Salt-Mediated Au-Cu Nanofoam and Au-Cu-Pd Porous Macrobeam Synthesis
Abstract
1. Introduction
2. Materials and Methods
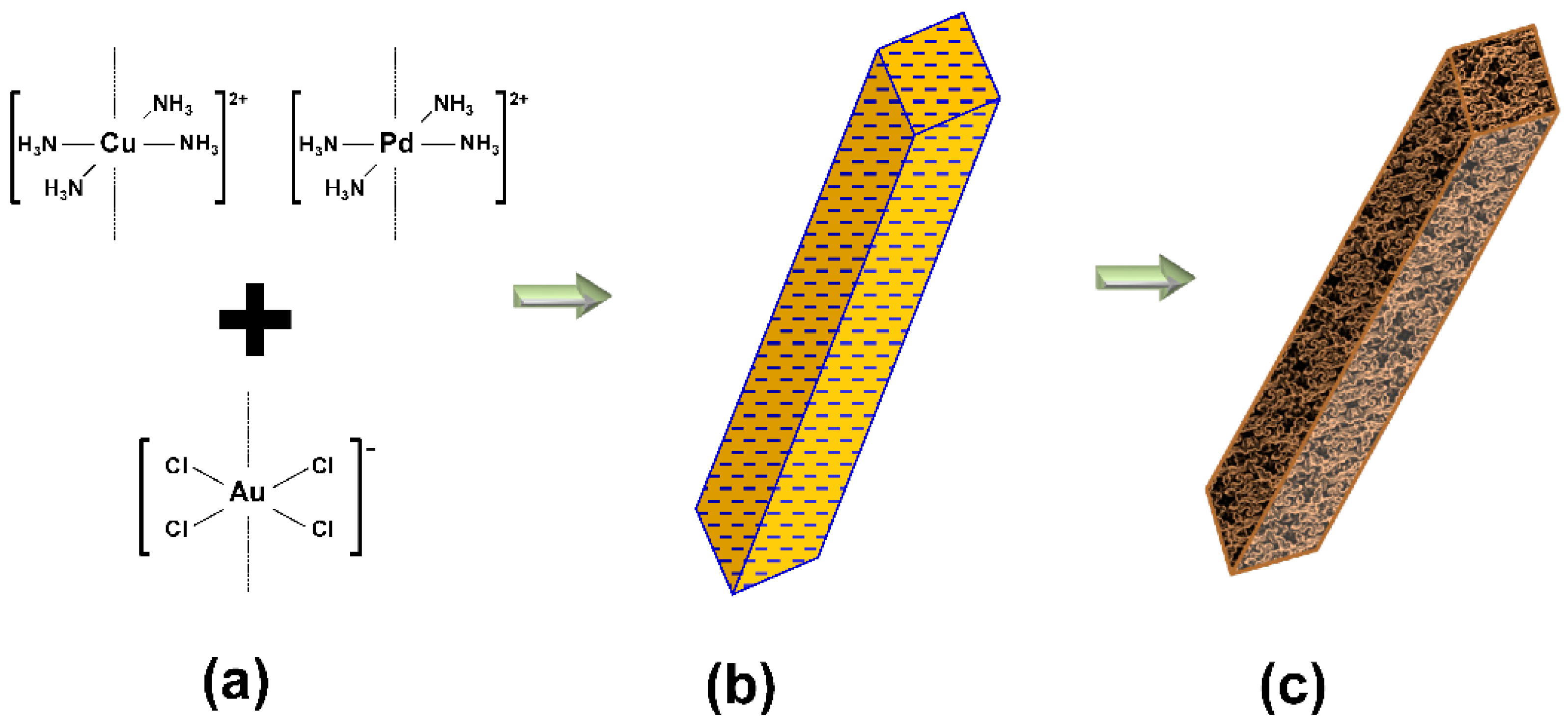
2.1. Au-Cu Nanofoam and Au-Cu-Pd Macrobeam Synthesis
2.2. Polarized Optical Microscopy
2.3. Scanning Electron Microscopy
2.4. X-ray Diffractometry
2.5. X-ray Fluorescence
2.6. UV-VIS
2.7. BET Analysis
2.8. Electrochemical Characterization
3. Results and Discussion
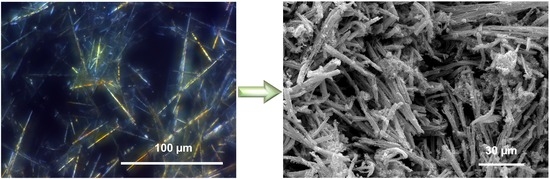
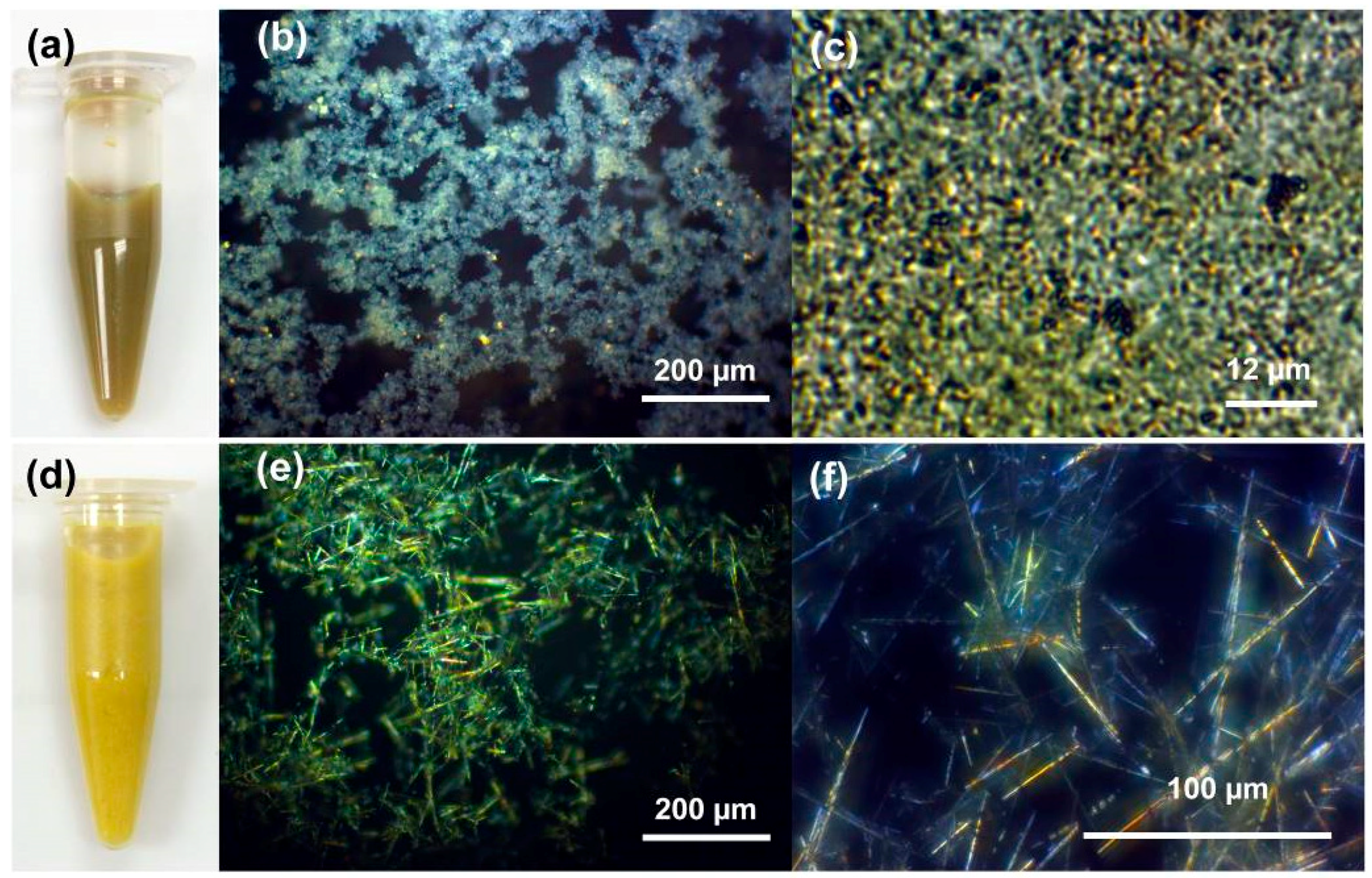
3.1. Nanofoam and Porous Macrobeam Synthesis
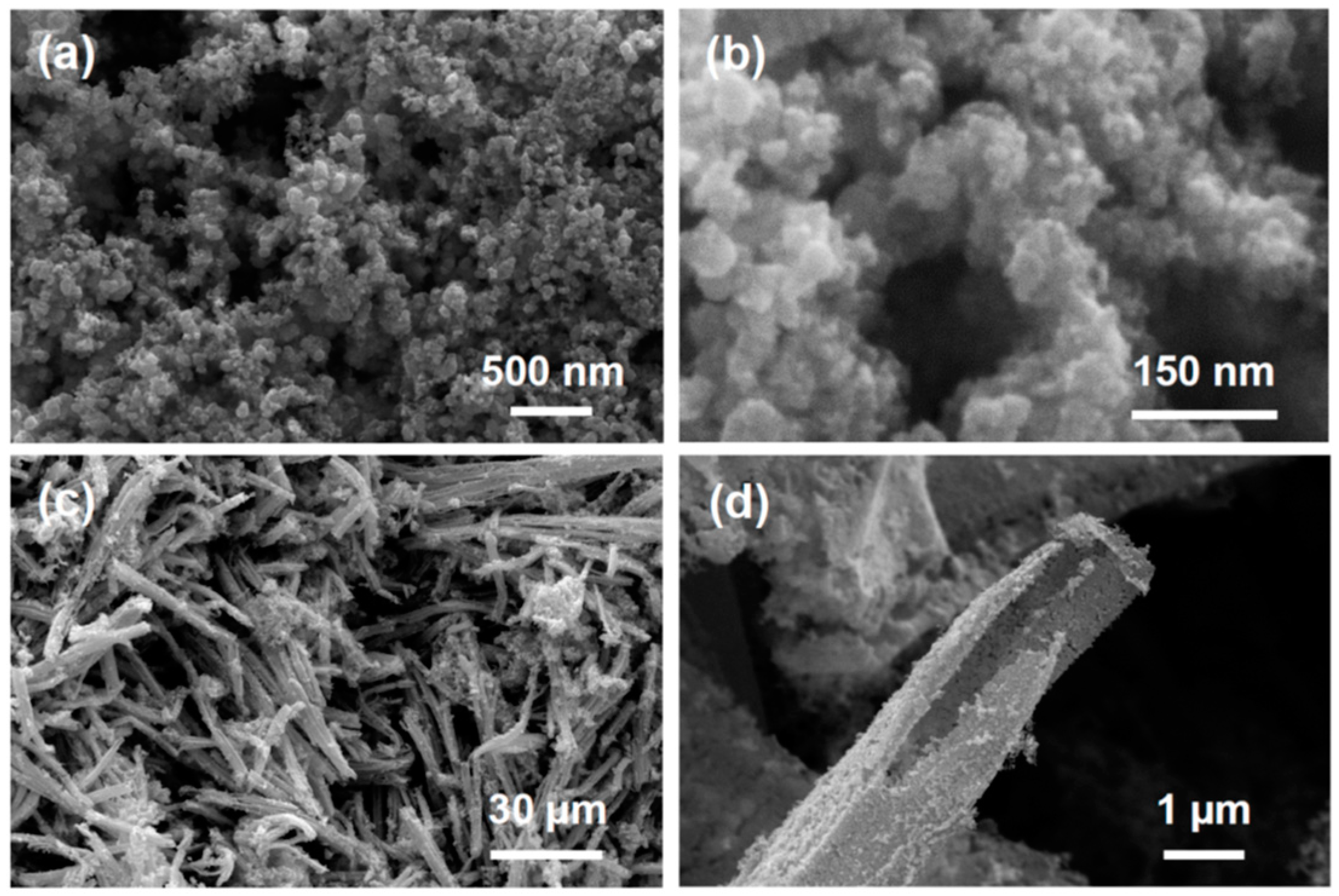
3.2. Scanning Electron Microscopy
3.3. X-ray Diffractometry
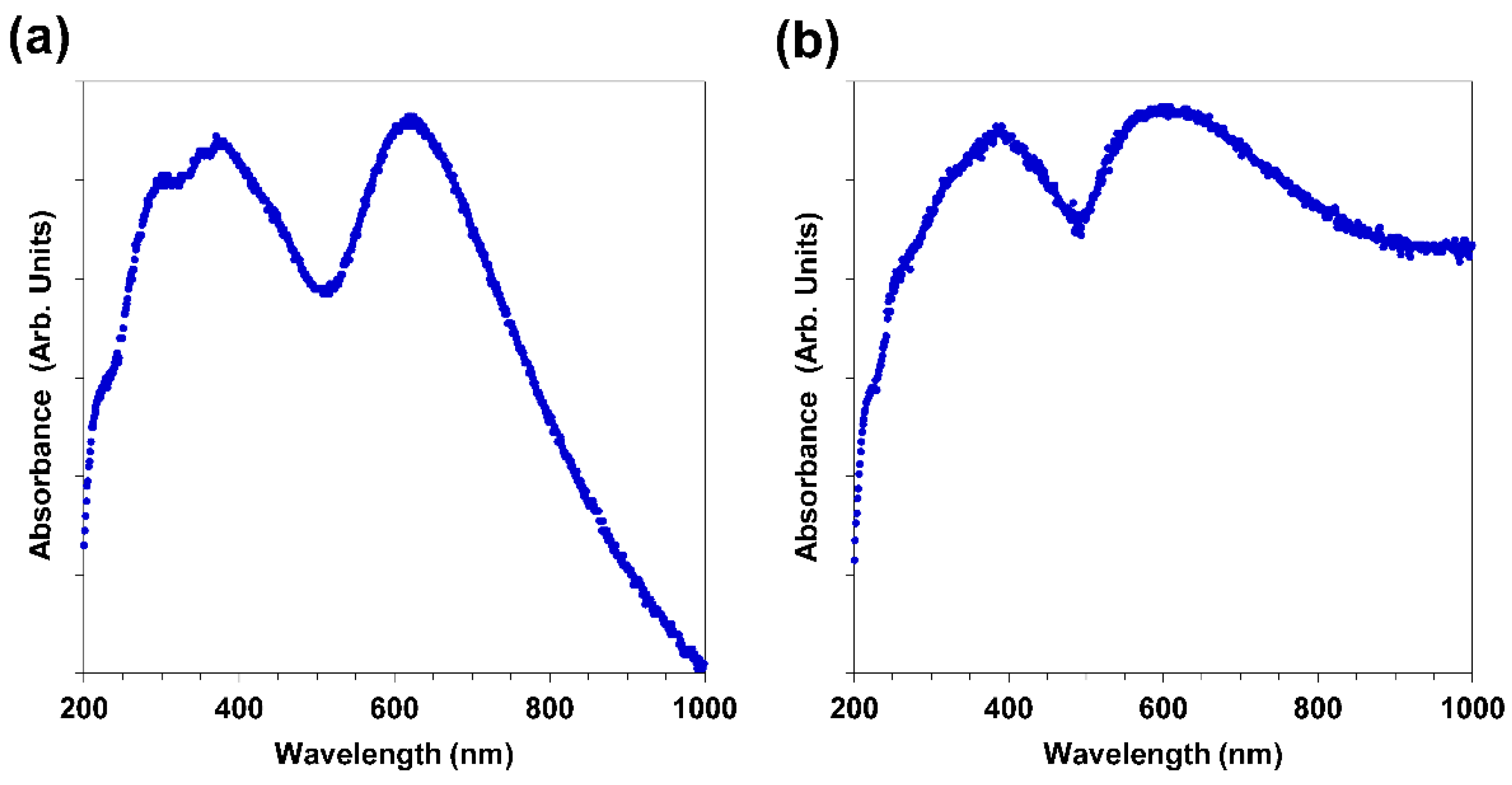
3.4. UV-VIS
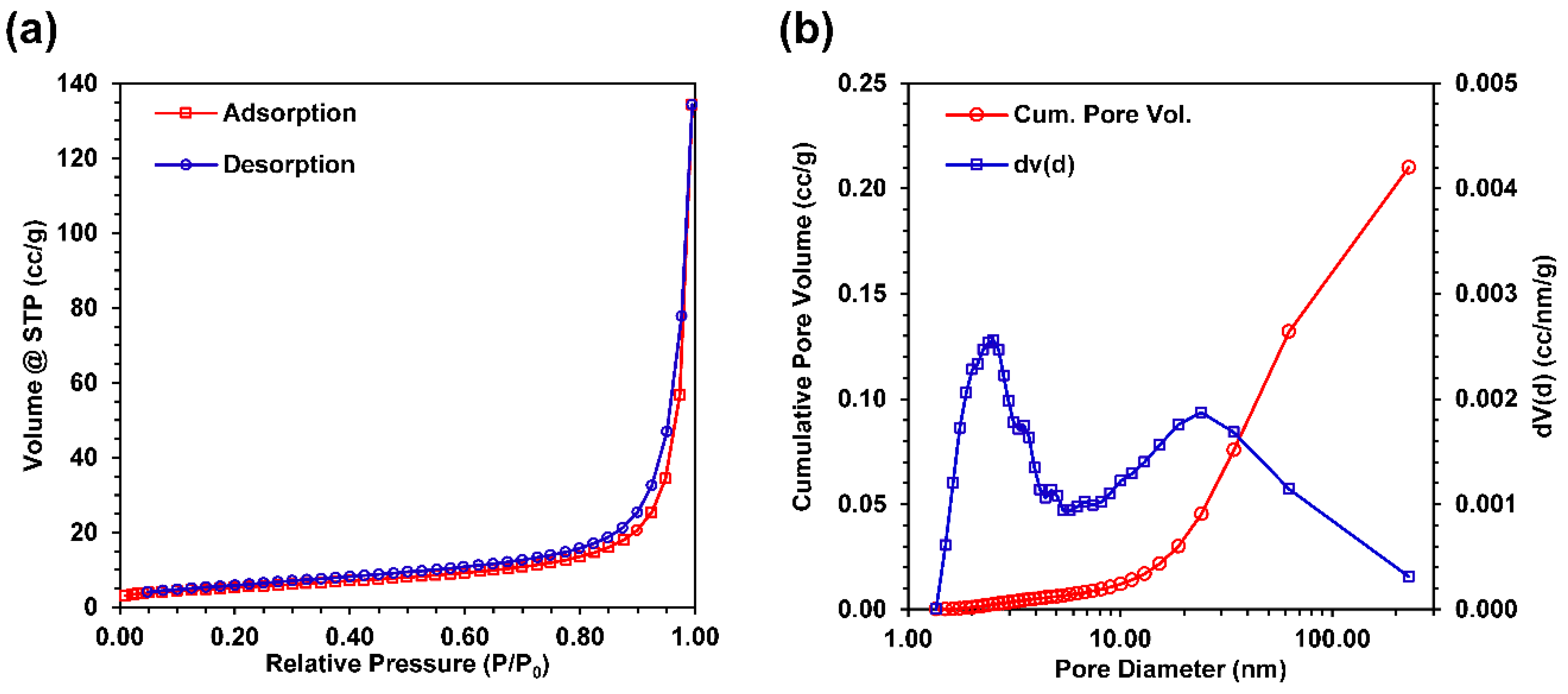
3.5. Nitrogen Gas Adsorption
3.6. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qiu, X.; Dai, Y.; Zhu, X.; Zhang, H.; Wu, P.; Tang, Y.; Wei, S. Template-engaged synthesis of hollow porous platinum–palladium alloy nanospheres for efficient methanol electro-oxidation. J. Power Sources 2016, 302, 195–201. [Google Scholar] [CrossRef]
- Jiang, B.; Kani, K.; Iqbal, M.; Abe, H.; Kimura, T.; Hossain, M.S.A.; Anjaneyulu, O.; Henzie, J.; Yamauchi, Y. Mesoporous Bimetallic RhCu Alloy Nanospheres Using a Sophisticated Soft-Templating Strategy. Chem. Mater. 2018, 30, 428–435. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Wang, L.; Xu, L.; Bian, X.; Ma, H.; Ding, Y. Nanotubular Mesoporous PdCu Bimetallic Electrocatalysts toward Oxygen Reduction Reaction. Chem. Mater. 2009, 21, 3110–3116. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Tonegawa, A.; Komatsu, M.; Wang, H.; Wang, L.; Nemoto, Y.; Suzuki, N.; Kuroda, K. Electrochemical Synthesis of Mesoporous Pt–Au Binary Alloys with Tunable Compositions for Enhancement of Electrochemical Performance. J. Am. Chem. Soc. 2012, 134, 5100–5109. [Google Scholar] [CrossRef] [PubMed]
