Isoflavones Production and Possible Mechanism of Their Exudation in Genista tinctoria L. Suspension Culture after Treatment with Vanadium Compounds
Abstract
1. Introduction
2. Results
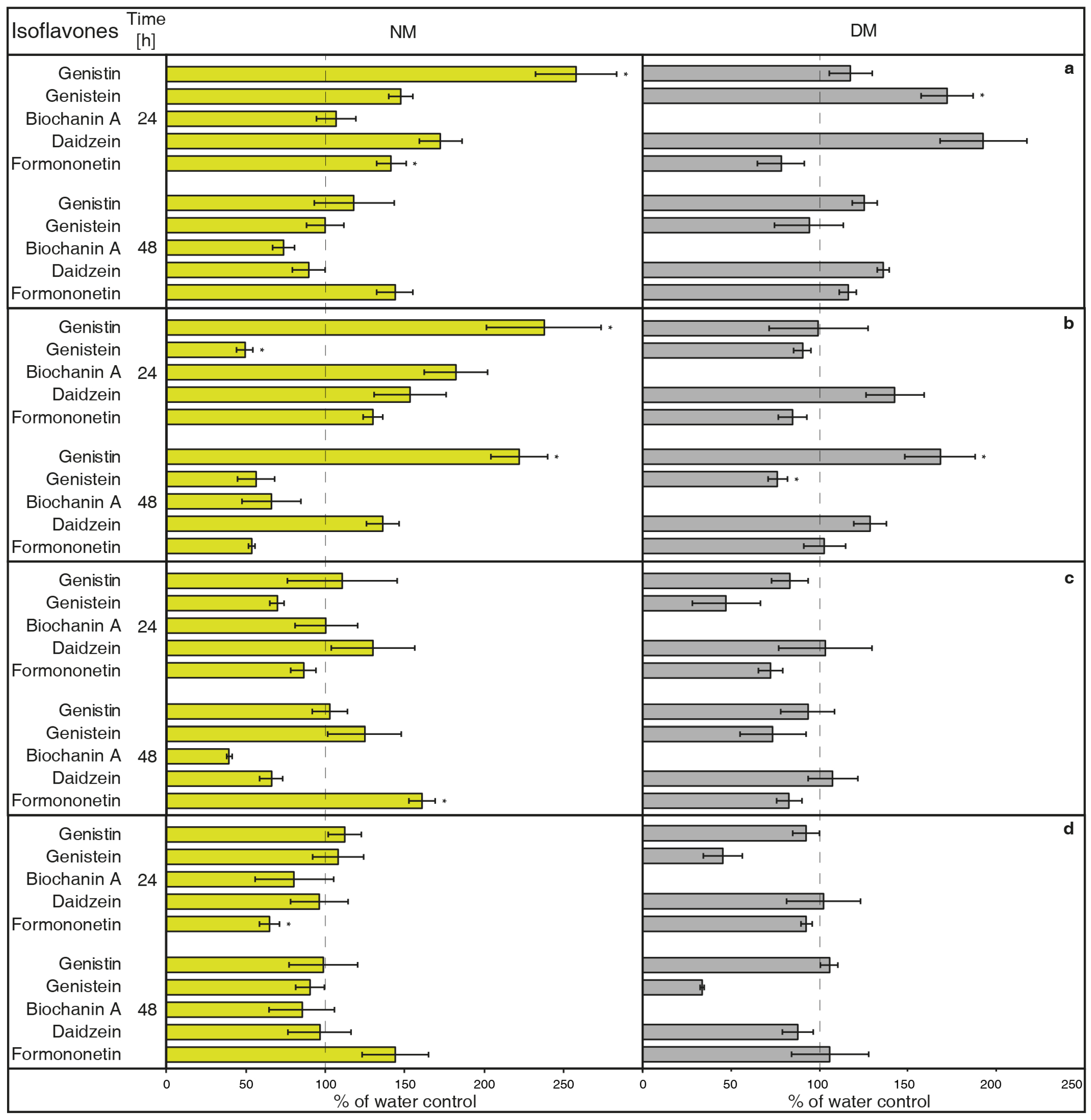
2.1. The Effect of Vanadium Compounds
- NH4VO3 (1 μM, Figure 1A) has the potential to increase isoflavone biosynthesis and possible metabolites exudation in G. tinctoria suspension culture. This compound significantly increased the content of genistin and formononetin in the NM. Genistein and daidzein content also visibly elevated in the NM after 24 h, but their release was statistically insignificant. Samples of DM only had significantly more genistein after 24 h, however, concentration of DM was higher in comparison with the rest of isoflavones. The amount of biochanin A was not traceable in DM and NH4VO3 (1 μM) took no effect on the release of this isoflavone. Overall, longer cultivation did not cause higher production or exudation of monitored isoflavones.
