Mechanism of Two-/Four-Electron Reduction of Nitroaromatics by Oxygen-Insensitive Nitroreductases: The Role of a Non-Enzymatic Reduction Step
Abstract
1. Introduction
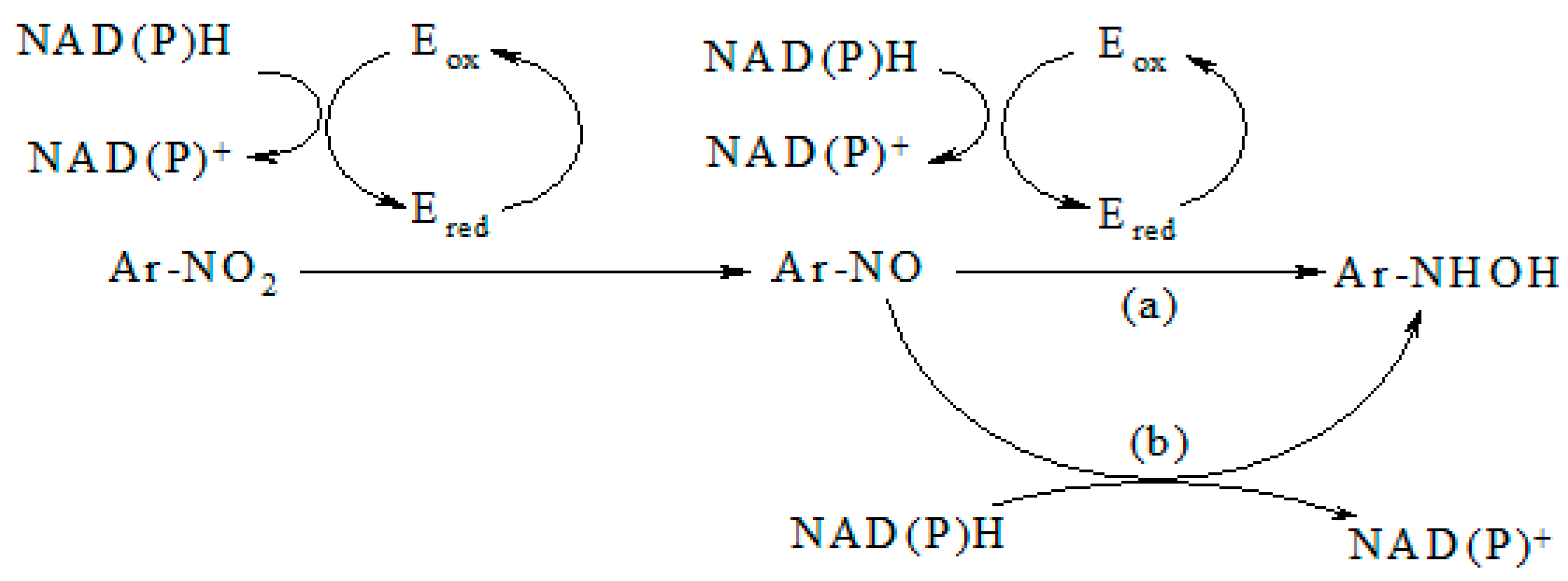
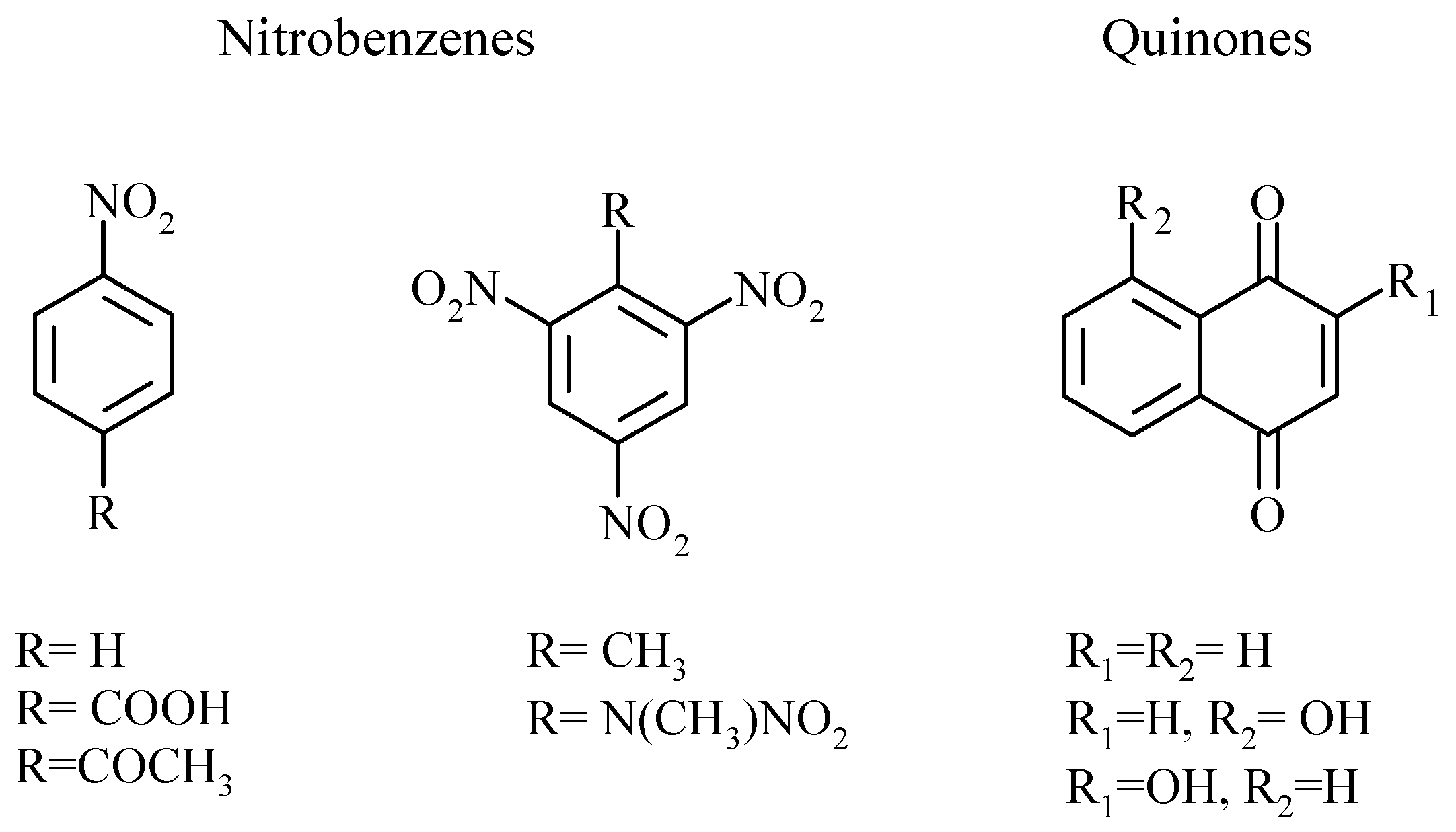
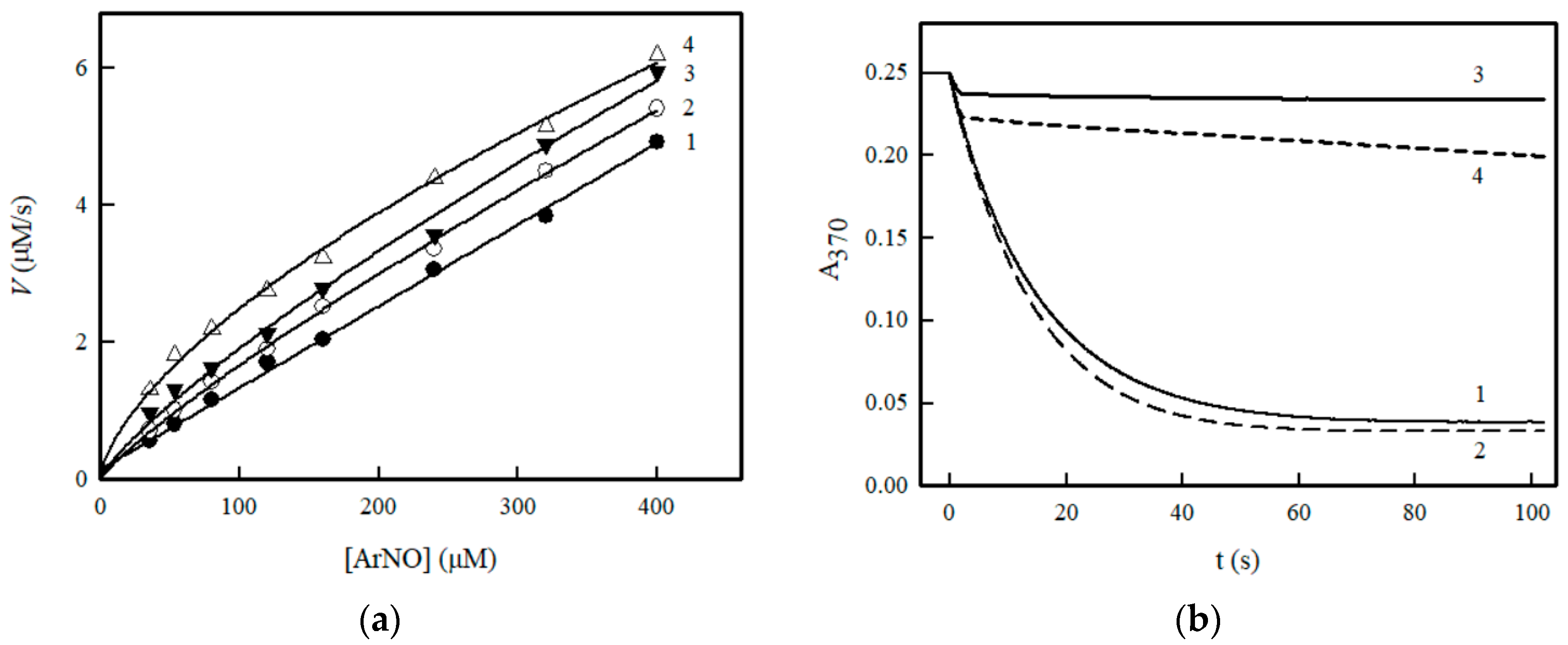
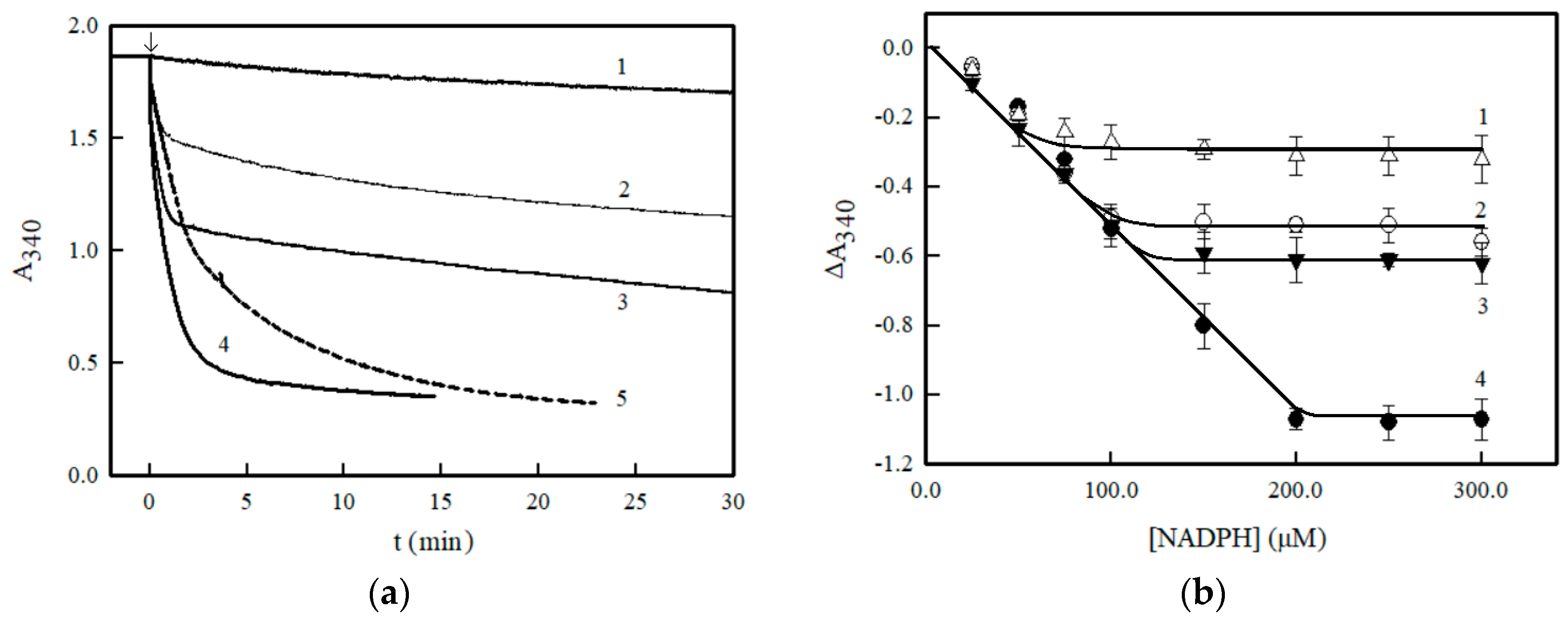
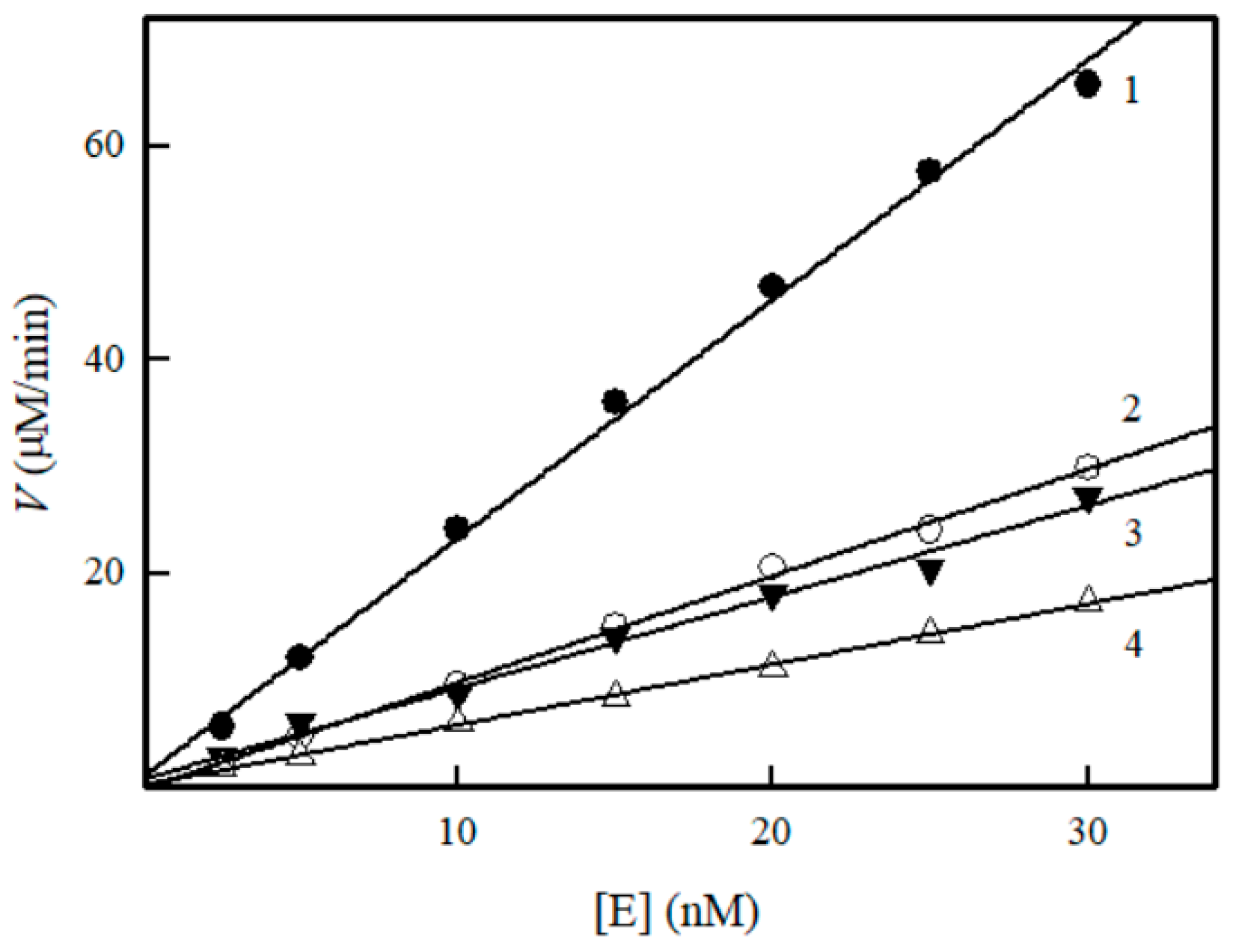
2. Results
3. Discussion
4. Materials and Methods
4.1. Enzymes and Chemicals
4.2. Enzymatic Assays
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 1,4-NQ | 1,4-naphthoquinone |
| 2-OH-1,4-NQ | 2-hydroxy-1,4-naphthoquinone |
| 5-OH-1,4-NQ | 5-hydroxy-1,4-naphthoquinone; |
| Ar-NO2 | aromatic nitrocompound |
| FMN | flavin mononucleotide |
| FNR | ferredoxin:NADP+ reductase |
| kcat(app.) | turnover number |
| kcat/Km(app.) | bimolecular rate constant |
| NAD(P)H | reduced nicotinamide adenine dinucleotide or its phosphate |
| NfsA | E. coli nitroreductase-A |
| P-450R | NADPH:cytochrome P-450 reductase |
| TNT | 2,4,6-trinitrotoluene |
References
- Čėnas, N.; Čėnienė, A.; Nivinskas, H.; Anusevičius, Ž.; Šarlauskas, J. Quantitative structure-activity relationships in enzymatic single-electron reduction of nitroaromatic explosives: Implications for their cytotoxicity. Biochim. Biophys. Acta 2001, 1528, 31–38. [Google Scholar] [CrossRef]