- Moshfegh, A.Z. Nanoparticle catalysts. J. Phy. D: Appl. Phys. 2009, 42, 233001. [Google Scholar] [CrossRef]
- Maillard, F.; Schreier, S.; Hanzlik, M.; Savinova, E.R.; Weinkauf, S.; Stimming, U. Influence of particle agglomeration on the catalytic activity of carbon-supported Pt nanoparticles in CO monolayer oxidation. Phys. Chem. Chem. Phys. 2005, 7, 385–393. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chua, C.K.; Bonanni, A.; Pumera, M. Electrochemistry of Graphene and Related Materials. Chem. Rev. 2014, 114, 7150–7188. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Herrmann, A.-K.; Bigall, N.C.; Rodriguez, P.; Wen, D.; Oezaslan, M.; Schmidt, T.J.; Gaponik, N.; Eychmüller, A. Noble Metal Aerogels—Synthesis, Characterization, and Application as Electrocatalysts. Acc. Chem. Res. 2015, 48, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Sacaliuc, E.; Beale, A.M.; Weckhuysen, B.M.; Nijhuis, T.A. Propene epoxidation over Au/Ti-SBA-15 catalysts. J. Catal. 2007, 248, 235–248. [Google Scholar] [CrossRef]
- Hughes, M.D.; Xu, Y.-J.; Jenkins, P.; McMorn, P.; Landon, P.; Enache, D.I.; Carley, A.F.; Attard, G.A.; Hutchings, G.J.; King, F.; et al. Tunable gold catalysts for selective hydrocarbon oxidation under mild conditions. Nature 2005, 437, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- González-Arellano, C.; Corma, A.; Iglesias, M.; Sánchez, F. Gold (I) and (III) catalyze Suzuki cross-coupling and homocoupling, respectively. J. Catal. 2006, 238, 497–501. [Google Scholar] [CrossRef]
- Corma, A.; Serna, P. Chemoselective Hydrogenation of Nitro Compounds with Supported Gold Catalysts. Science 2006, 313, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Bond, G.C.; Thompson, D.T. Gold-catalysed oxidation of carbon monoxide. Gold Bull. 2000, 33, 41–50. [Google Scholar] [CrossRef]