- NH4VO3 (10 μM, Figure 1B) also caused a significant increase of genistin content in the NM again after 24 h, but not of formononetin. Moreover, the content of genistein was significantly lower than water control in the NM after 24 h and in the DM after 48 h. In spite of previously used concentration of this elicitor, the content of genistin increased in both NM and DM after 48 h. NH4VO3 (10 μM) provided no evidence that it is capable of stimulating conceivable production or releasing remaining isoflavones, although daidzein content again was a little higher in both types of samples.
- VOSO4 (1 μM, Figure 1C), as the second tested vanadium compound, caused significant release in the case of formononetin content in the NM after 48 h. There was no verifiable difference of other isoflavones content in water control and tested samples after application of this elicitor.
- In a similar way, VOSO4 (10 μM, Figure 1D) again caused elevation of formononetin levels in the same samples after 48, but this result was insignificant. Moreover, cultivation for 24 h had a negative effect on this isoflavone presence in the NM. There was a non-significant reduction in genistein content in the DM after 24 h. This result was similar to the genistein value after VOSO4 (1 μM) application (Figure 1C); VOSO4 did not manifest as a potential elicitor for remaining isoflavones.
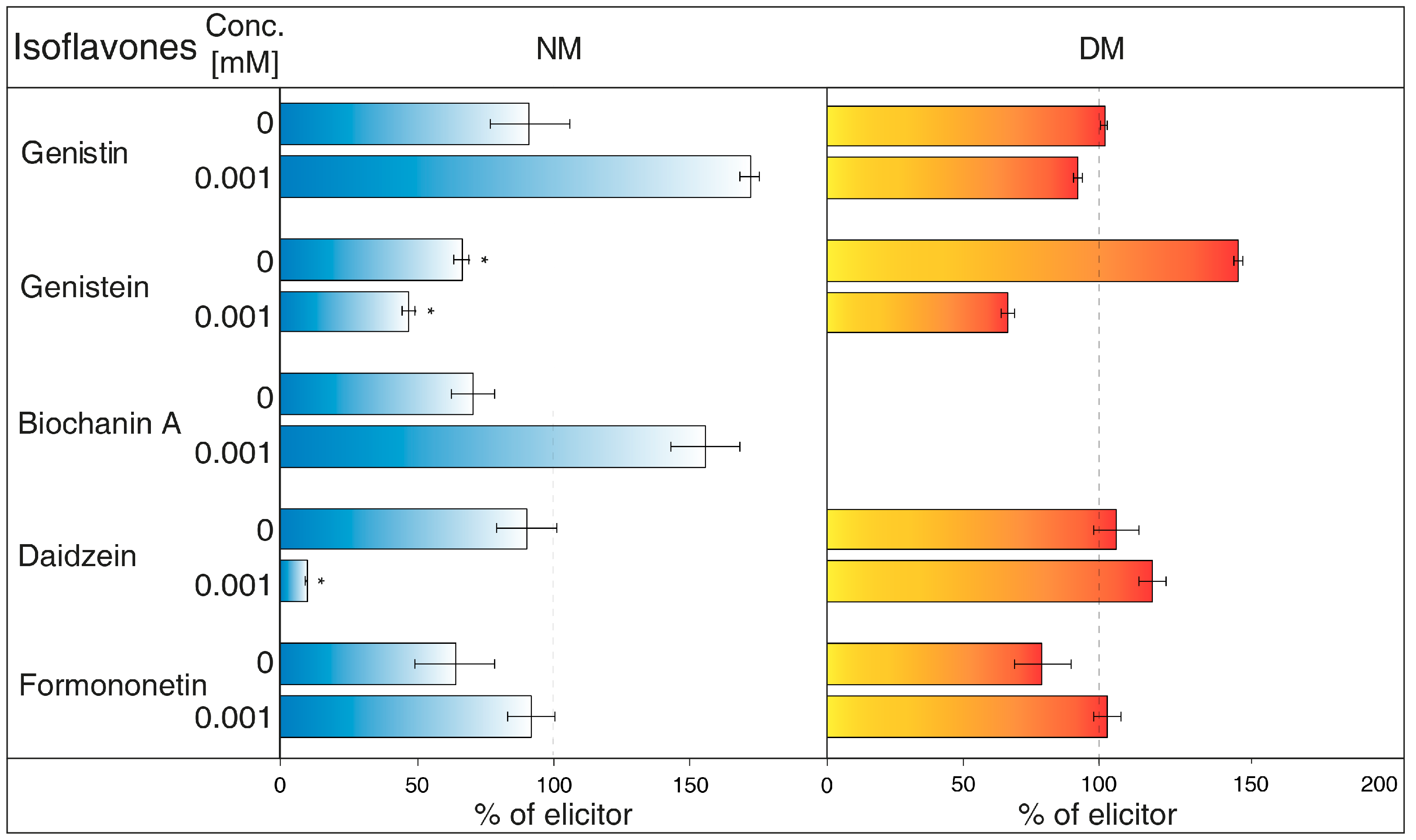
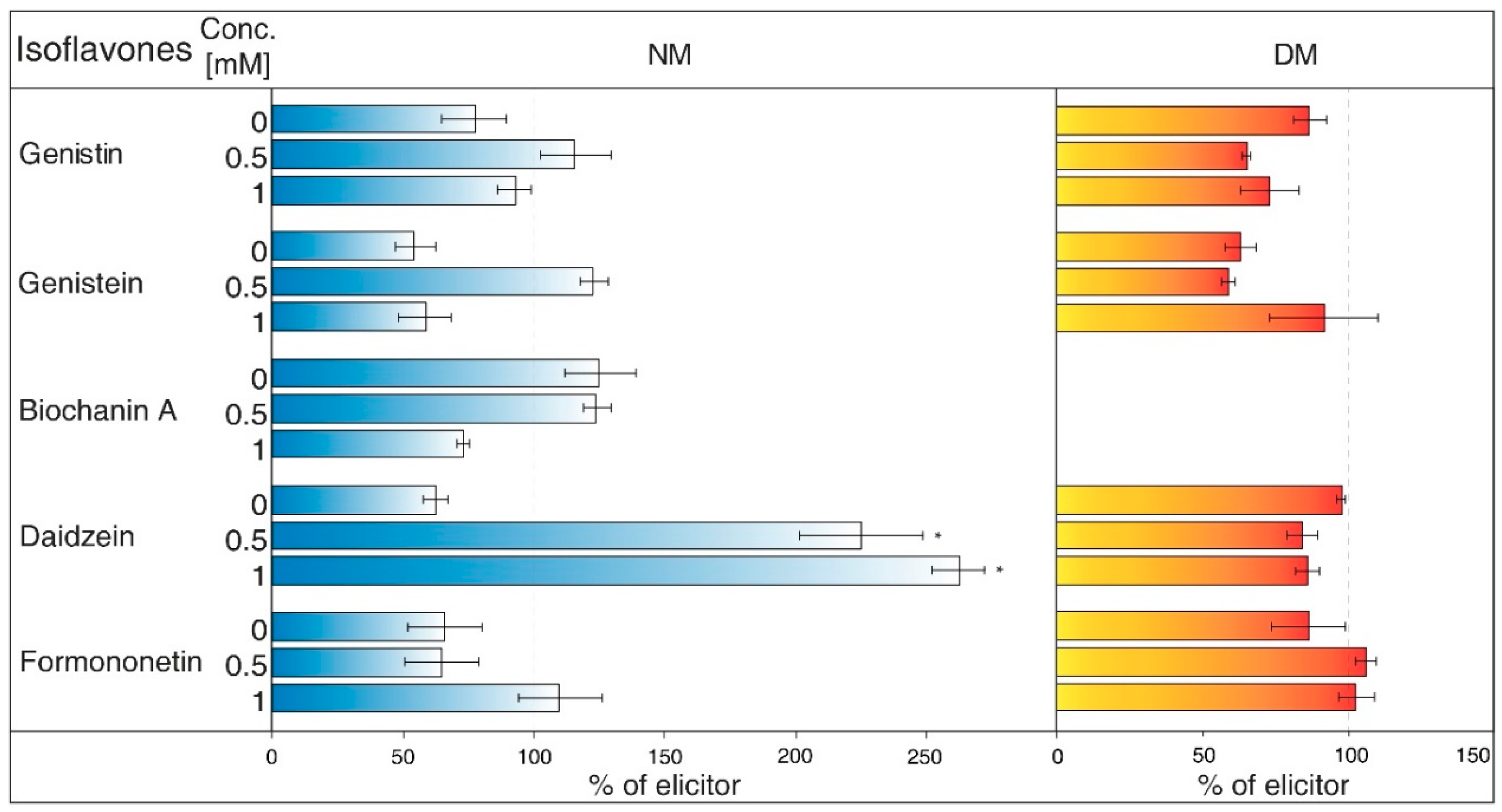
2.2. The Effects of Transport Mechanism Inhibitors
- NH4Cl (Figure 2) is ranked between protonophores [24]. It can disrupt the proton gradient and inhibit transport of certain substances, such as nicotine [25]. NH4Cl reduced the content of most isoflavones in NM of G. tinctoria cell culture, when only genistein (1 mM, 10 mM) and daidzein (10 mM) had significant values in comparison with NH4VO3 treated samples. In DM, NH4Cl had a specific effect, where it significantly decreased formononetin content. Increased genistin and genistein presence in DM was only evident in comparison with water control (not labelled in the figures).
