Isolation, Structural Elucidation of Three New Triterpenoids from the Stems and Leaves of Schisandra chinensis (Turcz) Baill.
Abstract
:1. Introduction
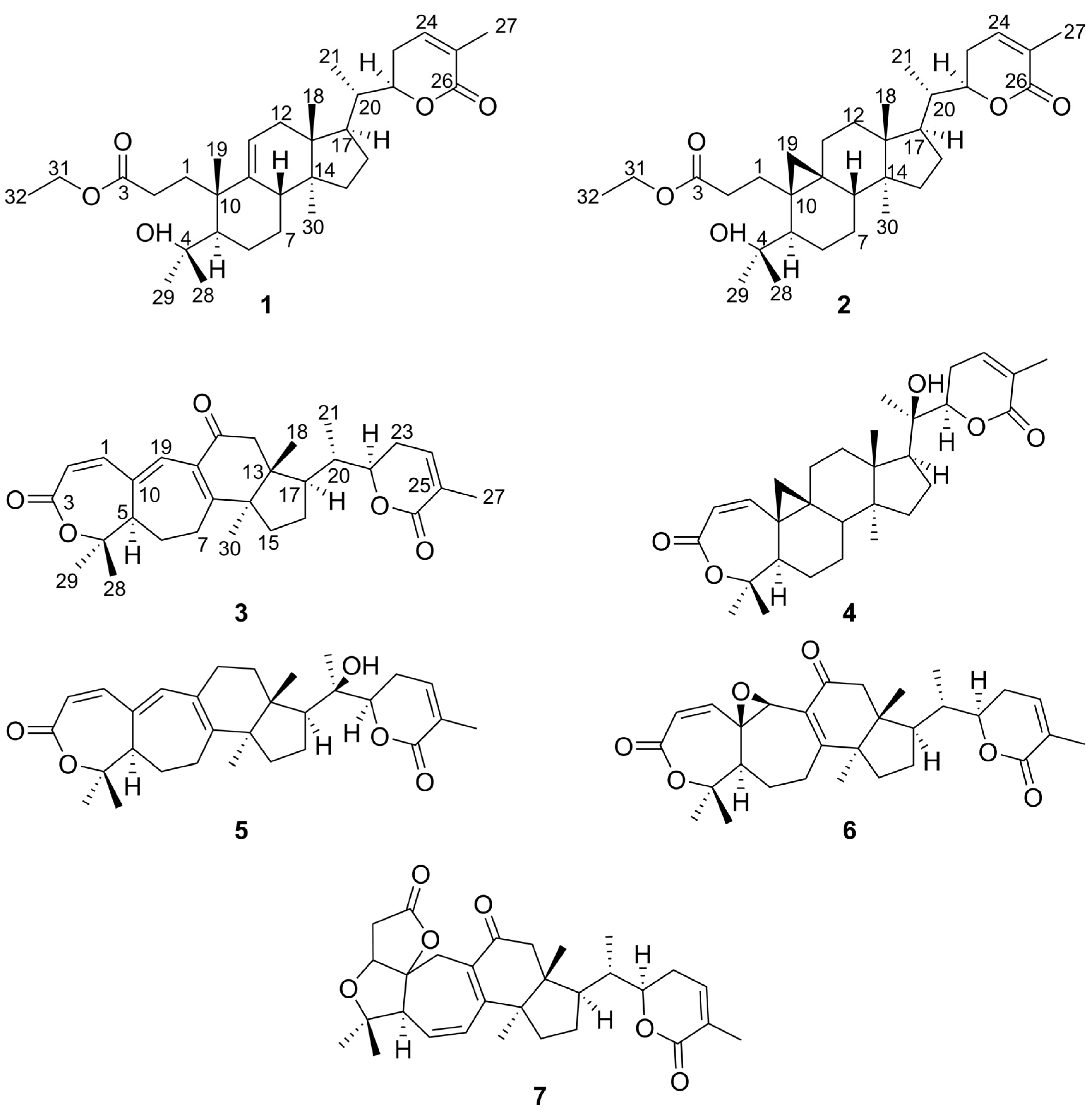
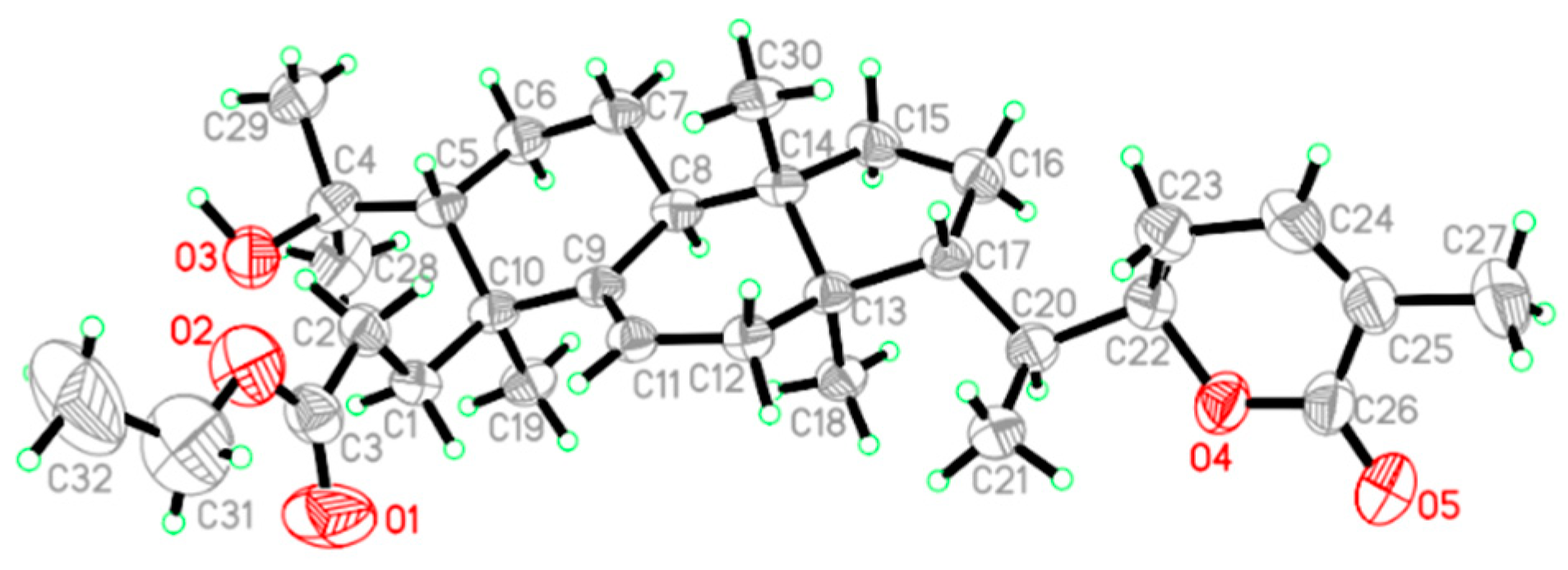
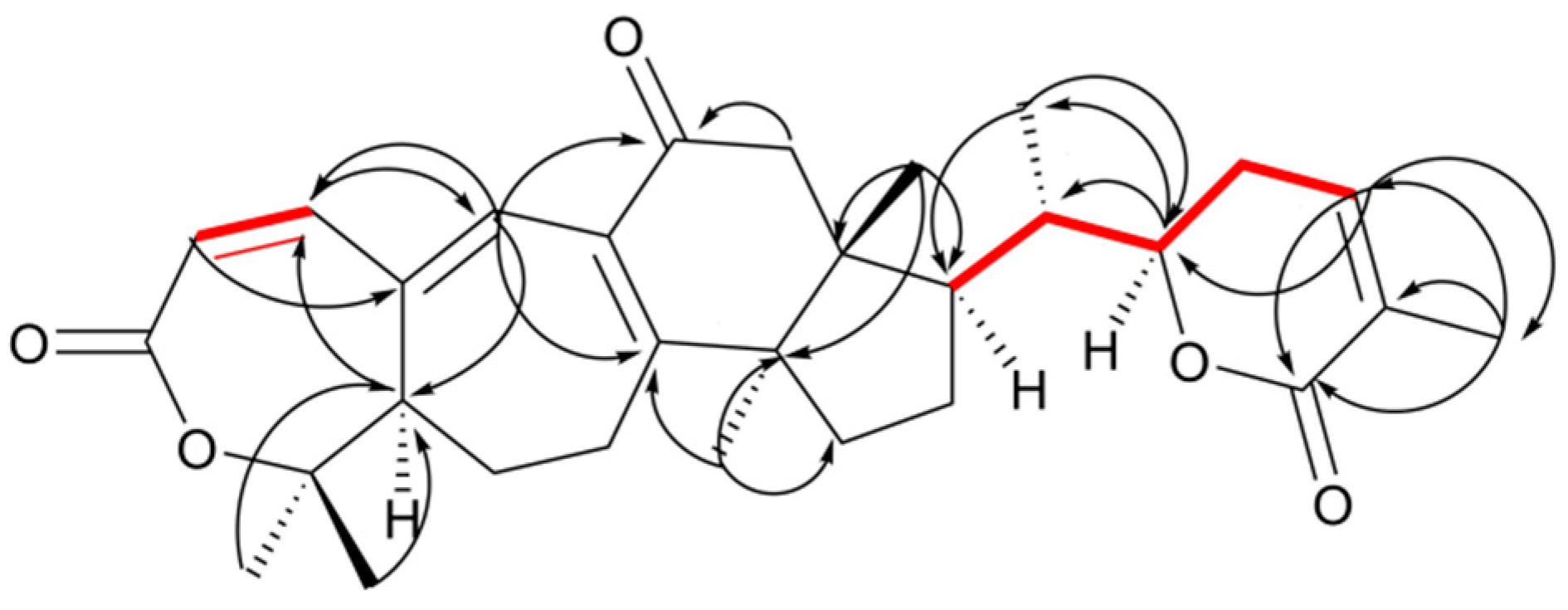
2. Results and Discussion
Structure Elucidation
3. Materials and Methods
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. MTT Assay for Measuring Cell Cytotoxicity
3.5. Experimental Data of Identified Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, S.X.; Li, R.T.; Liu, J.P.; Lu, Y.; Chang, Y.; Lei, C.; Xiao, W.L.; Yang, L.B.; Zheng, Q.T.; Sun, H.D. Isolation and characterization of biogenetically related highly oxygenated nortriterpenoids from Schisandra chinensis. Org. Lett. 2007, 9, 2079–2082. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Ekiert, R.; Ekiert, H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem. Rev. 2017, 16, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Klimek-Szczykutowicz, M.; Kokotkiewicz, A.; Maslanka, A.; Krol, A.; Luczkiewicz, M.; Ekiert, H. Phytochemical and biotechnological studies on Schisandra chinensis cultivar Sadova No. 1-a high utility medicinal plant. Appl. Microbiol. Biotechnol. 2018, 102, 5105–5120. [Google Scholar] [CrossRef] [PubMed]
- Hancke, J.L.; Burgos, R.A.; Ahumada, F. Schisandra chinensis (Turcz.) Baill. Fitoterapia 1999, 70, 451–471. [Google Scholar] [CrossRef]