- Schubert, M.M.; Hackenberg, S.; van Veen, A.C.; Muhler, M.; Plzak, V.; Behm, R.J. CO Oxidation over Supported Gold Catalysts—“Inert” and “Active” Support Materials and Their Role for the Oxygen Supply during Reaction. J. Catal. 2001, 197, 113–122. [Google Scholar] [CrossRef]
- Chen, M.S.; Goodman, D.W. The Structure of Catalytically Active Gold on Titania. Science 2004, 306, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Mihaylov, M.; Ivanova, E.; Hadjiivanov, K.; Knözinger, H.; Gates, B.C. CO oxidation catalyzed by gold supported on MgO: Spectroscopic identification of carbonate-like species bonded to gold during catalyst deactivation. J. Catal. 2009, 261, 137–149. [Google Scholar] [CrossRef]
- Zhu, W.; Michalsky, R.; Metin, Ö.; Lv, H.; Guo, S.; Wright, C.J.; Sun, X.; Peterson, A.A.; Sun, S. Monodisperse Au Nanoparticles for Selective Electrocatalytic Reduction of CO2 to CO. J. Am. Chem. Soc. 2013, 135, 16833–16836. [Google Scholar] [CrossRef] [PubMed]
- Gattrell, M.; Gupta, N.; Co, A. A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper. J. Electroanal. Chem. 2006, 594, 1–19. [Google Scholar] [CrossRef]
- Li, C.W.; Kanan, M.W. CO2 Reduction at Low Overpotential on Cu Electrodes Resulting from the Reduction of Thick Cu2O Films. J. Am. Chem. Soc. 2012, 134, 7231–7234. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050–7059. [Google Scholar] [CrossRef]
- Hori, Y.; Murata, A.; Takahashi, R. Formation of hydrocarbons in the electrochemical reduction of carbon dioxide at a copper electrode in aqueous solution. J. Chem. Soc. Faraday Trans. 1: Phys. Chem. Condens. Phases 1989, 85, 2309–2326. [Google Scholar] [CrossRef]
