C vs. saline treated heterozygote mice; p = 0.0033 |
| genotype F = 0.09, p = 0.76 | |||
| treatment F = 7.7, p = 0.006 | |||
| genotype × treatment F = 1.6, p = 0.20 | |||
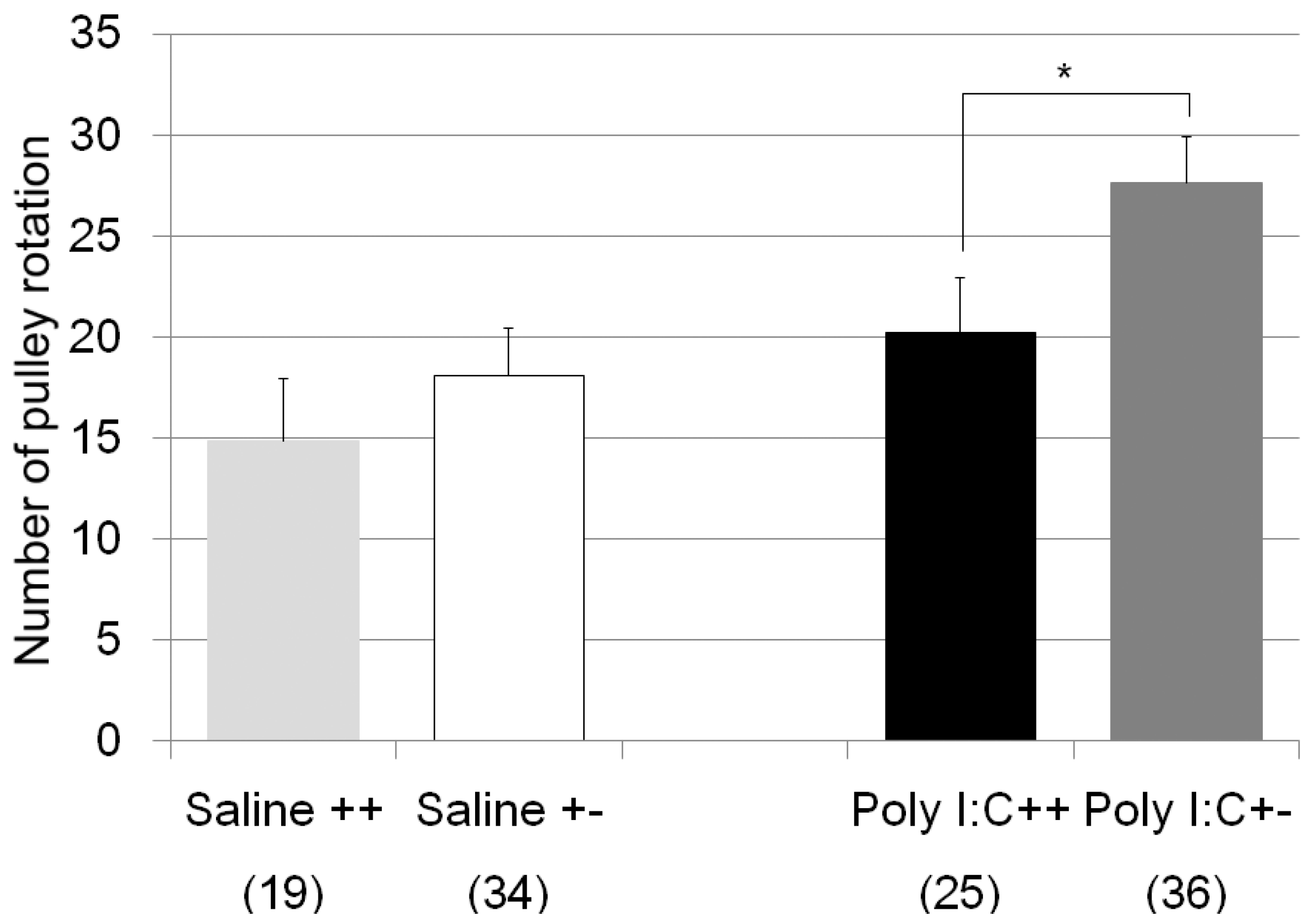
| pulley rotate test | Poly I:C/Cnr2 ko mice (Figure 2) | F3,110 = 4.8, p = 0.0034 | Poly I:C treated wildtype mice vs. heterozygote mice; p = 0.019 |
| genotype F = 4.2, p = 0.04 | |||
| treatment F = 8.2, p = 0.005 | |||
| genotype × treatment F = 0.6, p = 0.42 | |||
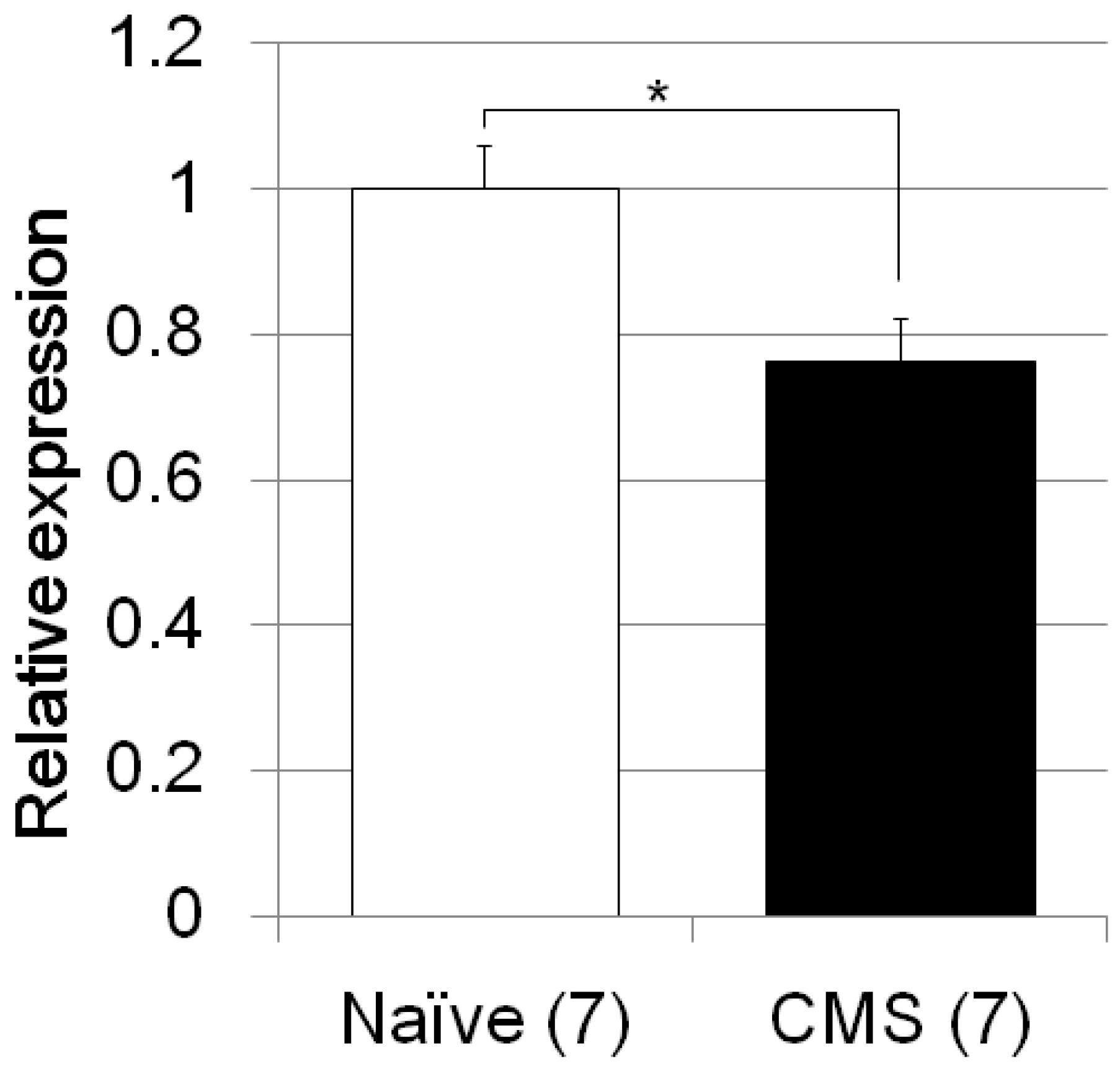