- Gramicidin (Figure 3) is an antibiotic formed by Bacillus brevis, which acts as a selective ionophore for cations. Gramicidin disrupted processes associated with potassium ions [26], and eventually transport nicotine in a similar way to NH4Cl [25]. The levels of genistein and daidzein significantly decreased in the NM after the application of this inhibitor. Gramicidin, however, did not affect the presence of these two aglycones in DM, as well as other isoflavones in the tested samples. The results also document that the content of genistin and biochanin A was statistically higher in NM after inhibitor application in comparison with the water control (not labelled in the figures).
- Brefeldin A (Figure 4) is a macrolide lactone produced by specific ascomycetes [27]. This inhibitor suppresses the guanine nucleotide exchange factor involved in the vesicular transport of molecules [28]. It also dissolves the Golgi apparatus that contributes on various molecules movement [29]. Brefeldin A suppressed content of some isoflavones content in the NM with significant decrease of genistein, daidzein (2.5 and 5 μM), and formononetin (5 μM). Reduced concentrations of these metabolites could not be verified in NM after treatment with other solutions of this inhibitor. On the other hand, brefeldin A caused a conclusive increase in the levels of genistein (5 μM) and biochanin A (2.5 and 5 μM) in the NM. This inhibitor has no significant effect on any isoflavone content in the DM.
- Na3VO4 (sodium orthovanadate; Figure 5) is known and used as a plasma membrane (PM) H+-ATPase inhibitor. Application of this substance to cell culture of Eschscholtzia californica caused gradual alkalisation of surrounding media and a lower rate of excretion of benzophenanthridine alkaloids [30]. In addition, the inhibition caused by Na3VO4 was discussed for the ABC transporter in Salmonella typhimurium [31] and similar transporters in the plants [32]. Na3VO4 affected isoflavone content according to used concentration. The application of Na3VO4 (1 mM) strongly reduced the content of genistein and daidzein in the NM, but the less concentrated solution only caused the decrease of genistein concentration. Na3VO4 had a negative effect on genistin and genistein content in DM, in a case of significant glycoside. Remaining isoflavones also had no greater difference in their amount in these samples.
- Verapamil (Figure 6) inhibits the activity of calcium transport channels, but also has a suppressing effect on multidrug resistance protein 1 (MDR1), a subfamily of ABC proteins. This effect could help with overcoming the tolerance of some drugs caused by P-glycoprotein [33], and Thalictrum minus accumulated alkaloid berberine within the cells after this inhibitor application [34]. Verapamil did not cause a significant decrease in any studied isoflavone levels in the NM, but a small reduction for all compounds, except for daidzein, was found. This particular aglycone had a higher concentration than the water control (not labelled in the figures). There was also no verifiable change of isoflavones amount in DM after verapamil application.
- Probenecid (Figure 7) primarily affects the excretion of uric acid in kidneys. This drug can have an effect on the ABC transporters, multidrug resistance-associated protein 1 and 2 subfamily (MRP1 and MRP2), and inhibits the transfer of organic anions [24]. After probenecid treatment, a statistically significant reduction of genistein (0.5 mM) and daidzein (0.5 and 1 mM) was measured in the NM. However, their content did not change significantly in the DM, as well as those of other isoflavones. On the other hand, this inhibitor also positively affects the concentration of genistin (1 mM) and biochanin A in a medium compared with water control samples.
- Glibenclamide (Figure 8) is a drug that acts primarily on ABC proteins in pancreatic B-cells. This drug inhibited the activity of MRP1 in the lung tumour [35], as well as AtMRP5 in Arabidopsis thaliana [36]. No isoflavone had a significantly lower content in the NM after glibenclamide application. Nevertheless, there was some reduction of isoflavones (genistein, biochanin A, formononetin). On the contrary, glibenclamide positively affected daidzein content in the NM after administration of both concentrations of the inhibitor, resulting in the opposite effect compared with probenecid. The same effect was found for genistein (0.5 mM) in spite of the water control.