- Williams, E.M.; Little, R.F.; Mowday, A.M.; Rich, M.H.; Chan-Hyams, J.V.E.; Copp, J.N.; Smaill, J.B.; Patterson, A.V.; Ackerley, D.F. Nitroreductase gene-directed enzyme prodrug therapy: Insights and advances toward clinical utility. Biochem. J. 2015, 471, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Talmage, S.S.; Opresko, D.M.; Maxwell, C.J.; Welsh, C.J.; Cretella, F.M.; Reno, P.H.; Daniel, F.B. Nitroaromatic munition compounds: Environmental effects and screening values. Rev. Environ. Contam. Toxicol. 1999, 161, 1–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Rylott, E.L.; Bruce, N.C.; Strand, S.E. Phytodetoxification of TNT by transplastomic tobacco (Nicotiana tabacum) expressing a bacterial nitroreductase. Plant Mol. Biol. 2017, 95, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Haynes, C.A.; Koder, R.L.; Miller, A.F.; Rodgers, D.W. Structures of nitroreductase in three states. Effects of inhibitor binding and reduction. J. Biol. Chem. 2002, 277, 11513–11520. [Google Scholar] [CrossRef] [PubMed]
- Koder, R.L.; Miller, A.F. Steady-state kinetic mechanism, stereospecificity, substrate and inhibitor specificity of Enterobacter cloacae nitroreductase. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1998, 1387, 395–405. [Google Scholar] [CrossRef]