| Zero maze test | CMS/C57B/JJmsSlc mice (Figure 3) | F4, 46 = 19.0, p < 0.0001 | CMS treated vs. naïve mice; p = 0.0016 |
| AM630 vs. Saline treated mice with CMS; p = 0.015 | |||
| JWH015 vs. Saline treated mice with CMS; p < 1 × 10−3 | |||
| Fluvoxamine vs. Saline treated mice with CMS; p < 1 × 10−3 | |||
| JWH015 vs. Fluvoxamine treated mice with CMS; n.s. | |||
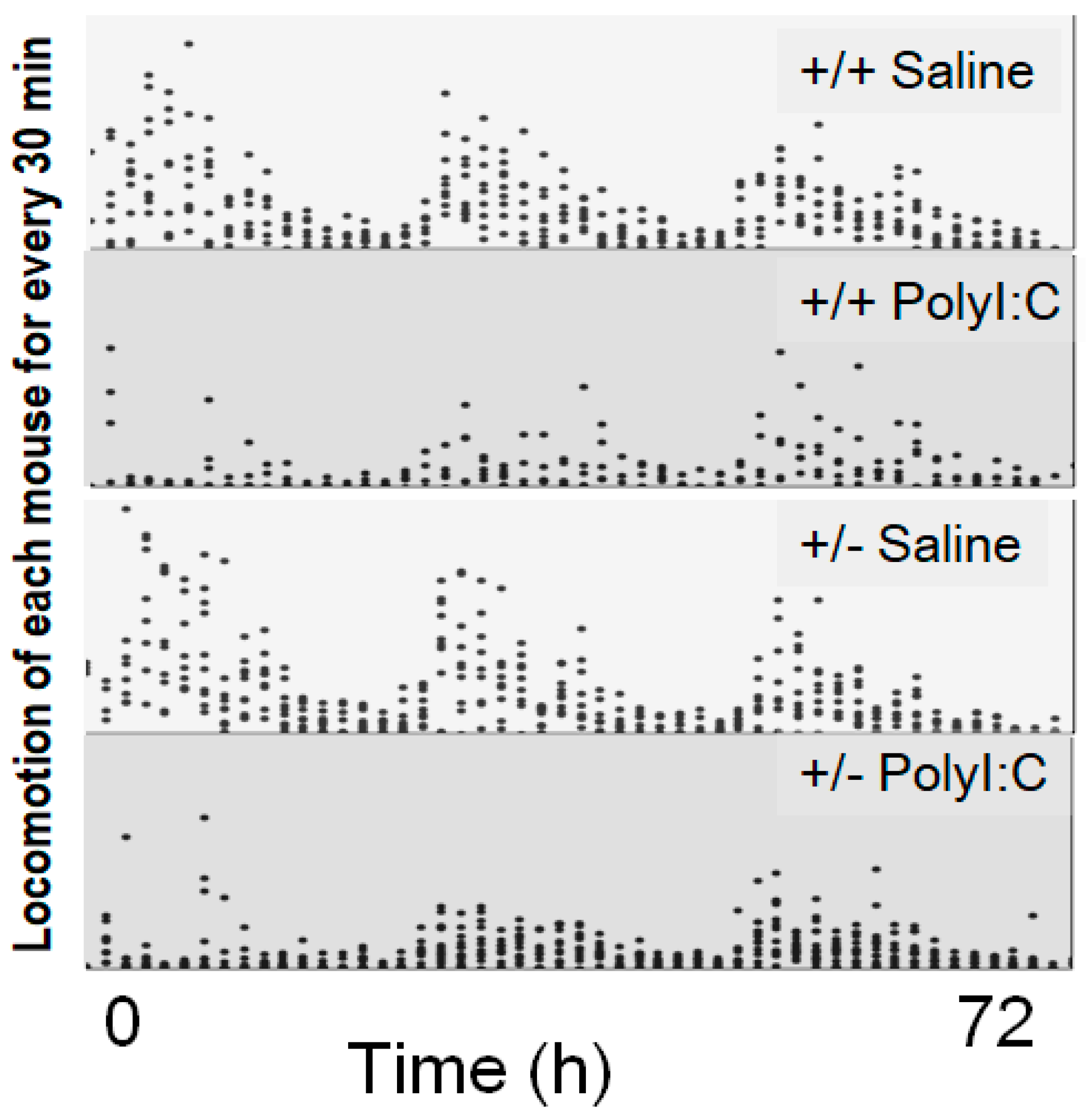
| Locomotion test | Poly I:C/Cnr2 ko mice (Figure 4) | t(1827) = 1.54, p (single sided) = 0.06 | |
| Gene expression | |||
| Fkbp5 | CMS/C57B/JJmsSlc mice (Figure 5) | F1, 12 = 8.4, p = 0.0134 | |
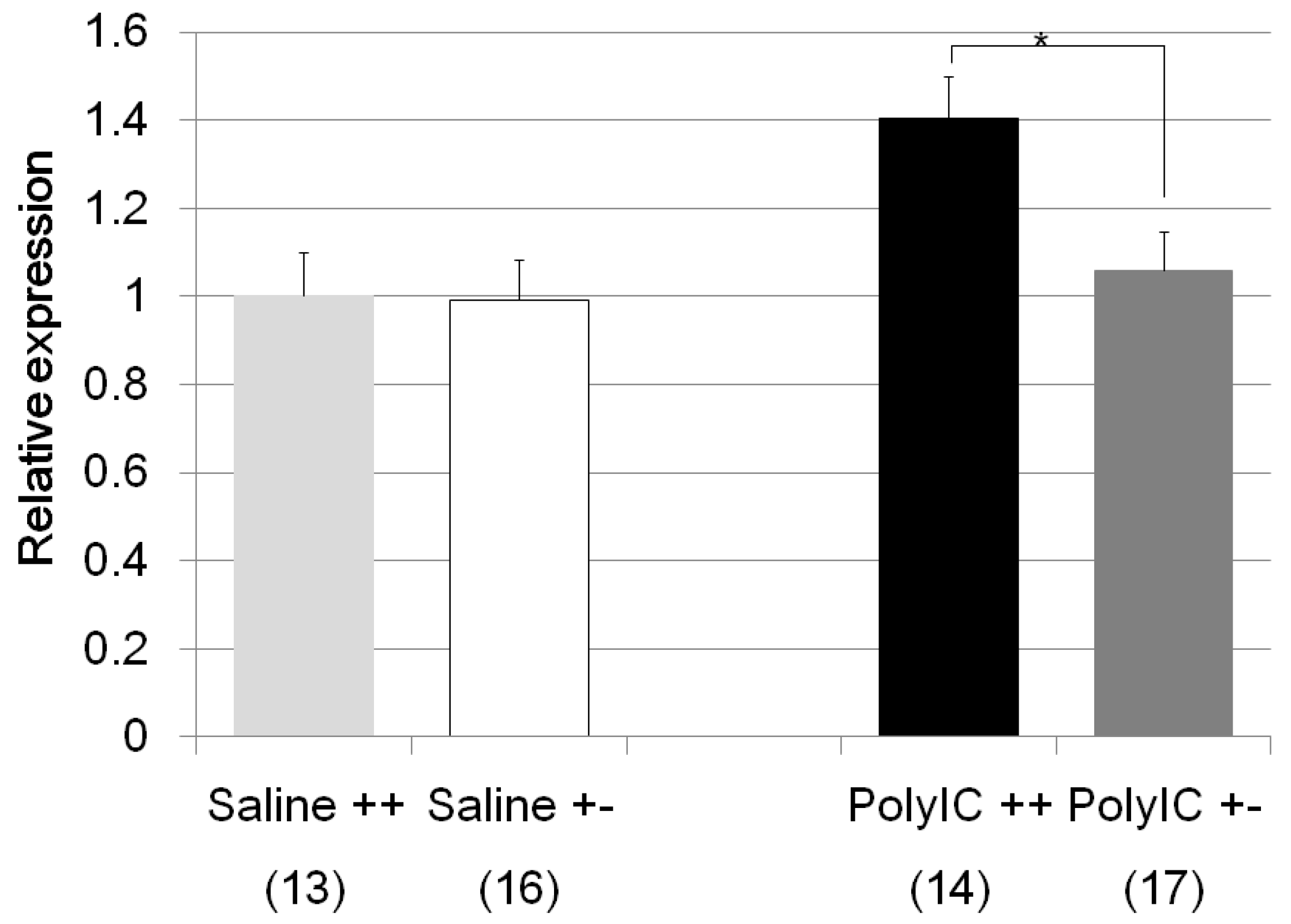