- Song, J.; Zhou, M.; Zhou, J.; Liang, J.J.; Peng, X.G.; Liu, J.; Ruan, H.L. Schincalactones A and B, Two 5/5/6/11/3 Fused Schinortriterpenoids with a 13-Membered Carbon Ring System from Schisandra incarnata. Org. Lett. 2018, 20, 2499–2502. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.L.; Yang, L.M.; Gong, N.B.; Wu, L.; Wang, R.R.; Pu, J.X.; Li, X.L.; Huang, S.X.; Zheng, Y.T.; Li, R.T.; et al. Rubriflordilactones A and B, two novel bisnortriterpenoids from Schisandra rubriflora and their biological activities. Org. Lett. 2006, 8, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Wikman, G. Pharmacology of Schisandra chinensis Bail: An overview of Russian research and uses in medicine. J. Ethnopharmacol. 2008, 118, 183–212. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.Y.; Hung, T.M.; Bae, K.H.; Shin, E.M.; Zhou, H.Y.; Hong, Y.N.; Kang, S.S.; Kim, H.P.; Kim, Y.S. Anti-inflammatory effects of schisandrin isolated from the fruit of Schisandra chinensis Baill. Eur. J. Pharmacol. 2008, 591, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, G.Z.; Chen, Y.; Liao, M.C.; Liu, X.M.; Chen, S.; Liu, L.; Lei, X.X. Terpenes from Schisandra sphenanthera. Helv. Chim. Acta 2011, 94, 491–496. [Google Scholar] [CrossRef]
- Shen, Y.C.; Lin, Y.C.; Chiang, M.Y.; Yeh, S.F.; Cheng, Y.B.; Liao, C.C. Kadsuphilactones A and B, two new triterpene dilactones from kadsuraphilippinensis. Org. Lett. 2005, 7, 3307–3310. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.S.; Huang, M.F. On the structures of schisanlactone C and schisanlactone D, two new triterpene lactones from Schisandra sp. Acta Chim. Sin. 1984, 42, 464–469. [Google Scholar]