- Lovering, A.L.; Hyde, E.I.; Searle, P.F.; White, S.A. The structure of Escherichia coli nitroreductase complexed with nicotinic acid: Three crystal forms at 1.7 Å, 1.8 Å and 2.4 Å resolution. J. Mol. Biol. 2001, 309, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Nivinskas, H.; Koder, R.L.; Anusevičius, Ž.; Šarlauskas, J.; Miller, A.F.; Čėnas, N. Quantitative structure-activity relationships in two-electron reduction of nitroaromatic compounds by Enterobacter cloacae NAD(P)H:nitroreductase. Arch. Biochem. Biophys. 2001, 385, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Valiauga, B.; Williams, E.M.; Ackerley, D.F.; Čėnas, N. Reduction of quinones and nitroaromatic compounds by Escherichia coli nitroreductase A (NfsA): Characterization of kinetics and substrate specificity. Arch. Biochem. Biophys. 2017, 614, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Koder, R.L.; Haynes, C.A.; Rodgers, M.E.; Rodgers, D.W.; Miller, A.F. Flavin thermodynamics explain the oxygen insensitivity of enteric nitroreductases. Biochemistry 2002, 41, 14197–14205. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, P.; Kassel, M.A.; Feinberg, B.A.; Corbett, M.D.; McClelland, R.A. Reduction potentials in relation to physiological activities of benzenoid and heterocyclic nitroso compounds: Comparison with the nitro precursors. Bioorg. Chem. 1990, 18, 265–275. [Google Scholar] [CrossRef]
