Spectral Characterization and Molecular Dynamics Simulation of Pesticides Based on Terahertz Time-Domain Spectra Analyses and Density Functional Theory (DFT) Calculations
Abstract
1. Introduction
2. Results and Discussion
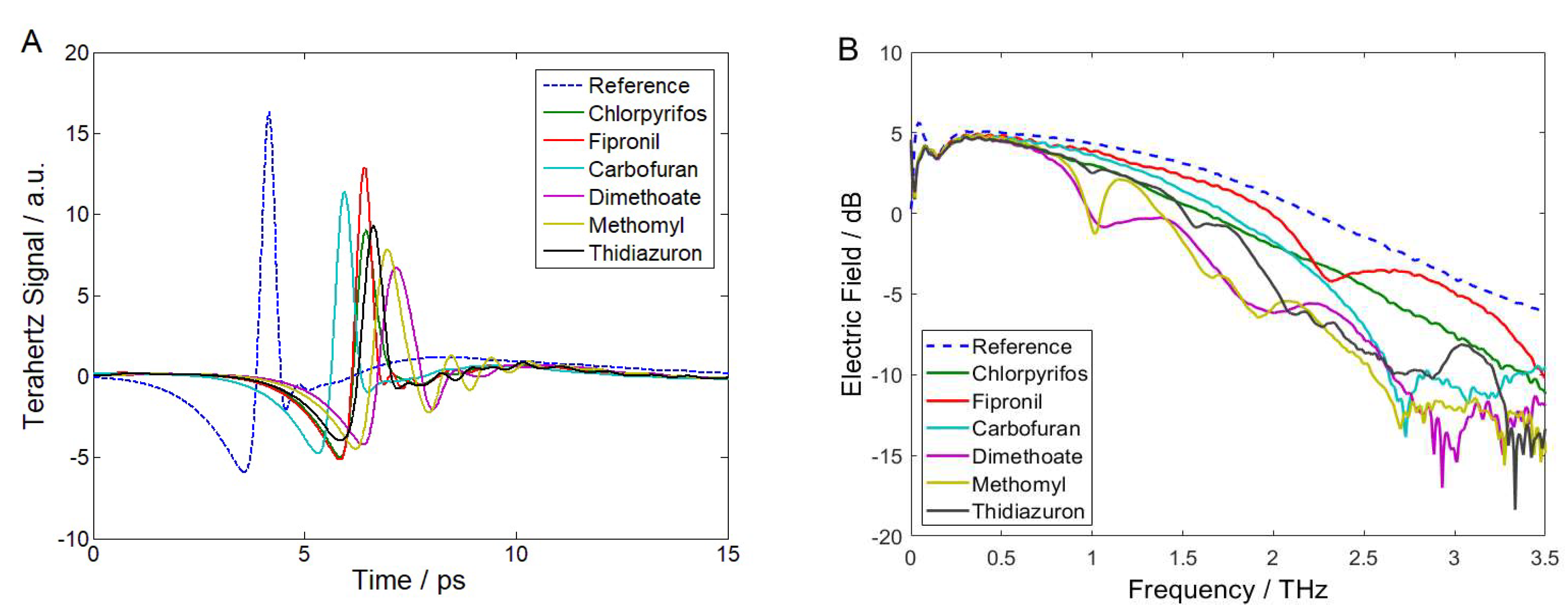
2.1. THz Time-Domain Waveforms and Frequency-Domain Spectra
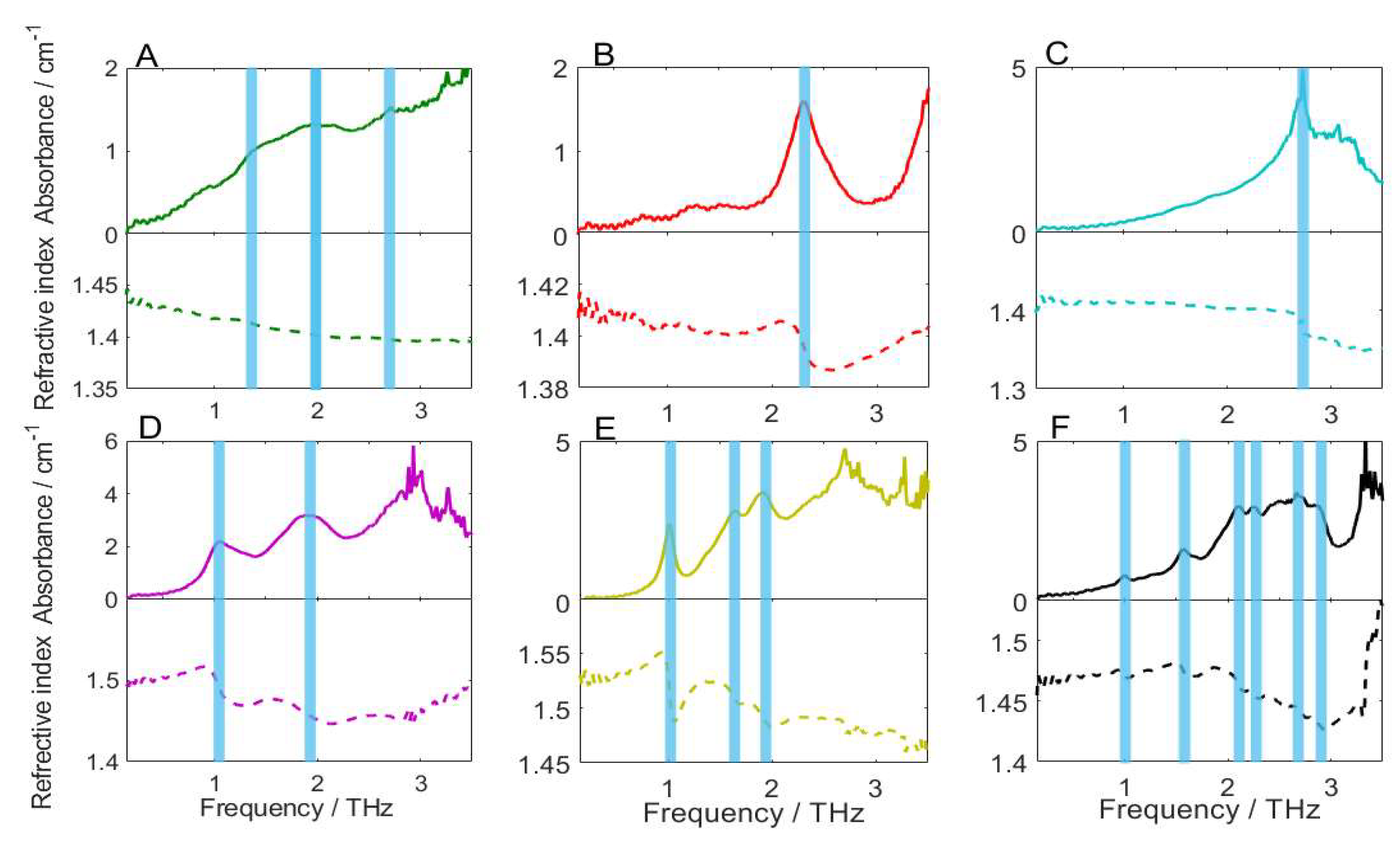
2.2. Analysis of Absorption and Refraction Characteristics
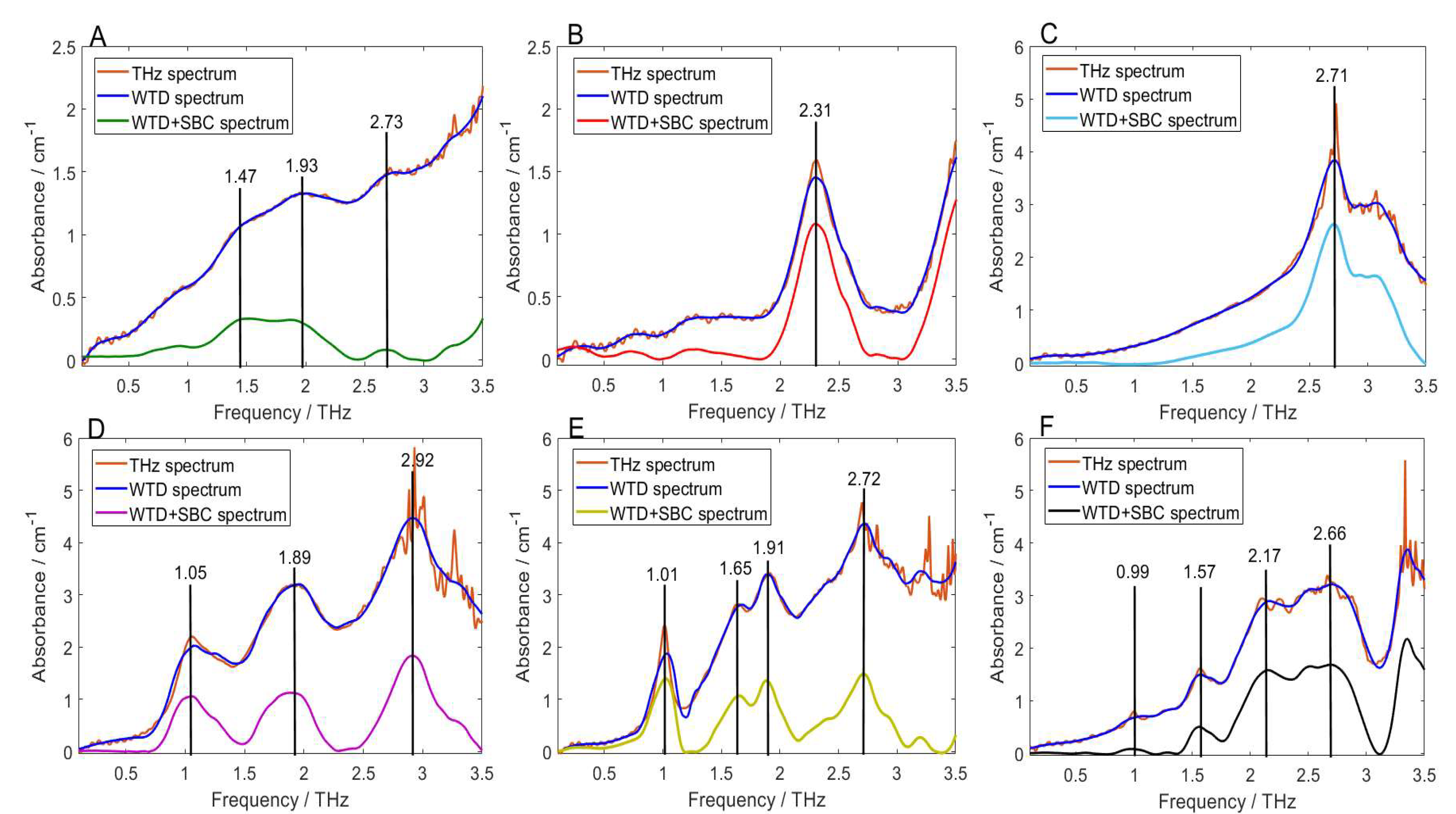
2.3. Spectral De-noising and Baseline Correction
2.4. Molecular Dynamics Simulations by DFT
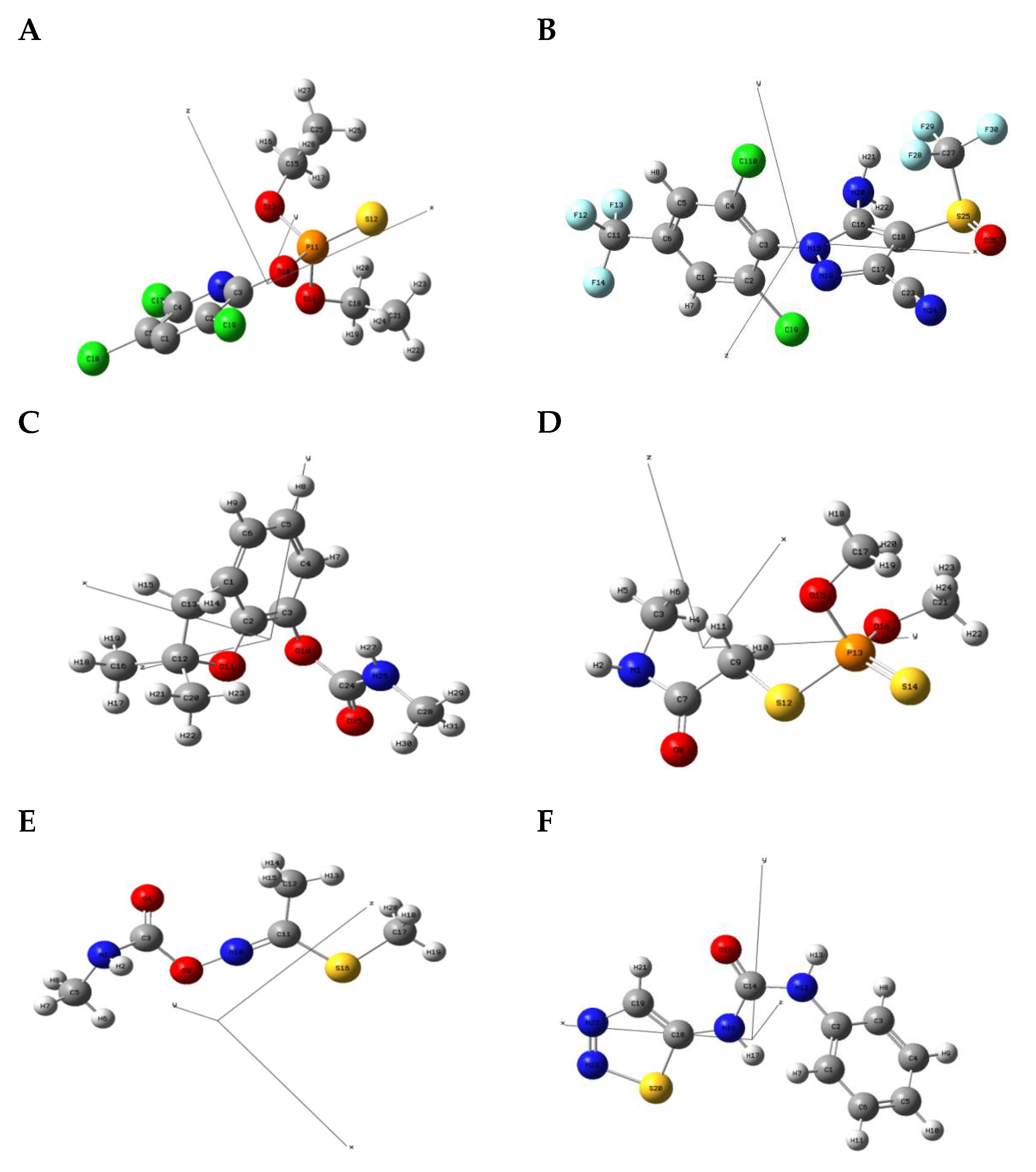
2.4.1. Molecular Geometric Configuration
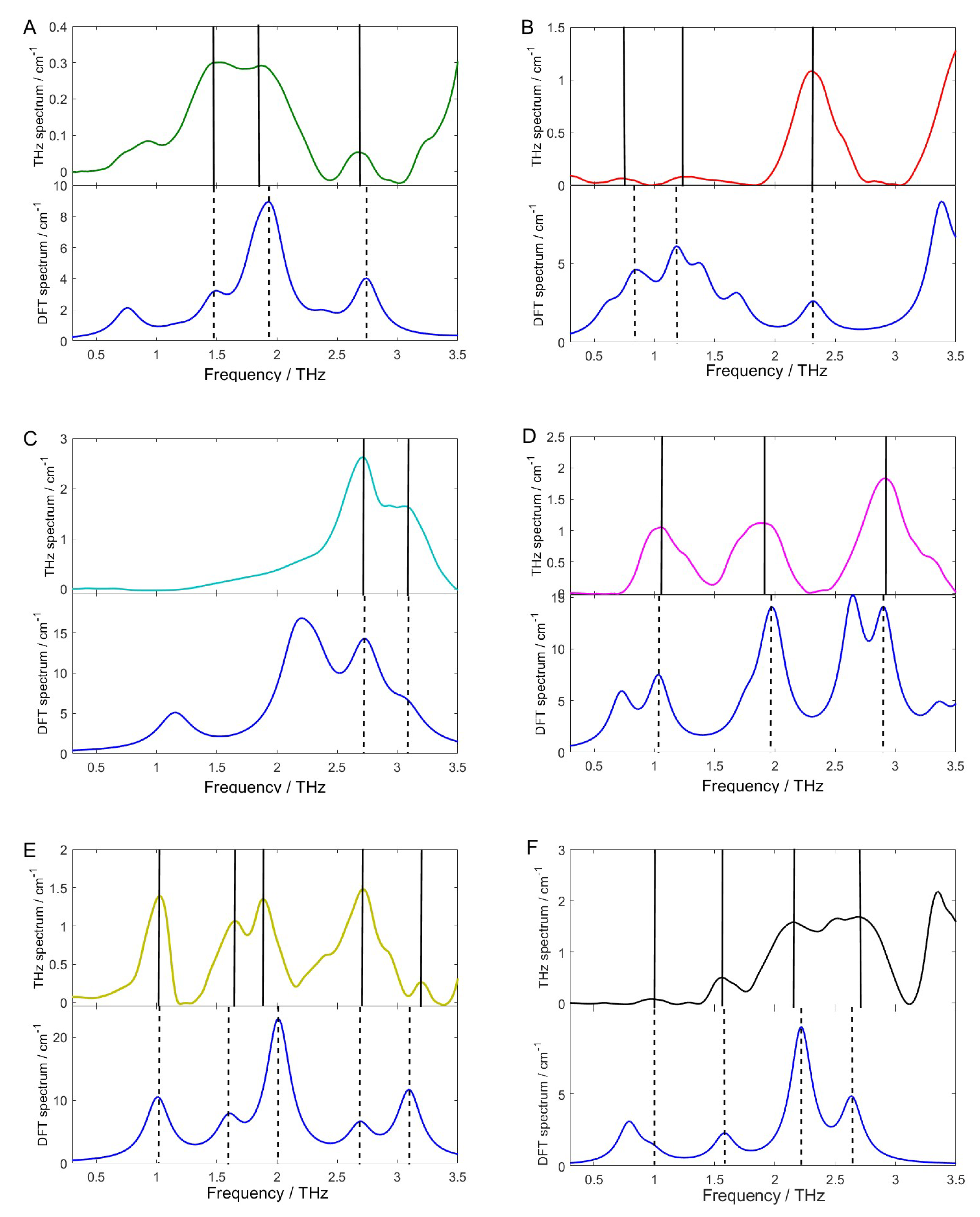
2.4.2. Comparison of Experimental and Theoretical Spectra
2.4.3. Assignment of Absorption Peaks
3. Materials and Methods
3.1. Sample Preparation
3.2. THz Spectral Acquisition
3.3. THz Spectral Processing
3.4. Density Functional Theory
