High-Pressure Homogenization Pretreatment before Enzymolysis of Soy Protein Isolate: the Effect of Pressure Level on Aggregation and Structural Conformations of the Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Different Pretreatments of SPI Suspensions
2.3. Preparation of SPI Suspensions with HPH
2.4. Determination of the Degree of Hydrolysis (DH)
2.5. Determination of Antioxidant Activity of SPI Hydrolysates
2.6. Light Scattering
2.6.1. Apparatus of Light Scattering
2.6.2. Dynamic Light Scattering
2.6.3. Static Light Scattering
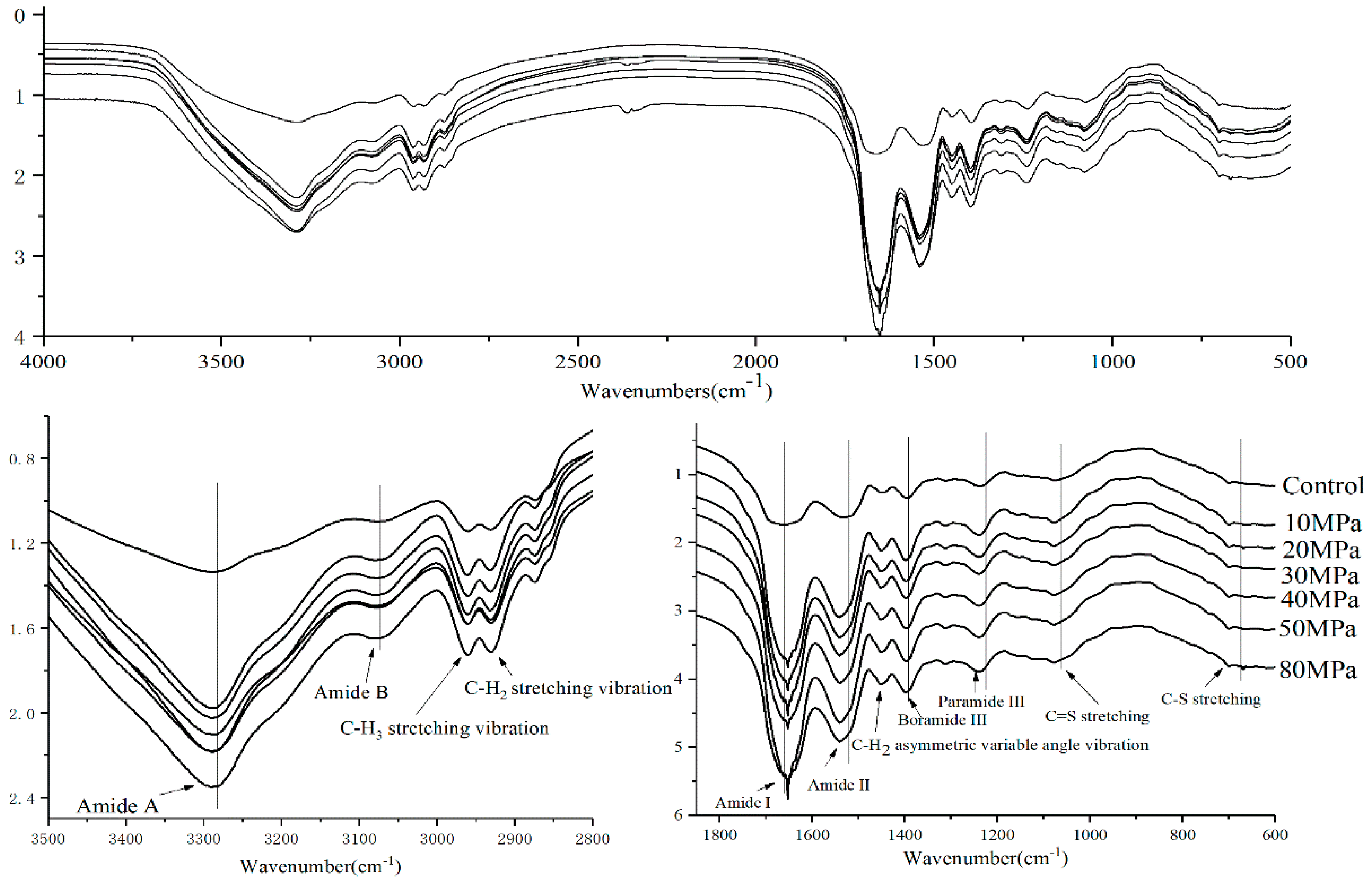
2.7. Fourier Transformed Infrared Spectroscopy
2.8. Scanning Electron Microscopy
2.9. Data Analysis
3. Results and Discussion
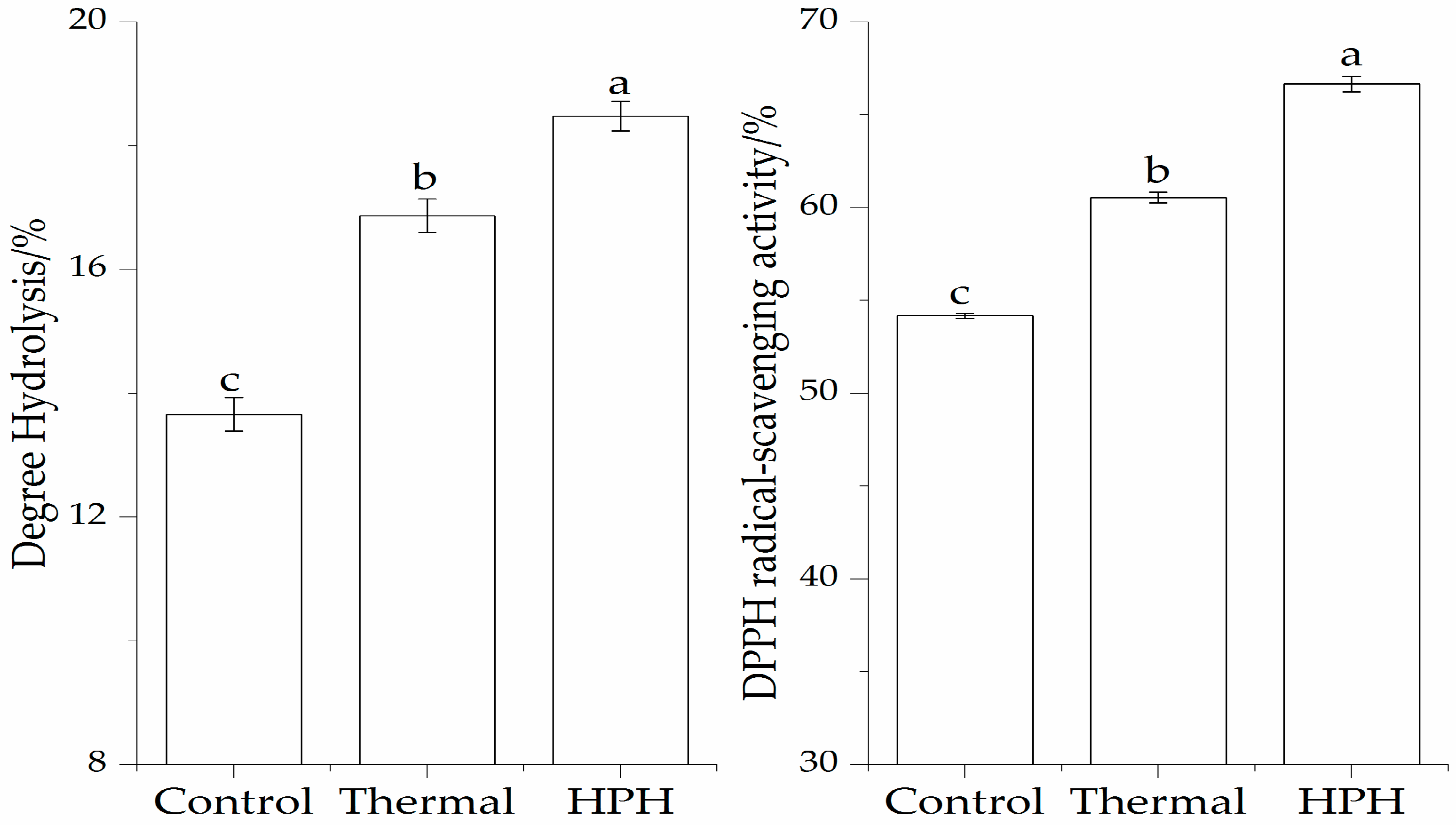
3.1. Effect of Pretreatment on Enzymatic Hydrolysis and DPPH Radical-Scavenging Activity of the SPI Hydrolysates
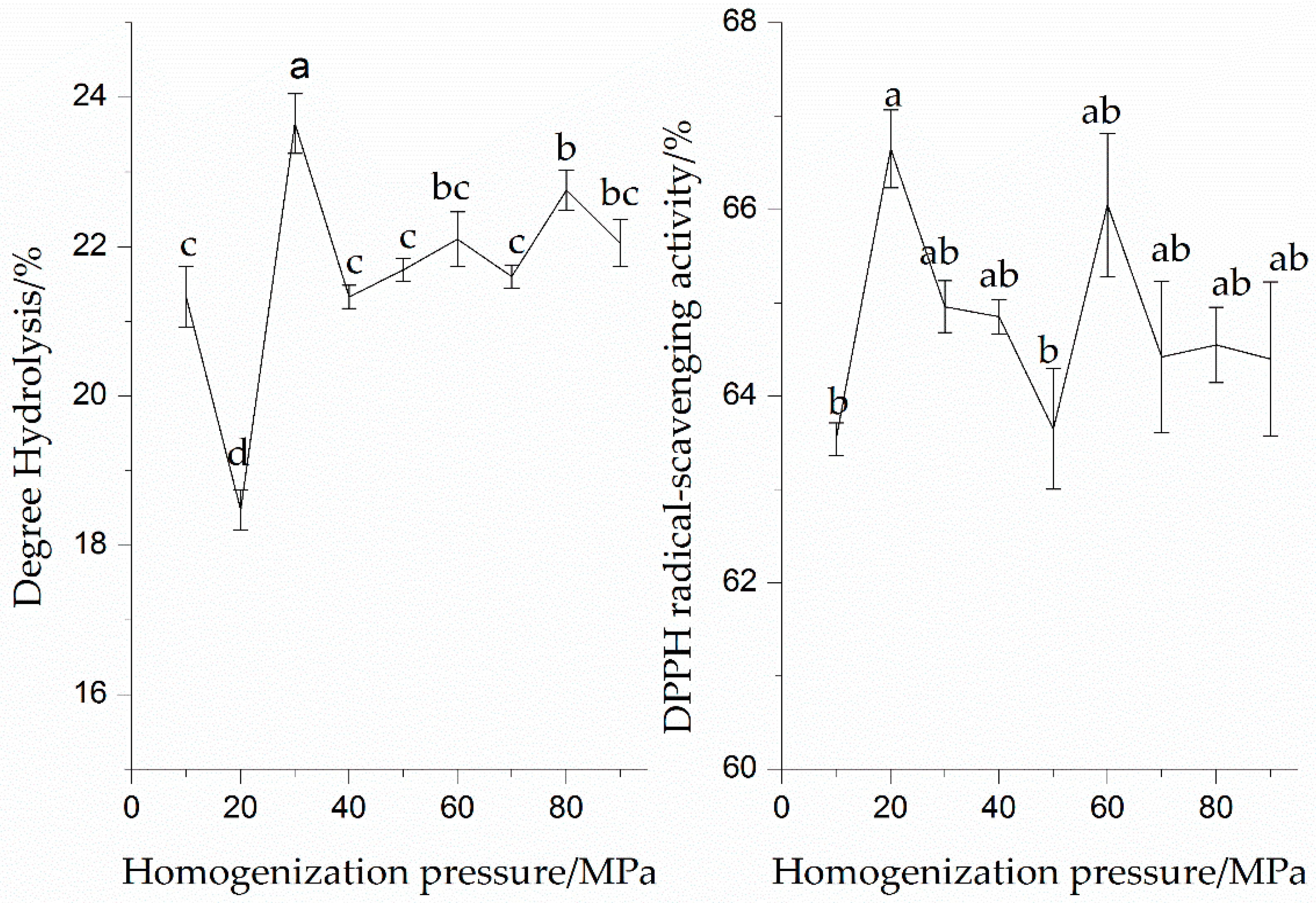
3.1.1. Effect of Pressure on Enzymatic Hydrolysis and DPPH Radical-Scavenging Activity of the SPI Hydrolysates
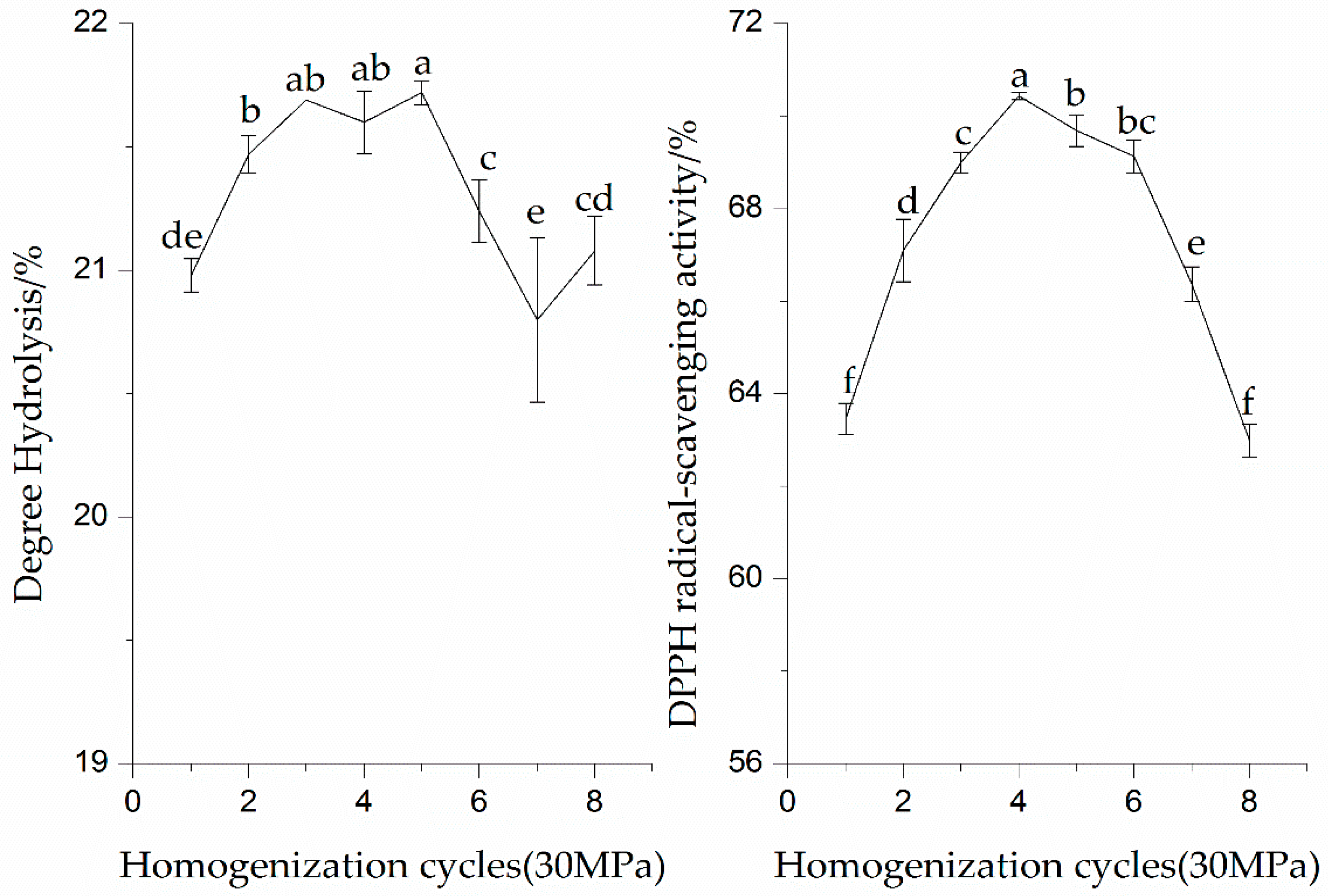
3.1.2. Effect of Homogenization Cycles on Enzymatic Hydrolysis and DPPH Radical-Scavenging Activity of the SPI Hydrolysates
3.2. Light Scattering
3.2.1. Static Light Scattering Analysis
3.2.2. Dynamic Light Scattering Analysis
3.2.3. The Conformation of SPI Aggregates
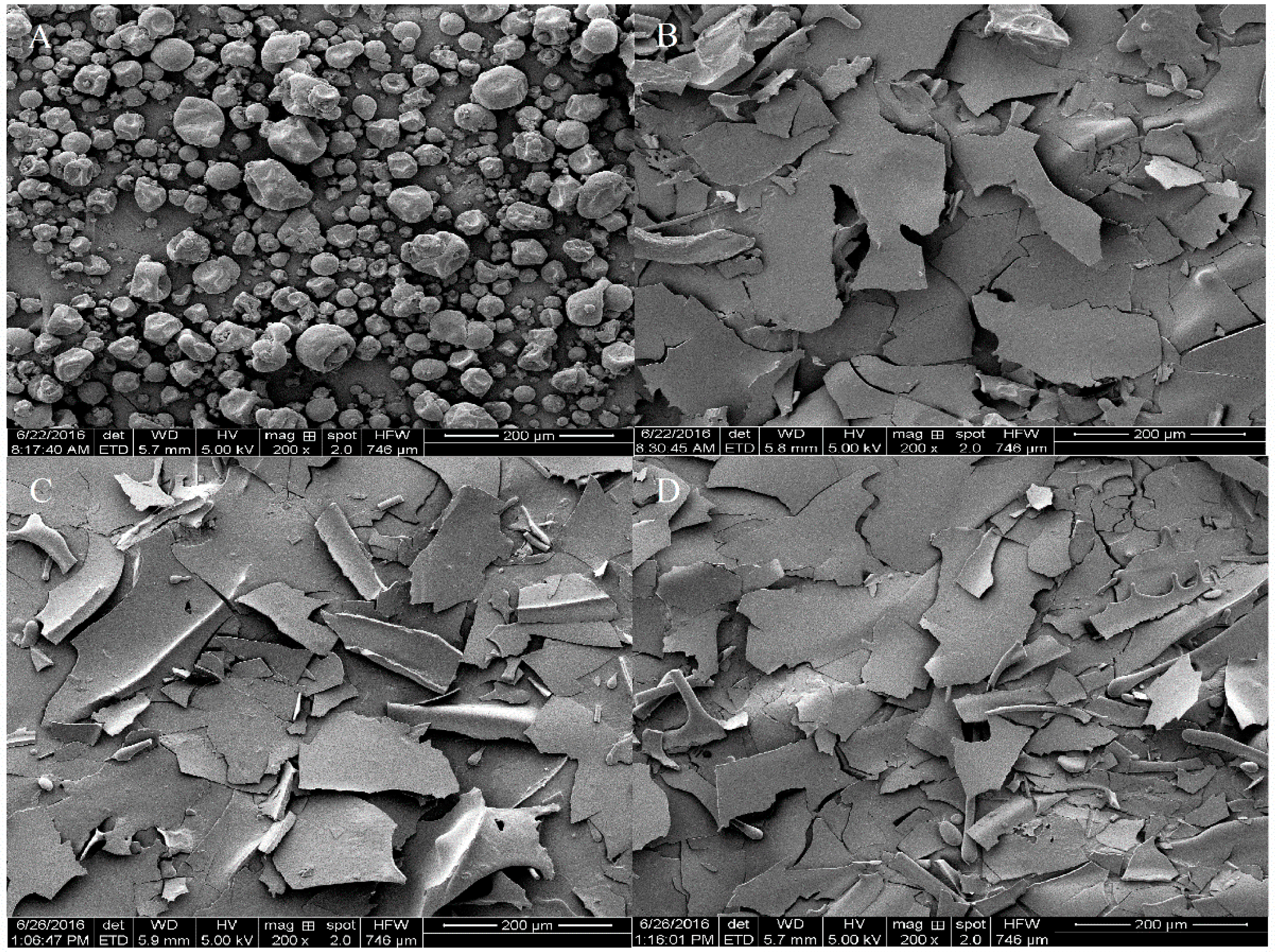
3.3. Microscopic Appearance and Structural Analysis of SPI