- Tang, W.; Peterson, A.A.; Varela, A.S.; Jovanov, Z.P.; Bech, L.; Durand, W.J.; Dahl, S.; Norskov, J.K.; Chorkendorff, I. The importance of surface morphology in controlling the selectivity of polycrystalline copper for CO2 electroreduction. Phys. Chem. Chem. Phys. 2012, 14, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Ranu, B.C.; Dey, R.; Chatterjee, T.; Ahammed, S. Copper Nanoparticle-Catalyzed Carbon-Carbon and Carbon-Heteroatom Bond Formation with a Greener Perspective. ChemSusChem 2012, 5, 22–44. [Google Scholar] [CrossRef] [PubMed]
- Decan, M.R.; Impellizzeri, S.; Marin, M.L.; Scaiano, J.C. Copper nanoparticle heterogeneous catalytic ‘click’ cycloaddition confirmed by single-molecule spectroscopy. Nat. Commun. 2014, 5, 4612. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajadi, S.M.; Khalaj, M. Green synthesis of copper nanoparticles using aqueous extract of the leaves of Euphorbia esula L and their catalytic activity for ligand-free Ullmann-coupling reaction and reduction of 4-nitrophenol. RSC Adv. 2014, 4, 47313–47318. [Google Scholar] [CrossRef]
- Damodara, D.; Arundhathi, R.; Likhar, P.R. Copper Nanoparticles from Copper Aluminum Hydrotalcite: An Efficient Catalyst for Acceptor- and Oxidant-Free Dehydrogenation of Amines and Alcohols. Adv. Synth. Catal. 2014, 356, 189–198. [Google Scholar] [CrossRef]
- He, R.; Wang, Y.-C.; Wang, X.; Wang, Z.; Liu, G.; Zhou, W.; Wen, L.; Li, Q.; Wang, X.; Chen, X.; et al. Facile synthesis of pentacle gold–copper alloy nanocrystals and their plasmonic and catalytic properties. Nat. Commun. 2014, 5, 4327. [Google Scholar] [CrossRef] [PubMed]
- Motl, N.E.; Ewusi-Annan, E.; Sines, I.T.; Jensen, L.; Schaak, R.E. Au−Cu Alloy Nanoparticles with Tunable Compositions and Plasmonic Properties: Experimental Determination of Composition and Correlation with Theory. J. Phys. Chem. C 2010, 114, 19263–19269. [Google Scholar] [CrossRef]