- Song, Q.Y.; Jiang, K.; Zhao, Q.Q.; Gao, K.; Jin, X.J.; Yao, X.J. Eleven new highly oxygenated triterpenoids from the leaves and stems of Schisandra chinensis. Org. Biomol. Chem. 2013, 11, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Y.; Wang, W.G.; Li, H.M.; Zhang, R.B.; Li, H.Z.; Li, R.T. Schisanlactone H and sphenanthin A, new metabolites from Schisandra sphenanthera. J. Asian Nat. Prod. Res. 2009, 11, 861–866. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Pu, J.X.; Huang, S.X.; Wang, Y.Y.; Xiao, W.L.; Li, L.M.; Liu, J.P.; Zhang, H.B.; Li, Y.; Sun, H.D. Schinalactone A, a new cytotoxic triterpenoid from Schisandra sphenanthera. Org. Lett. 2010, 12, 1208–1211. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.F.; Zhang, S.X.; Wang, H.K.; Zhang, S.Y.; Sun, Q.Z.; Cosentino, L.M.; Lee, K.H. Novel anti-HIV lancilactone C and related triterpenes from Kadsura lancilimba. J. Nat. Prod. 1999, 62, 94–97. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |
| 1 | 2.03, 2.73, m | 33.66 | 1.33, 2.70, m | 31.55 | 6.94, d, (12.32) | 145.70 |
| 2 | 2.30, 2.53, m | 30.90 | 2.19, 2.71, m | 33.18 | 5.82, d, (12.09) | 119.11 |
| 3 | 177.05 | 176.31 | 169.08 | |||
| 4 | 76.05 | 76.84 | 82.15 | |||
| 5 | 1.46, m | 51.46 | 1.88, m | 46.59 | 2.52, m | 50.01 |