- Race, P.R.; Lovering, A.L.; Green, R.M.; Ossor, A.; White, S.A.; Searle, P.F.; Wrighton, C.J.; Hyde, E.I. Structural and mechanistic studies of Escherichia coli nitroreductase with the antibiotic nitrofurazone: Reversed binding orientations in different redox states of the enzyme. J. Biol. Chem. 2005, 280, 13256–13264. [Google Scholar] [CrossRef] [PubMed]
- Leskovac, V.; Svirčevič, J.; Trivić, S.; Popović, M.; Radulović, M. Reduction of aryl-nitroso compounds by pyridine and flavin coenzymes. Int. J. Biochem. 1989, 21, 825–834. [Google Scholar] [CrossRef]
- Knox, R.; Friedlos, F.; Biggs, P.; Flitter, W.; Gaskell, M.; Goddard, P.; Davies, L.; Jarman, M. Identification, synthesis and properties of 5-(aziridin-1-yl)-2-nitro-4-nitrosobenzamide, a novel DNA crosslinking agent derived from CB1954. Biochem. Pharmacol. 1993, 46, 797–803. [Google Scholar] [CrossRef]
- Uršič, S.; Vrčik, V.; Ljubas, D.; Vinkovič, I. Interaction of L-ascorbate with substituted nitrosobenzenes. Role of the ascorbate 2-OH group in antioxidant reactions. New J. Chem. 1998, 22, 221–223. [Google Scholar] [CrossRef]