- Hsia, C.-F.; Madasu, M.; Huang, M.H. Aqueous Phase Synthesis of Au–Cu Core–Shell Nanocubes and Octahedra with Tunable Sizes and Noncentrally Located Cores. Chem. Mater. 2016, 28, 3073–3079. [Google Scholar] [CrossRef]
- Rice, K.P.; Walker, E.J.; Stoykovich, M.P.; Saunders, A.E. Solvent-Dependent Surface Plasmon Response and Oxidation of Copper Nanocrystals. J. Phys. Chem. C 2011, 115, 1793–1799. [Google Scholar] [CrossRef]
- Ou, T.-C.; Chang, F.-W.; Roselin, L.S. Production of hydrogen via partial oxidation of methanol over bimetallic Au–Cu/TiO2 catalysts. J. Mol. Catal. A: Chem. 2008, 293, 8–16. [Google Scholar] [CrossRef]
- Liu, X.; Wang, A.; Li, L.; Zhang, T.; Mou, C.-Y.; Lee, J.-F. Structural changes of Au–Cu bimetallic catalysts in CO oxidation: In situ XRD, EPR, XANES, and FT-IR characterizations. J. Catal. 2011, 278, 288–296. [Google Scholar] [CrossRef]
- Kim, D.; Resasco, J.; Yu, Y.; Asiri, A.M.; Yang, P. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold–copper bimetallic nanoparticles. Nat. Commun. 2014, 5, 4948. [Google Scholar] [CrossRef] [PubMed]
- Bakır, Ç.C.; Şahin, N.; Polat, R.; Dursun, Z. Electrocatalytic reduction of oxygen on bimetallic copper–gold nanoparticles–multiwalled carbon nanotube modified glassy carbon electrode in alkaline solution. J. Electroanal. Chem. 2011, 662, 275–280. [Google Scholar] [CrossRef]
- Xu, Z.; Lai, E.; Shao-Horn, Y.; Hamad-Schifferli, K. Compositional dependence of the stability of AuCu alloy nanoparticles. Chem. Commun. 2012, 48, 5626–5628. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Tachikawa, T.; Fujitsuka, M.; Majima, T. Photochemical Formation of Au/Cu Bimetallic Nanoparticles with Different Shapes and Sizes in a Poly(vinyl alcohol) Film. Adv. Funct. Mater. 2007, 17, 857–862. [Google Scholar] [CrossRef]