| 6 | 1.21, 1.60, m | 28.38 | 0.72, 1.44, m | 26.44 | 2.15, 2.43 ,m | 30.73 |
| 7 | 1.54, 1.74, m | 27.36 | 1.04, 1.29, m | 27.03 | 2.27, 2.58, m | 39.19 |
| 8 | 2.15, m | 44.84 | 1.36, m | 50.24 | 176.40 | |
| 9 | 146.02 | 23.88 | 133.01 | |||
| 10 | 45.83 | 28.01 | 142.70 | |||
| 11 | 5.46, d, (5.85) | 118.63 | 1.27, 2.21, m | 27.64 | 199.85 | |
| 12 | 1.98, 2.23, m | 38.96 | 1.72, m | 34.28 | 2.52, 2.83, m | 49.93 |
| 13 | 45.29 | 46.57 | 48.61 | |||
| 14 | 48.13 | 49.77 | 54.39 | |||
| 15 | 1.42, 1.45, m | 34.95 | 1.39, m | 37.23 | 1.62, 1.88, m | 31.89 |
| 16 | 1.45, 1.83, m | 27.69 | 1.41, 1.83, m | 27.97 | 1.56, 1.98, m | 26.34 |
| 17 | 1.72, m | 48.01 | 1.69, m | 49.46 | 1.96, m | 47.10 |
| 18 | 3H, 0.75, s | 14.93 | 3H, 1.05, s | 18.91 | 3H, 0.92, s | 17.50 |
| 19 | 3H, 1.21, s | 27.77 | 0.55, 0.72, m | 32.40 | 6.72, s | 138.92 |
| 20 | 1.97, m | 40.59 | 1.96, m | 40.64 | 1.99, m | 40.64 |
| 21 | 3H, 1.00, d, (6.61) | 13.49 | 3H, 0.98, d, (6.49) | 13.37 | 3H, 0.99, d, (6.43) | 13.70 |
| 22 | 4.51, dt, (3.57, 13.22) | 82.24 | 4.51, dt, (3.60,13.24) | 82.28 | 4.51, dt, (3.53,13.14) | 81.69 |
| 23 | 2.23, 2.40, m | 24.37 | 2.23, 2.40, m | 24.39 | 2.26, 2.38, m | 24.41 |
| 24 | 6.76, d, (6.47) | 142.32 | 6.76, d, (6.47) | 142.38 | 6.74, d, (6.38) | 142.18 |
| 25 | 128.61 | 128.64 | 128.66 | |||
| 26 | 168.87 | 168.94 | 168.70 | |||
| 27 | 3H, 1.87, s | 16.96 | 3H, 1.86, s | 16.99 | 3H, 1,84, s | 16.95 |
| 28 | 3H, 1.27, s | 28.26 | 3H, 1.20, s | 26.23 | 3H, 1.50, s | 26.28 |