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, P.H. Terahertz technology in biology and medicine. IEEE Trans. Microw. Theory Tech. 2004, 52, 2438–2447. [Google Scholar] [CrossRef]
- Naftaly, M.; Miles, R.E. Terahertz time-domain spectroscopy for material characterization. Proc. IEEE 2007, 95, 1658–1665. [Google Scholar] [CrossRef]
- Mccrindle, I.J.H.; Grant, J.; Drysdale, T.D.; Cumming, D.R.S. Multi-spectral materials: Hybridisation of optical plasmonic filters and a terahertz metamaterial absorber. Adv. Opt. Mater. 2014, 2, 149–153. [Google Scholar] [CrossRef]
- Popov, A.K.; Myslivets, S.A. Generation, amplification, frequency conversion, and reversal of propagation of THz photons in nonlinear hyperbolic metamaterial. Opt. Lett. 2017, 42, 4151–4154. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Fukunaga, K.; Hosako, I. Terahertz spectroscopy and its applications to dielectric and electrical insulating materials. In Proceedings of the 2008 International Symposium on Electrical Insulating Materials, Mie, Japan, 7–11 September 2008; IEEE: Piscataway, NJ, USA, 2008; pp. 613–616. [Google Scholar]
- Nie, P.; Qu, F.; Lin, L.; Dong, T.; He, Y.; Shao, Y.; Zhang, Y. Detection of water content in rapeseed leaves using terahertz spectroscopy. Sensors 2017, 17, 2830. [Google Scholar] [CrossRef] [PubMed]
- Duvillaret, L.; Garet, F.; Coutaz, J.L. A reliable method for extraction of material parameters in terahertz time-domain spectroscopy. IEEE J. Sel. Top. Quantum Electron. 2002, 2, 739–746. [Google Scholar] [CrossRef]
- De Cumis, U.S.; Xu, J.H.; Masini, L.; Degl'innocenti, R.; Pingue, P.; Beltram, F.; Tredicucci, A.; Vitiello, M.S.; Benedetti, P.A.; Beere, H.E.; et al. Terahertz confocal microscopy with a quantum cascade laser source. Opt. Express 2012, 20, 21924–21931. [Google Scholar] [CrossRef] [PubMed]