- Yang, J.; Zhan, J.; Bai, J.; Liu, P.; Xue, Y.; Yang, Q. Residue Phe42 is critical for the catalytic activity of Escherichia coli major nitroreductase NfsA. Biotechnol. Lett. 2013, 35, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.N.R.; Harman, L.; Motten, A.; Schreiber, J.; Mason, R.P. Generation of radical anions of nitrofurantoin, misonidazole, and metronidazole by ascorbate. Arch. Biochem. Biophys. 1987, 255, 419–427. [Google Scholar] [CrossRef]
- Nivinskas, H.; Staškevičienė, S.; Šarlauskas, J.; Koder, R.L.; Miller, A.F.; Čėnas, N. Two-electron reduction of quinones by Enterobacter cloacae NAD(P)H:nitroreductase: Quantitative structure-activity relationships. Arch. Biochem. Biophys. 2002, 403, 249–258. [Google Scholar] [CrossRef]
- O’Brien, P.J. Molecular mechanisms of quinone cytotoxicity. Chem. Biol. Interact. 1991, 80, 1–41. [Google Scholar] [CrossRef]
- Roginsky, V.A.; Barsukova, T.K.; Stegmann, H.B. Kinetics of redox interaction between substituted quinones and ascorbate under aerobic conditions. Chem. Biol. Interact. 1999, 121, 177–197. [Google Scholar] [CrossRef]
- Awano, H.; Hirabayashi, T.; Tagaki, W. Acid catalyzed reduction of nitrosobenzene by 3,5-dipyrrolidinocarbamoyl-N-benzyl-1,4-dihydropyridine as a NADH analog. Tetrahedron Lett. 1984, 25, 2005–2008. [Google Scholar] [CrossRef]
- Cleland, W.W. Steady State Kinetics. Enzymes 1970, 2, 1–65. [Google Scholar] [CrossRef]