| 29 | 3H, 1.28, s | 33.44 | 3H, 1.21, s | 31.30 | 3H, 1.37, s | 29.11 |
| 30 | 3H, 0.79, s | 18.81 | 3H, 0.98, s | 20.09 | 3H, 1.32, s | 27.25 |
| 31 | 4.11, q, (7.13) | 61.40 | 4.08, q, (7.12) | 61.39 | ||
| 32 | 3H, 1.25, t, (7.14) | 14.57 | 3H, 1.22, t, (7.15) | 14.58 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, F.; Liu, H.; Duan, H.; Chen, P.; Lu, S.-J.; Yang, G.-Z.; Lei, X.-X. Isolation, Structural Elucidation of Three New Triterpenoids from the Stems and Leaves of Schisandra chinensis (Turcz) Baill. Molecules 2018, 23, 1624. https://doi.org/10.3390/molecules23071624
Qiu F, Liu H, Duan H, Chen P, Lu S-J, Yang G-Z, Lei X-X. Isolation, Structural Elucidation of Three New Triterpenoids from the Stems and Leaves of Schisandra chinensis (Turcz) Baill. Molecules. 2018; 23(7):1624. https://doi.org/10.3390/molecules23071624
Chicago/Turabian StyleQiu, Feng, Han Liu, Huan Duan, Pian Chen, Shao-Juan Lu, Guang-Zhong Yang, and Xin-Xiang Lei. 2018. "Isolation, Structural Elucidation of Three New Triterpenoids from the Stems and Leaves of Schisandra chinensis (Turcz) Baill." Molecules 23, no. 7: 1624. https://doi.org/10.3390/molecules23071624
APA StyleQiu, F., Liu, H., Duan, H., Chen, P., Lu, S.-J., Yang, G.-Z., & Lei, X.-X. (2018). Isolation, Structural Elucidation of Three New Triterpenoids from the Stems and Leaves of Schisandra chinensis (Turcz) Baill. Molecules, 23(7), 1624. https://doi.org/10.3390/molecules23071624