| Fkbp5 | Poly I:C/Cnr2 ko mice (Figure 6) | F3,58 = 4.6, p = 0.008 | Poly I:C treated wildtype mice vs. heterozygote mice; p = 0.048 |
| genotype F = 2.6, p = 0.01 | Poly I:C treated vs. Saline treated wildtype mice; p = 0.025 | ||
| treatment F = −1.9, p = 0.06 | Poly I:C treated wildtype mice vs. Saline treated heterozygote mice; p = 0.013 | ||
| genotype × treatment F = −1.7, p = 0.08 | |||
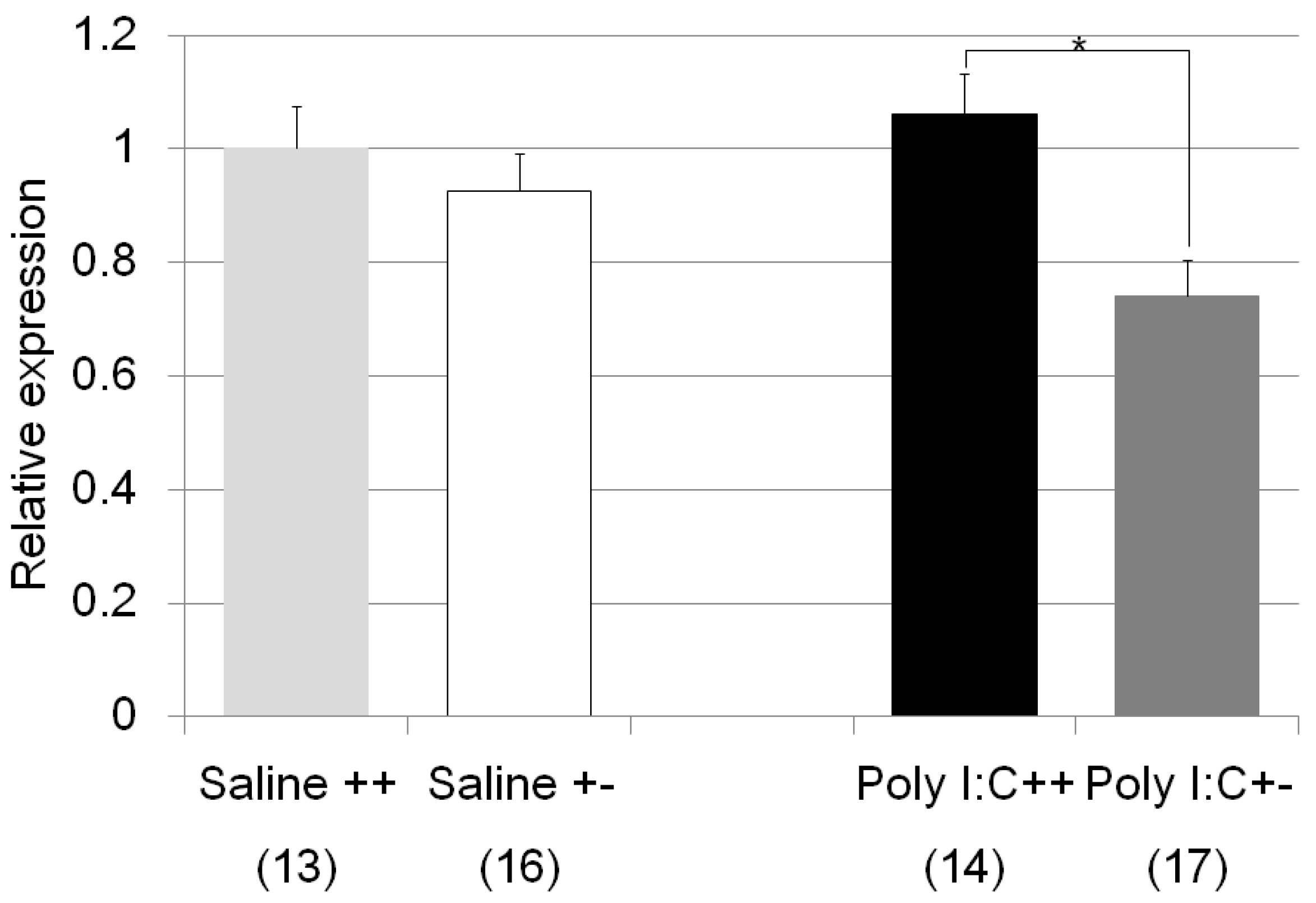
| Nr3c1 | Poly I:C/Cnr2 ko mice (Figure 7) | F3,59 = 4.4, p = 0.008 | Poly I:C treated wildtype mice vs. heterozygote mice; p = 0.007 |