3. Discussion
3.1. Impact of Vanadium Compounds
3.2. Transport of Isoflavones across Membranes
4. Materials and Methods
4.1. In Vitro Culture Preparation
4.2. Vanadium Treatment
4.3. Transport Mechanism Inhibitors Treatment
4.4. Extracts Preparation and HPLC Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Troalen, L.G.; Phillips, A.S.; Peggie, D.A.; Barran, P.E.; Hulme, A.N. Historical textile dyeing with Genista tinctoria L.: A comprehensive study by UPLC-MS/MS analysis. Anal. Methods 2014, 6, 8915–8923. [Google Scholar] [CrossRef]
- Fakir, H.; Korkmaz, M.; Güller, B. Medicinal plant diversity of western Mediterrenean region in Turkey. J. Appl. Biol. Sci. 2009, 3, 30–40. [Google Scholar]
- Tero-Vescan, A.; Vari, C.E.; Vlase, L. Alkaloid content of some potential isoflavonoids sources (native genista species). Long-term safety implications. Farmacia 2014, 62, 1109–1117. [Google Scholar]
- Wink, M. Quinolizidine alkaloids: Biochemistry, metabolism, and function in plants and cell suspension cultures. Planta Med. 1987, 53, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Tero-Vescan, A.; Imre, S.; Vari, C.E.; Oşan, A.; Dogaru, M.T.; Csedö, C. Determination of some isoflavonoids and flavonoids from Genista tinctoria L. by HPLC-UV. Farmacia 2009, 57, 120–127. [Google Scholar]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Schulze, E.D.; Beck, E.; Hohenstein, K.M. Environment as stress factor: Stress physiology of plants. In Plant Ecology; Czeschlik, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 7–21. ISBN 978-3-540-20833-4. [Google Scholar]
- Davies, K.M.; Schwinn, K.E. Molecular biology and biotechnology of flavonoid biosynthesis. In Flavonoids: Chemistry, Biochemistry and Applications; Andersen, Ø.M., Markham, K.R., Eds.; CRC Taylor and Francis: Boca Raton, FL, USA, 2006; pp. 143–218. ISBN 0-8493-2021-6. [Google Scholar]
- Viehweger, K. How plants cope with heavy metals. Bot. Stud. 2014, 55, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Panichev, N.; Mandiwana, K.; Moema, D.; Molatlhegi, R.; Ngobeni, P. Distribution of vanadium(V) species between soil and plants in the vicinity of vanadium mine. J. Hazard. Mater. 2006, 137, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.A.; Baken, S.; Gustafsson, J.P.; Hadialhejazi, G.; Smolders, E. Vanadium bioavailability and toxicity to soil microorganisms and plants. Environ. Toxicol. Chem. 2013, 32, 2266–2273. [Google Scholar] [CrossRef] [PubMed]
- Crans, D.C.; Smee, J.J.; Gaidamauskas, E.; Yang, L. The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chem. Rev. 2004, 104, 849–902. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Baranowska-Bosiacka, I.; Gutowska, I.; Chlubek, D. Biochemical and medical importance of vanadium compounds. Acta Biochim. Pol. 2012, 59, 195–200. [Google Scholar] [PubMed]
- Gaspar, T.; Franck, T.; Bisbis, B.; Kevers, C. Concepts in plant stress physiology. Application to plant tissue cultures. Plant Growth Regul. 2002, 37, 263–286. [Google Scholar] [CrossRef]
- Smith, J.I.; Smart, N.J.; Misawa, M.; Kurz, W.G.W.; Tallevi, S.G.; DiCosmo, F. Increased accumulation of indole alkaloids by some cell lines of Catharanthus roseus in response to addition of vanadyl sulphate. Plant Cell Rep. 1987, 6, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Palazón, J.; Cusidó, R.M.; Bonfill, M.; Mallol, A.; Moyano, E.; Morales, C.; Piñol, M.T. Elicitation of different Panax ginseng transformed root phenotypes for an improved ginsenoside production. Plant Physiol. Biochem. 2003, 41, 1019–1025. [Google Scholar] [CrossRef]
- Sánchez, T.; Martín, S.; Saco, D. Some responses of two Nicotiana tabacum L. cultivars exposed to vanadium. J. Plant Nutr. 2014, 37, 777–784. [Google Scholar] [CrossRef]
- Hattori, T.; Ohta, Y. Induction of phenylalanine ammonia-lyase activation and isoflavone glucoside accumulation in suspension-cultured cells of red bean, Vigna angularis, by phytoalexin elicitors, vanadate, and elevation of medium pH. Plant Cell Physiol. 1985, 26, 1101–1110. [Google Scholar] [CrossRef]
- Hagendoorn, M.J.M.; Traas, T.P.; Boon, J.J.; van der Plas, L.H.W. Orthovanadate induced lignin production, in batch and continuous cultures of Petunia hybrida. J. Plant Physiol. 1990, 137, 72–80. [Google Scholar] [CrossRef]