- Ganjali, M.; Ganjali, M.; Khoby, S.; Meshkot, M.A. Synthesis of Au-Cu Nano-Alloy from Monometallic Colloids by Simultaneous Pulsed Laser Targeting and Stirring. Nano-Micro Lett. 2011, 3, 256–263. [Google Scholar] [CrossRef]
- De, G.; Mattei, G.; Mazzoldi, P.; Sada, C.; Battaglin, G.; Quaranta, A. Au−Cu Alloy Nanocluster Doped SiO2 Films by Sol−Gel Processing. Chem. Mater. 2000, 12, 2157–2160. [Google Scholar] [CrossRef]
- Burpo, F.J.; Nagelli, E.A.; Morris, L.A.; McClure, J.P.; Ryu, M.Y.; Palmer, J.L. Direct solution-based reduction synthesis of Au, Pd, and Pt aerogels. J. Mater. Res. 2017, 32, 4153–4165. [Google Scholar] [CrossRef]
- Burpo, F.J.; Nagelli, E.A.; Winter, S.J.; McClure, J.P.; Bartolucci, S.F.; Burns, A.R.; O‘Brien, S.F.; Chu, D.D. Salt-Templated Hierarchically Porous Platinum Macrotube Synthesis. ChemistrySelect 2018, 3, 4542–4546. [Google Scholar] [CrossRef]
- Burpo, F.J.; Mitropoulos, A.N.; Nagelli, E.A.; Ryu, M.Y.; Palmer, J.L. Gelatin biotemplated platinum aerogels. MRS Adv. 2018, 1–6. [Google Scholar] [CrossRef]
- Burpo, F.; Mitropoulos, A.; Nagelli, E.; Palmer, J.; Morris, L.; Ryu, M.; Wickiser, J. Cellulose Nanofiber Biotemplated Palladium Composite Aerogels. Molecules 2018, 23, 1405. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Brunauer, B.; Emmett, P.; Teller, P. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.; Joyner, L.; Halenda, P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Ostwald, W. Catalysis. Phys. Z. 1901, 3, 313–322. [Google Scholar]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV−Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Gonella, F.; Mattei, G.; Mazzoldi, P.; Sada, C.; Battaglin, G.; Cattaruzza, E. Au–Cu alloy nanoclusters in silica formed by ion implantation and annealing in reducing or oxidizing atmosphere. Appl. Phys. Lett. 1999, 75, 55–57. [Google Scholar] [CrossRef]