3.4. SPI Film Macromorphology
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boen, Y.; Ren, J.; Zhao, M.; Luo, D.; Gu, L. Effects of limited enzymatic hydrolysis with pepsin and on the functional properties of soybean protein isolate. LWT Food Sci. Technol. 2012, 46, 453–459. [Google Scholar]
- Rickert, D.A.; Johnson, L.A.; Murphy, P.A. Functional properties of improved glycinin and b-conglycinin fractions. J. Food Sci. 2004, 69, 303–311. [Google Scholar]
- Ngoh, Y.-Y.; Lim, T.S.; Gan, C.-Y. Screening and identification of five peptides from pinto bean with inhibitory activities against α-amylase using phage display technique. Enzym. Microb. Technol. 2016, 89, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Floury, J.; Bellettre, J.; Legrand, J.; Anne, D. Analysis of a new type of high pressure homogeniser: A study of the flow pattern. Chem. Eng. Sci. 2004, 59, 843–853. [Google Scholar] [CrossRef]
- Tang, C.-H.; Ma, C.-Y. Effect of high pressure treatment on aggregation and structural properties of soy protein isolate. LWT Food Sci. Technol. 2009, 42, 606–611. [Google Scholar] [CrossRef]
- Luo, D.; Zhao, Q.; Zhao, M.; Yang, B.; Long, X.; Ren, J.; Zhao, H. Effects of limited proteolysis and high-pressure homogenisation on structural and functional characteristics of glycinin. Food Chem. 2010, 122, 25–30. [Google Scholar] [CrossRef]