- Wagner, G.J.; Hrazdina, G. Endoplasmic reticulum as a site of phenylpropanoid and flavonoid metabolism in Hippeastrum. Plant Physiol. 1984, 74, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J. Flavonoid transport mechanisms: How to go, and with whom. Trends Plant Sci. 2015, 20, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Prieto, D.; Corchete, P. Transport of flavonolignans to the culture medium of elicited cell suspensions of Silybum marianum. J. Plant Physiol. 2014, 171, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Martinoia, E.; Hoffmann-Thoma, G.; Weissenböck, G. A membrane-potential dependent ABC-like transporter mediates the vacuolar uptake of rye flavone glucuronides: Regulation of glucuronide uptake by glutathione and its conjugates. Plant J. 2000, 21, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Inai, K.; Yazaki, Y.; Sato, Y.; Takase, H.; Shitan, N.; Yazaki, K.; Goto, Y.; Toyooka, K.; Matsuoka, K.; et al. Multidrug and toxic compound extrusion-type transporters implicated in vacuolar sequestration of nicotine in tobacco roots. Plant. Physiol. 2008, 149, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.V.; Mills, J.D. Uptake of salicylic acid 2-O-β-d-glucose into soybean tonoplast vesicles by an ATP-binding cassette transporter-type mechanism. Physiol. Plant. 2004, 120, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Wu, Y.F.; Xue, F.; Wu, Z.X.; Xue, Y.P.; Zheng, Y.G.; Shen, Y.C. Isolation of brefeldin A from Eupenicillium brefeldianum broth using macroporous resin adsorption chromatography. J. Chromatogr. B 2012, 895–896, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Mossessova, E.; Corpina, R.A.; Goldberg, J. Crystal structure of ARF1·Sec7 complexed with Brefeldin A and its implications for the guanine nucleotide exchange mechanism. Mol. Cell 2003, 12, 1403–1411. [Google Scholar] [CrossRef]
- Klausner, R.D.; Donaldson, J.G.; Lippincott-Schwartz, J. Brefeldin A: Insights into the control of membrane traffic and organelle structure. J. Cell Biol. 1992, 116, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Villegas, M.; Sommarin, M.; Brodelius, P.E. Effects of sodium orthovanadate on benzophenanthridine alkaloid formation and distribution in cell suspension cultures of Eschscholtzia californica. Plant Physiol. Biochem. 2000, 38, 233–241. [Google Scholar] [CrossRef]
- Hunke, S.; Dröse, S.; Schneider, E. Vanadate and bafilomycin A1are potent inhibitors of the ATPase activity of the reconstituted bacterial ATP-Binding Cassette transporter for maltose (MalFGK2). Biochem. Biophys. Res. Commun. 1995, 216, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Frangne, N.; Eggmann, T.; Koblischke, C.; Weissenbock, G.; Martinoia, E.; Klein, M. Flavone glucoside uptake into barley mesophyll and Arabidopsis cell culture vacuoles. Energization occurs by H+-antiport and ATP-binding cassette-type mechanisms. Plant Physiol. 2002, 128, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Potschka, H.; Baltes, S.; Löscher, W. Inhibition of multidrug transporters by verapamil or probenecid does not alter blood-brain barrier penetration of levetiracetam in rats. Epilepsy Res. 2004, 58, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Terasaka, K.; Sakaia, K.; Sato, F.; Yamamoto, H.; Yazaki, K. Thalictrum minus cell cultures and ABC-like transporter. Phytochemistry 2003, 62, 483–489. [Google Scholar] [CrossRef]
- Payen, L.; Delugin, L.; Courtois, A.; Trinquart, Y.; Guillouzo, A.; Fardel, O. The sulphonylurea glibenclamide inhibits multidrug resistance protein (MRP1) activity in human lung cancer cells. Br. J. Pharmacol. 2001, 132, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Gaedeke, N.; Klein, M.; Kolukisaoglu, U.; Forestier, C.; Müller, A.; Ansorge, M.; Becker, D.; Mamnun, Y.; Kuchler, K.; Schulz, B.; et al. The Arabidopsis thaliana ABC transporter AtMRP5 controls root development and stomata movement. EMBO J. 2001, 20, 1875–1887. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.I.; Adriano, D.C.; Carlson, C.L.; Sajwan, K.S. Vanadium: Toxicity and accumulation by beans. Water. Air. Soil Pollut. 1990, 49, 81–91. [Google Scholar] [CrossRef]
- Saco, D.; Martín, S.; San José, P. Vanadium distribution in roots and leaves of Phaseolus vulgaris: Morphological and ultrastructural effects. Biol. Plant. 2013, 57, 128–132. [Google Scholar] [CrossRef]
- Schützendübel, A.; Polle, A. Plant responses to abiotic stresses: Heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 2002, 53, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Dalal, N.S. NADPH-dependent flavoenzymes catalyze one electron reduction of metal ions and molecular oxygen and generate hydroxyl radicals. FEBS Lett. 1990, 276, 189–191. [Google Scholar] [CrossRef]