- Guo, J.; Yu, H.; Dong, F.; Zhu, B.; Huang, W.; Zhang, S. High efficiency and stability of Au-Cu/hydroxyapatite catalyst for the oxidation of carbon monoxide. RSC Adv. 2017, 7, 45420–45431. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Asim, T.; Chen, Y. Plant Mediated Green Synthesis of CuO Nanoparticles: Comparison of Toxicity of Engineered and Plant Mediated CuO Nanoparticles towards Daphnia magna. Nanomaterials 2016, 6, 205. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Guo, X.; Jia, Z.; Xu, Y.; Liu, L.; Zhao, D.; Qin, G.; Qin, W. Gold nanorods as saturable absorbers for all-fiber passively Q-switched erbium-doped fiber laser. Opt. Mater. Express 2013, 3, 1986–1991. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Chang, S.-S.; Lee, C.-L.; Wang, C.R.C. Gold Nanorods: Electrochemical Synthesis and Optical Properties. J. Phys. Chem. B 1997, 101, 6661–6664. [Google Scholar] [CrossRef]
- De Levie, R. On porous electrodes in electrolyte solutions—IV. Electrochim. Acta 1964, 9, 1231–1245. [Google Scholar] [CrossRef]
- Keiser, H.; Beccu, K.D.; Gutjahr, M.A. Abschätzung der porenstruktur poröser elektroden aus impedanzmessungen. Electrochim. Acta 1976, 21, 539–543. [Google Scholar] [CrossRef]
- Daikhin, L.I.; Kornyshev, A.A.; Urbakh, M. Double-layer capacitance on a rough metal surface. Phys. Rev. E 1996, 53, 6192–6199. [Google Scholar] [CrossRef]
- Sun, X.; Li, D.; Ding, Y.; Zhu, W.; Guo, S.; Wang, Z.L.; Sun, S. Core/Shell Au/CuPt Nanoparticles and Their Dual Electrocatalysis for Both Reduction and Oxidation Reactions. J. Am. Chem. Soc. 2014, 136, 5745–5749. [Google Scholar] [CrossRef] [PubMed]