| genotype F = 8.4, p = 0.005 | |||
| treatment F = 0.83, p = 0.37 | |||
| genotype x treatment F = 3.2, p = 0.078 | |||
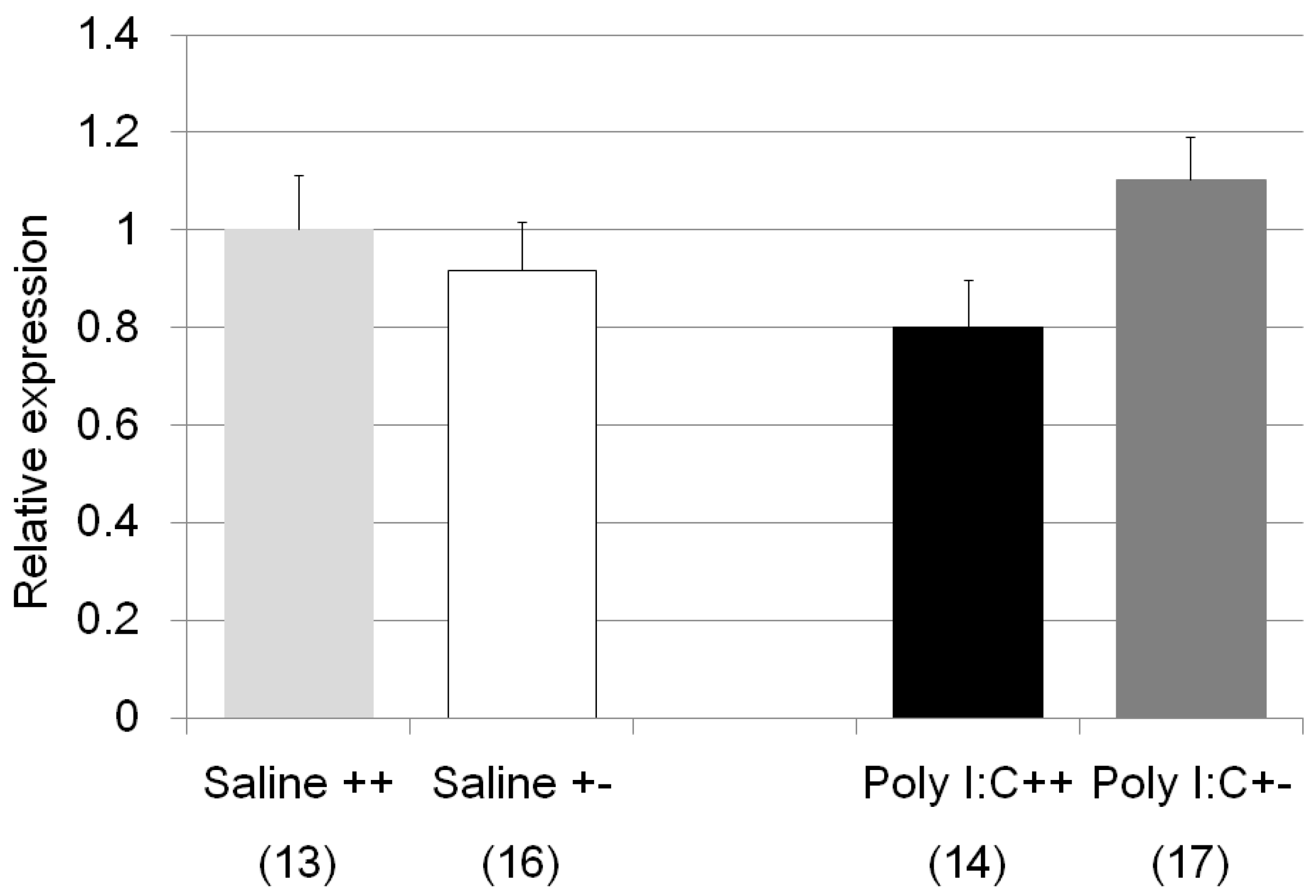
| Il1b | Poly I:C/Cnr2 ko mice (Figure 8) | F3,58 = 1.8, p = 0.17 | n.s. (Poly I:C treated wildtype mice vs. heterozygote mice; p = 0.14) |
| genotype F = 0.0004, p = 0.98 | |||
| treatment F = 0.98, p = 0.33 | |||
| genotype × treatment F = 4.05, p = 0.049 | |||
| Bdnf | Poly I:C/Cnr2 ko mice (no figure) | F3,59 = 0.15, p = 0.93 | n.s. |
| Crf | Poly I:C/Cnr2 ko mice (no figure) | F3,59 = 0.54, p = 0.66 | n.s. |