- Dong, X.; Zhao, M.; Shi, J.; Yang, B.; Li, J.; Luo, D.; Jiang, G.; Jiang, Y. Effects of combined high-pressure homogenization and enzymatic treatment on extraction yield, hydrolysis and function properties of peanut proteins. Innov. Food Sci. Emerg. Technol. 2011, 12, 478–483. [Google Scholar] [CrossRef]
- Kuhn, K.R.; Cunha, R.L. Flaxseed oil-Whey protein isolate emulsions: Effect of high pressure homogenization. J. Food Eng. 2012, 111, 449–457. [Google Scholar] [CrossRef]
- Hayes, M.G.; Fox, P.F.; Kelly, A.L. Potential applications of high pressure homogenizationin-processing of liquid milk. J. Dairy Res. 2005, 72, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Leonard, M.; Agnese, P.; Edwin, T.; Musabe, A.V.L.; Marc, H. Carotenoid transfer to oil upon high pressure homogenisation of tomato and carrot based matrices. J. Funct. Foods 2015, 19, 775–785. [Google Scholar]
- Saioa, A.-S.; Inigo, M.D.M.; Juan-Carlos, A. Impact of high pressure homogenisation (HPH) on inulin gelling properties, stability and development during storage. Food Hydrocoll. 2015, 44, 333–344. [Google Scholar]
- Thomas, S.H.L.; Zhou, M.; Zhou, D.; Muthupandian, A.; Gregory, J.O.M. The formation of double emulsions in skim milk using minimal foodgrade emulsifiers—A comparison between ultrasonic and high pressure homogenisation efficiencies. J. Food Eng. 2018, 219, 81–92. [Google Scholar]
- Zhao, X.; Feng, Z. Determination for degree of hydrolysis of the protein hydrolysates. Food Sci. 1994, 15, 65–67. [Google Scholar]