- Haïdour, A.; Ramos, J.L. Identification of products resulting from the biological reduction of 2,4,6-trinitrotoluene, 2,4-dinitrotoluene, and 2,6-dinitrotoluene by Pseudomonas sp. Environ. Sci. Technol. 1996, 30, 2365–2370. [Google Scholar] [CrossRef]
- Sayama, M.; Mori, M.; Nakada, Y.; Kagamimori, S.; Kozuka, H. Metabolism of 2,4-dinitrotoluene by Salmonella typhimurium strains TA98, TA98NR and TA98/1,8-DNP6, and mutagenicity of the metabolites of 2,4-dinitrotoluene and related compounds to strains TA98 and TA100. Mutat. Res. Lett. 1991, 264, 147–153. [Google Scholar] [CrossRef]
- Prosser, G.A.; Copp, J.N.; Syddall, S.P.; Williams, E.M.; Smaill, J.B.; Wilson, W.R.; Patterson, A.V.; Ackerley, D.F. Discovery and evaluation of Escherichia coli nitroreductases that activate the anti-cancer prodrug CB1954. Biochem. Pharmacol. 2010, 79, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Kröger, M.; Fels, G. 14C-TNT synthesis revisited. J. Label. Compd. Radiopharm. 2000, 43, 217–227. [Google Scholar] [CrossRef]
- Hughes, E.D.; Ingold, C.; Pearson, R.B. Nitration at nitrogen and oxygen centres. Part I. Kinetics and mechanism of the conversion of secondary amines into nitroamines. J. Chem. Soc. 1958, 4357–4365. [Google Scholar] [CrossRef]
- Alizadeh, M.H.; Tayebee, R. Catalytic oxidation of aniline by aqueous hydrogen peroxide in the presence of some heteropolyoxometalates. J. Braz. Chem. Soc. 2005, 16, 108–111. [Google Scholar] [CrossRef]