| genotype F = 0.49, p = 0.63 | |||
| treatment F = 0.54, p = 0.59 | |||
| genotype × treatment F = 0.97, p = 0.34 | |||
| Stressors | Experimental Treatment | Behavioral Tests | Gene Expression Analysis |
|---|---|---|---|
| Physical/Emotional (Chronic mild stress) | Control (10) AM630 (9) JWH015 (8) Fluvoxamine (11) Saline (10) | 1. Zero maze | Fkbp5 Control (7) vs. Saline (7) |
| Immune | Poly I:C (20) Saline (19) | 1. Locomotion in home cage | Fkbp5, Il1b, Nr3c1, Bdnf, Crf Poly I:C (31) vs. Saline (29) |
| Poly I:C (70) Saline (52) | 2. Zero maze | ||
| Poly I:C (61) Saline (53) | 3. Pulley rotating test (originally developed test) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishiguro, H.; Horiuchi, Y.; Tabata, K.; Liu, Q.-R.; Arinami, T.; Onaivi, E.S. Cannabinoid CB2 Receptor Gene and Environmental Interaction in the Development of Psychiatric Disorders. Molecules 2018, 23, 1836. https://doi.org/10.3390/molecules23081836
Ishiguro H, Horiuchi Y, Tabata K, Liu Q-R, Arinami T, Onaivi ES. Cannabinoid CB2 Receptor Gene and Environmental Interaction in the Development of Psychiatric Disorders. Molecules. 2018; 23(8):1836. https://doi.org/10.3390/molecules23081836
Chicago/Turabian StyleIshiguro, Hiroki, Yasue Horiuchi, Koichi Tabata, Qing-Rong Liu, Tadao Arinami, and Emmanuel S. Onaivi. 2018. "Cannabinoid CB2 Receptor Gene and Environmental Interaction in the Development of Psychiatric Disorders" Molecules 23, no. 8: 1836. https://doi.org/10.3390/molecules23081836
APA StyleIshiguro, H., Horiuchi, Y., Tabata, K., Liu, Q.-R., Arinami, T., & Onaivi, E. S. (2018). Cannabinoid CB2 Receptor Gene and Environmental Interaction in the Development of Psychiatric Disorders. Molecules, 23(8), 1836. https://doi.org/10.3390/molecules23081836