- Yamagucei, T.; Tasamura, H.; Matob, A.; Junji, T. HPLC method for evaluation of the free radical-scavenging activity of foods by using l, l-dipheny l-2-picrylhydrazy. Biosci. Biotechnol. Biochem. 1998, 62, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Steve, W.; Wang, C.Q.; Rickey, Y.Y. Studies of aggregation behaviours of cereal β-glucans in dilute aqueous solutions by light scattering: Part I., Structure effects. Food Hydrocoll. 2011, 25, 189–195. [Google Scholar] [CrossRef]
- García-Risco, M.R.; Ramos, M.; López-Fandino, R. Modifications in milk proteins induced by heat treatment and homogenization and their influence on susceptibility to proteolysis. Int. Dairy J. 2002, 12, 679–688. [Google Scholar] [CrossRef]
- Elena, P.; Guadalupe, P.; Maria, L.B.; Maria, I.M.-M.; Rosario, G. Effects of combined high pressure and enzymatic treatments on the hydrolysis and immunoreactivity of dairy whey proteins. Int. Dairy J. 2006, 16, 831–839. [Google Scholar]
- Song, X.; Zhou, C.; Fu, F.; Chen, Z.; Wu, Q. Effect of high-pressure homogenization on particle size and film properties of soy protein isolate. Ind. Crops Prod. 2013, 43, 538–544. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, B.; Wei, Y.; Li, S. Effects of specific mechanical energy on soy protein aggregation during extrusion process studied by size exclusion chromatography coupled with multi-angle laser light scattering. J. Food Eng. 2013, 115, 220–225. [Google Scholar] [CrossRef]