| No | Oxidant | E17 (V) | kcat (s−1) | kcat/Km (M−1·s−1) |
|---|---|---|---|---|
| 1 | Tetryl | −0.16 | 110.0 ± 13.0 1 | 7.9 ± 0.9 × 106 |
| 2 | TNT | −0.25 | 135.1 ± 12.3 1 | 2.7 ± 0.3 × 106 |
| 3 | p-Nitroacetophenone | −0.36 | 59.4 ± 5.2 2 | 1.5 ± 0.2 × 105 |
| 4 | p-Nitrobenzoic acid | −0.43 | 64.3 ± 7.0 2 | 2.5 ± 0.5 × 103 |
| 5 | Nitrobenzene | −0.49 | 14.2 ± 2.0 2 | 1.5 ± 0.3 × 103 |
| No | Oxidant | −Ascorbate | +Ascorbate | ||
|---|---|---|---|---|---|
| kcat(app.) (s−1) | kcat/Km (M−1·s−1) | kcat(app.) (s−1) | kcat/Km (M−1·s−1) | ||
| 1 | TNT (50 µM) | 52.0 ± 5.5 | 3.85 ± 0.36 × 106 | 23.0 ± 3.5 | 1.56 ± 0.22 × 106 |
| 2 | Tetryl (50 µM) | 41.8 ± 7.0 | 3.78 ± 0.50 × 106 | 16.1 ± 3.0 | 2.08 ± 0.23 × 106 |
| 3 | p-Nitroacetophenone (0.5 mM) | 50.8 ± 6.2 | 3.20 ± 0.42 × 106 | 20.1 ± 3.5 | 1.62 ± 0.21 × 106 |
| 4 | p-Nitrobenzoic acid (2.0 mM) | 30.6 ± 4.5 | 3.30 ± 0.42 × 106 | 19.7 ± 1.2 | 2.05 ± 0.30 × 106 |
| 5 | Nitrobenzene (1.5 mM) | 4.6 ± 0.3 1 | n.d. 2 | 3.1 ± 0.2 1 | n.d. 2 |
| 6 | 5-OH-1,4-NQ (50 µM | 19.4 ± 2.9 | 1.60 ± 0.22 × 106 | 18.3 ± 2.2 1 | n.d. 3 |
| 7 | 1,4-NQ (50 µM) | 26.0 ± 3.2 | 1.52 ± 0.18 × 106 | 22.0 ± 3.0 1 | n.d. 3 |