- Ye, Y.; Ding, Y.; Jiang, Q.; Wang, F.; Sun, J.; Zhu, C. The role of receptor-like protein kinases (RLKs) in abiotic stress response in plants. Plant Cell Rep. 2017, 36, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Steffens, M.; Ettl, F.; Kranz, D.; Kindl, H. Vanadate mimics effects of fungal cell wall in eliciting gene activation in plant cell cultures. Planta 1989, 177, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Armero, J.; Tena, M. Possible role of plasma membrane H+-ATPase in the elicitation of phytoalexin and related isoflavone root secretion in chickpea (Cicer arietinum L.) seedlings. Plant Sci. 2001, 161, 791–798. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Pawlak-Sprada, S.; Stobiecki, M.; Deckert, J. Activation of phenylpropanoid pathway in legume plants exposed to heavy metals. Part II. Profiling of isoflavonoids and their glycoconjugates induced in roots of lupine (Lupinus luteus) seedlings treated with cadmium and lead. Acta Biochim. Pol. 2011, 58, 217–223. [Google Scholar] [PubMed]
- Huang, C.; Zhong, J.J. Elicitation of ginsenoside biosynthesis in cell cultures of Panax ginseng by vanadate. Process Biochem. 2013, 48, 1227–1234. [Google Scholar] [CrossRef]
- Namdeo, A.G. Plant cell elicitation for production of secondary metabolites: A review. Pharmacogn. Rev. 2007, 1, 69–79. [Google Scholar] [CrossRef]
- Cai, Z.; Kastell, A.; Knorr, D.; Smetanska, I. Exudation: An expanding technique for continuous production and release of secondary metabolites from plant cell suspension and hairy root cultures. Plant Cell Rep. 2012, 31, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Luczkiewicz, M.; Kokotkiewicz, A.; Glod, D. Plant growth regulators affect biosynthesis and accumulation profile of isoflavone phytoestrogens in high-productive in vitro cultures of Genista tinctoria. Plant Cell. Tissue Organ Cult. 2014, 118, 419–429. [Google Scholar] [CrossRef]
- Sugiyama, A.; Shitan, N.; Yazaki, K. Involvement of a soybean ATP-binding cassette-type transporter in the secretion of genistein, a signal flavonoid in legume-Rhizobium symbiosis. Plant Physiol. 2007, 144, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dixon, R.A. MATE transporters facilitate vacuolar uptake of epicatechin 3′-O-glucoside for proanthocyanidin biosynthesis in Medicago truncatula and Arabidopsis. Plant Cell Online 2009, 21, 2323–2340. [Google Scholar] [CrossRef] [PubMed]
- Sirikantaramas, S.; Sudo, H.; Asano, T.; Yamazaki, M.; Saito, K. Transport of camptothecin in hairy roots of Ophiorrhiza pumila. Phytochemistry 2007, 68, 2881–2886. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Hébrard, C.; Deville, M.A.; Cordelier, S.; Dorey, S.; Aziz, A.; Crouzet, J. Deciphering the role of phytoalexins in plant-microorganism interactions and human health. Molecules 2014, 19, 18033–18056. [Google Scholar] [CrossRef] [PubMed]
- Buer, C.S.; Muday, G.K.; Djordjevic, M.A. Flavonoids are differentially taken up and transported long distances in Arabidopsis. PLANT Physiol. 2007, 145, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Shitan, N. Secondary metabolites in plants: Transport and self-tolerance mechanisms. Biosci. Biotechnol. Biochem. 2016, 80, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Banasiak, J.; Biała, W.; Staszków, A.; Swarcewicz, B.; Kȩpczyńska, E.; Figlerowicz, M.; Jasiński, M. A Medicago truncatula ABC transporter belonging to subfamily G modulates the level of isoflavonoids. J. Exp. Bot. 2013, 64, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Alejandro, S.; Lee, Y.; Tohge, T.; Sudre, D.; Osorio, S.; Park, J.; Bovet, L.; Lee, Y.; Geldner, N.; Fernie, A.R.; et al. AtABCG29 is a monolignol transporter involved in lignin biosynthesis. Curr. Biol. 2012, 22, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.C.; Liu, C.J. ATP-binding cassette-like transporters are involved in the transport of lignin precursors across plasma and vacuolar membranes. Proc. Natl. Acad. Sci. USA 2010, 107, 22728–22733. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Yonekura-Sakakibara, K.; Nakabayashi, R.; Higashi, Y.; Yamazaki, M.; Tohge, T.; Fernie, A.R. The flavonoid biosynthetic pathway in Arabidopsis: Structural and genetic diversity. Plant Physiol. Biochem. 2013, 72, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Huhman, D.; Shadle, G.; He, X.Z.; Sumner, L.W.; Tang, Y.; Dixon, R.A. MATE2 Mediates Vacuolar Sequestration of Flavonoid Glycosides and Glycoside Malonates in Medicago truncatula. Plant Cell 2011, 23, 1536–1555. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.D.; Casati, P.; Walbot, V. A multidrug resistance-associated protein involved in anthocyanin transport in Zea mays. Plant Cell 2004, 16, 1812–1826. [Google Scholar] [CrossRef] [PubMed]