- Peter, C.G.; Beatrice, C.; Mervat, S.I.; Josephine, C.A. Probing the interaction of nanoparticles with mucin for drug delivery applications using dynamic light scattering. Eur. J. Pharm. Biopharm. 2015, 97, 218–222. [Google Scholar]
- Caterina, B.; Ulderico, W.; Giovanna, D.A.; Cristina, C.; Simona, R. Study of the dynamical behavior of sodium alginate/myoglobin aqueous solutions: A dynamic light scattering study. J. Mol. Liq. 2015, 209, 294–300. [Google Scholar]
- Enoki, T.A.; Henriques, V.B.; Lamy, M.T. Light scattering on the structural characterization of DMPG vesicles along the bilayer anomalous phase transition. Chem. Phys. Lipids 2012, 165, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Burchard, W. Light scattering techniques. In Physical Techniques for the Study of Food Biopolymers; Ross-Murphy, S.B., Ed.; Springer: Boston, MA, USA, 1994; pp. 151–213. [Google Scholar]
- Li, W.; Wang, Q.C.; Cui, S.W.; Huang, X.; Kakuda, Y. Elimination of aggregates of (1→3) (1→4)-beta-d-glucan in dilute solutions for light scattering and size exclusion chromatography study. Food Hydrocoll. 2006, 20, 361–368. [Google Scholar] [CrossRef]
- Sonia, Z.; Ingo, L.; Muhammad, I.S. Soluble aramid containing ether linkages: Synthesis, static and dynamic light scattering studies. Chem. Phys. 2008, 344, 202–208. [Google Scholar]