| 8 | 2-OH-1,4-NQ (20 µM) | 26.5 ± 1.4 | 1.70 ± 0.22 × 106 | 26.2 ± 3.7 | 1.46 ± 0.23 × 106 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valiauga, B.; Misevičienė, L.; Rich, M.H.; Ackerley, D.F.; Šarlauskas, J.; Čėnas, N. Mechanism of Two-/Four-Electron Reduction of Nitroaromatics by Oxygen-Insensitive Nitroreductases: The Role of a Non-Enzymatic Reduction Step. Molecules 2018, 23, 1672. https://doi.org/10.3390/molecules23071672
Valiauga B, Misevičienė L, Rich MH, Ackerley DF, Šarlauskas J, Čėnas N. Mechanism of Two-/Four-Electron Reduction of Nitroaromatics by Oxygen-Insensitive Nitroreductases: The Role of a Non-Enzymatic Reduction Step. Molecules. 2018; 23(7):1672. https://doi.org/10.3390/molecules23071672
Chicago/Turabian StyleValiauga, Benjaminas, Lina Misevičienė, Michelle H. Rich, David F. Ackerley, Jonas Šarlauskas, and Narimantas Čėnas. 2018. "Mechanism of Two-/Four-Electron Reduction of Nitroaromatics by Oxygen-Insensitive Nitroreductases: The Role of a Non-Enzymatic Reduction Step" Molecules 23, no. 7: 1672. https://doi.org/10.3390/molecules23071672
APA StyleValiauga, B., Misevičienė, L., Rich, M. H., Ackerley, D. F., Šarlauskas, J., & Čėnas, N. (2018). Mechanism of Two-/Four-Electron Reduction of Nitroaromatics by Oxygen-Insensitive Nitroreductases: The Role of a Non-Enzymatic Reduction Step. Molecules, 23(7), 1672. https://doi.org/10.3390/molecules23071672