- Francisco, R.M.; Regalado, A.; Ageorges, A.; Burla, B.J.; Bassin, B.; Eisenach, C.; Zarrouk, O.; Vialet, S.; Marlin, T.; Chaves, M.M.; et al. ABCC1, an ATP binding cassette protein from grape berry, transports anthocyanidin 3-O-glucosides. Plant Cell 2013, 25, 1840–1854. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.; Liu, H.; Kang, L.; Geng, J.; Gai, Y.; Ding, Y.; Sun, H.; Li, Y. Identification and expression analysis of MATE genes involved in flavonoid transport in blueberry plants. PLoS ONE 2015, 10, e0118578. [Google Scholar] [CrossRef] [PubMed]
- Tsuyama, T.; Kawai, R.; Shitan, N.; Matoh, T.; Sugiyama, J.; Yoshinaga, A.; Takabe, K.; Fujita, M.; Yazaki, K. Proton-dependent coniferin transport, a common major transport event in differentiating xylem tissue of woody plants. Plant Physiol. 2013, 162, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Searle, B.M.; Higashino, H.; Khalil, F.; Bogden, J.D.; Tokushige, A.; Tamura, H.; Kino, M.; Aviv, A. Vanadate effect on the Na,K-ATPase and the Na-K pump in in vitro-grown rat vascular smooth muscle cells. Circ. Res. 1983, 53, 186–191. [Google Scholar] [CrossRef] [PubMed]
- O’neill, S.D.; Spanswick, R.M. Effects of vanadate on the plasma membrane ATPase of red beet and corn. Plant Physiol. 1984, 75, 586–591. [Google Scholar] [CrossRef] [PubMed]
- North, P.; Post, R.L. Inhibition of (Na,K)-ATPase by tetravalent vanadium. J. Biol. Chem. 1984, 259, 4971–4978. [Google Scholar] [PubMed]
- Udvardi, M.K.; Day, D.A. Electrogenic ATPase Activity on the Peribacteroid Membrane of Soybean (Glycine max L.) Root Nodules. Plant Physiol. 1989, 90, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, S. Transport of flavonoids: From cytosolic synthesis to vacuolar accumulation. In The Science of Flavonoids; Grotewold, E., Ed.; Springer: New York, NY, USA, 2006; pp. 123–146. ISBN 9780387288222. [Google Scholar]
- Ichino, T.; Fuji, K.; Ueda, H.; Takahashi, H.; Koumoto, Y.; Takagi, J.; Tamura, K.; Sasaki, R.; Aoki, K.; Shimada, T.; et al. GFS9/TT9 contributes to intracellular membrane trafficking and flavonoid accumulation in Arabidopsis thaliana. Plant J. 2014, 80, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Filippi, A.; Petrussa, E.; Braidot, E. Flavonoid facilitated/passive transport: Characterization of quercetin microsomal uptake by a DPBA-dependent assay. Biochim. Biophys. Acta Bioen. 2016, 1857, e64. [Google Scholar] [CrossRef]
- Schenk, R.U.; Hildebrandt, A.C. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Kubeš, J.; Tůmová, L.; Martin, J.; Vildová, A.; Hendrychová, H.; Sojková, K. The production of isoflavonoids in Genista tinctoria L. cell suspension culture after abiotic stressors treatment. Nat. Prod. Res. 2014, 28, 2253–2263. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skalicky, M.; Kubes, J.; Hejnak, V.; Tumova, L.; Martinkova, J.; Martin, J.; Hnilickova, H. Isoflavones Production and Possible Mechanism of Their Exudation in Genista tinctoria L. Suspension Culture after Treatment with Vanadium Compounds. Molecules 2018, 23, 1619. https://doi.org/10.3390/molecules23071619
Skalicky M, Kubes J, Hejnak V, Tumova L, Martinkova J, Martin J, Hnilickova H. Isoflavones Production and Possible Mechanism of Their Exudation in Genista tinctoria L. Suspension Culture after Treatment with Vanadium Compounds. Molecules. 2018; 23(7):1619. https://doi.org/10.3390/molecules23071619
Chicago/Turabian StyleSkalicky, Milan, Jan Kubes, Vaclav Hejnak, Lenka Tumova, Jaroslava Martinkova, Jan Martin, and Helena Hnilickova. 2018. "Isoflavones Production and Possible Mechanism of Their Exudation in Genista tinctoria L. Suspension Culture after Treatment with Vanadium Compounds" Molecules 23, no. 7: 1619. https://doi.org/10.3390/molecules23071619
APA StyleSkalicky, M., Kubes, J., Hejnak, V., Tumova, L., Martinkova, J., Martin, J., & Hnilickova, H. (2018). Isoflavones Production and Possible Mechanism of Their Exudation in Genista tinctoria L. Suspension Culture after Treatment with Vanadium Compounds. Molecules, 23(7), 1619. https://doi.org/10.3390/molecules23071619