- Kirkwood, J.G.; Riseman, J. The intrinsic viscosities and diffusion constants of flexible macromolecules in solution. J. Chem. Phys. 1948, 16, 565–573. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, X.; Akihiro, N.; Walther, B.; Hallett, F.R. Molecular characterisation of soybean polysaccharides: An approach by size exclusion chromatography, dynamicand static light scattering methods. Carbohydr. Res. 2005, 340, 2637–2644. [Google Scholar] [CrossRef] [PubMed]
- Buckow, R.; Sikes, A.; Tume, R. Effect of high pressure on physicochemical properties of meat. Crit. Rev. Food Sci. Nutr. 2013, 53, 770–786. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; Xu, Y. Fourier Transform Infrared Spectroscopy, 3rd ed.; Chemistry Industry Press: Beijing, China, 2016; pp. 490–507. [Google Scholar]
- Wang, C.; Jiang, L.; Wei, D.; Li, Y.; Sui, X.; Wang, Z.; Li, D. Effect of Secondary Structure determined by FTIR Spectra on Surface Hydrophobicity of Soybean Protein Isolate. Procedia Eng. 2018, 15, 4819–4827. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Z.; Cai, Q.; Peng, B. Effects of high hydrostatic pressure on structural and physical properties of nisin-SPI film. Int. J. Biol. Macromol. 2018, 111, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Sobhan, S.; Anna, B.; Nitin, M.; Stefan, K. Structural modification in condensed soy glycinin systems following application of high pressure. Food Hydrocoll. 