- Cherevko, S.; Kulyk, N.; Chung, C.-H. Nanoporous Pt@AuxCu100–x by Hydrogen Evolution Assisted Electrodeposition of AuxCu100–x and Galvanic Replacement of Cu with Pt: Electrocatalytic Properties. Langmuir 2012, 28, 3306–3315. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.Z.; Ambrosi, A.; Pumera, M. Direct electrochemistry of copper oxide nanoparticles in alkaline media. Electrochem. Commun. 2013, 28, 51–53. [Google Scholar] [CrossRef]
- Giri, S.D.; Sarkar, A. Electrochemical Study of Bulk and Monolayer Copper in Alkaline Solution. J. Electrochem. Soc. 2016, 163, H252–H259. [Google Scholar] [CrossRef]
Sample Availability: Samples of the Au-Cu nanofoams and Au-Cu-Pd macrobeams are available from the authors. |



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burpo, F.J.; Nagelli, E.A.; Morris, L.A.; Woronowicz, K.; Mitropoulos, A.N. Salt-Mediated Au-Cu Nanofoam and Au-Cu-Pd Porous Macrobeam Synthesis. Molecules 2018, 23, 1701. https://doi.org/10.3390/molecules23071701
Burpo FJ, Nagelli EA, Morris LA, Woronowicz K, Mitropoulos AN. Salt-Mediated Au-Cu Nanofoam and Au-Cu-Pd Porous Macrobeam Synthesis. Molecules. 2018; 23(7):1701. https://doi.org/10.3390/molecules23071701
Chicago/Turabian StyleBurpo, Fred J., Enoch A. Nagelli, Lauren A. Morris, Kamil Woronowicz, and Alexander N. Mitropoulos. 2018. "Salt-Mediated Au-Cu Nanofoam and Au-Cu-Pd Porous Macrobeam Synthesis" Molecules 23, no. 7: 1701. https://doi.org/10.3390/molecules23071701
APA StyleBurpo, F. J., Nagelli, E. A., Morris, L. A., Woronowicz, K., & Mitropoulos, A. N. (2018). Salt-Mediated Au-Cu Nanofoam and Au-Cu-Pd Porous Macrobeam Synthesis. Molecules, 23(7), 1701. https://doi.org/10.3390/molecules23071701