2016, 53, 115–124. [Google Scholar]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Tabilo-Munizaga, G.; Gordon, T.A.; Villalobos-Carvajal, R.; Moreno-Osorio, L.; Salazar, F.N.; Perez-Won, M.; Sergio, A. Effects of high hydrostatic pressure (HHP) on the protein structure and thermal stability of Sauvignon blanc wine. Food Chem. 2014, 155, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Hailiang, Z.; Jianbing, P.; Jinwen, H.; Hongbo, L.; Yanwu, C.; Fenghui, L. Removal of copper(II) using deacetylated konjac glucomannan conjugated soy protein isolate. Int. J. Biol. Macromol. 2016, 86, 338–344. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
| Sample Code | Mw (g/mol × 107) | Rg (nm) | Rh (nm) | A2 (×10-4) | ρ (Rg/Rh) | Structure |
|---|---|---|---|---|---|---|
| Control | 0.29 ± 0.17 e | 96.0 ± 5.4 e | 122.97 | –3.28 ± 0.65 | 0.78 | hard sphere |
| HPH-10 MPa | 5.90 ± 0.22 a | 310.0 ± 6.4 a | 155.67 | 1.60 ± 0.41 | 1.99 | GCP |
| HPH-20 MPa | 4.36 ± 0.34 b | 161.0 ± 8.3 b | 140.18 | 0.72 ± 0.18 | 1.15 | GCM |
| HPH-30 MPa | 2.44 ± 0.14 d | 141.0 ± 6.0 c | 117.14 | 1.45 ± 0.5 | 1.2 | GCM |
| HPH-40 MPa | 2.24 ± 0.08 d | 122.2 ± 3.4 d | 115.94 | 1.38 ± 0.32 | 1.05 | hollow sphere |
| HPH-60 MPa | 2.89 ± 0.11 c | 114.3 ± 3.7 d | 109.11 | 0.74 ± 0.22 | 1.05 | hollow sphere |
| Pressure (MPa) | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Coil (%) |
|---|---|---|---|---|
| 0 | 20.21 a | 27.23 a | 33.33 c | 19.23 a |
| 10 | 16.99 d | 22.84 e | 43.86 a | 16.99 c |
| 20 | 17.27 c | 22.74 e | 43.78 a | 16.18 e |
| 30 | 16.43 e | 22.99 d | 43.69 a | 16.89 c |
| 40 | 16.15 f | 23.42 c | 44.04 a | 16.39 d |
| 50 | 17.82 b | 22.57 f | 43.47 b | 16.13 e |
| 80 | 15.29 g | 23.67 b | 43.25 b | 17.79 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Zhang, D.; Li, X.; Dong, H. High-Pressure Homogenization Pretreatment before Enzymolysis of Soy Protein Isolate: the Effect of Pressure Level on Aggregation and Structural Conformations of the Protein. Molecules 2018, 23, 1775. https://doi.org/10.3390/molecules23071775
Zhao F, Zhang D, Li X, Dong H. High-Pressure Homogenization Pretreatment before Enzymolysis of Soy Protein Isolate: the Effect of Pressure Level on Aggregation and Structural Conformations of the Protein. Molecules. 2018; 23(7):1775. https://doi.org/10.3390/molecules23071775
Chicago/Turabian StyleZhao, Fei, Daofang Zhang, Xiangyang Li, and Haizhou Dong. 2018. "High-Pressure Homogenization Pretreatment before Enzymolysis of Soy Protein Isolate: the Effect of Pressure Level on Aggregation and Structural Conformations of the Protein" Molecules 23, no. 7: 1775. https://doi.org/10.3390/molecules23071775
APA StyleZhao, F., Zhang, D., Li, X., & Dong, H. (2018). High-Pressure Homogenization Pretreatment before Enzymolysis of Soy Protein Isolate: the Effect of Pressure Level on Aggregation and Structural Conformations of the Protein. Molecules, 23(7), 1775. https://doi.org/10.3390/molecules23071775