- Kawase, K.; Ogawa, Y.; Watanabe, Y.; Inoue, H. Non-destructive terahertz imaging of illicit drugs using spectral fingerprints. Opt. Express 2003, 11, 2549–2554. [Google Scholar] [CrossRef] [PubMed]
- Neumaier, P.F.; Schmalz, K.; Borngräber, J.; Wylde, R.; Hübers, H.W. Terahertz gas-phase spectroscopy: chemometrics for security and medical applications. Analyst 2015, 140, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Missori, M.; Bagniuk, J.; Clerici, M.; Lojewska, J.; Misiti, M.C.; Peters, L.; Morandotti, R.; Conte, A.M.; Pulci, O.; Teodonio, L.; et al. Terahertz Waves for Ancient Manuscripts Conservation. In Proceedings of the 2015 European Conference on Lasers and Electro-Optics-European Quantum Electronics Conference, Munich, Germany, 21–25 June 2015; Optical Society of America: Washington, DC, USA, 2015. [Google Scholar]
- Recur, B.; Lewis, D.; Darracq, F.; Balacey, H.; Sleiman, J.B.; Guillet, J.P.; Mounaix, P. Low-frequency noise effect on terahertz tomography using thermal detectors. Appl. Opt. 2015, 54, 6758–6762. [Google Scholar]
- Pan, C.; Zhang, R.; Luo, H.; Shen, H. Baseline correction of vibration acceleration signals with inconsistent initial velocity and displacement. Adv. Mech. Eng. 2016, 8. [Google Scholar] [CrossRef]
- Kokkoniemi, J.; Lehtomäki, J.; Juntti, M. A discussion on molecular absorption noise in the terahertz band. Nano Commun. Netw. 2016, 8, 35–45. [Google Scholar] [CrossRef]
- Blanchard, P.; Brüning, E. Density Functional Theory of Atoms and Molecules. In Mathematical Methods in Physics; Birkhäuser: Boston, MA, USA, 2003. [Google Scholar]
- Wiberg, K.B. Comparison of density functional theory models’ ability to reproduce experimental 13C-NMR shielding values. J. Comput. Chem. 2015, 20, 1299–1303. [Google Scholar] [CrossRef]
- Witko, E.M.; Buchanan, W.D.; Korter, T.M. Terahertz spectroscopy and solid-state density functional theory simulations of the improvised explosive oxidizers potassium nitrate and ammonium nitrate. J. Phys. Chem. A 2011, 115, 12410–12418. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Security 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Maeng, I.; Baek, S.H.; Kim, H.Y.; Ok, G.S.; Choi, S.W.; Chun, H.S. Feasibility of using terahertz spectroscopy to detect seven different pesticides in wheat flour. J. Food Prot. 2014, 77, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Ju, H.K.; Hwang, Y.H.; Kang, M.O.; Kwak, K.; Chun, H.S. Detection of methomyl, a carbamate insecticide, in food matrices using terahertz time-domain spectroscopy. J. Infrared Millim. Terahertz Waves 2016, 37, 486–497. [Google Scholar] [CrossRef]
- Qin, J.; Ying, Y.; Xie, L. The Detection of Agricultural Products and Food Using Terahertz Spectroscopy: A Review. Appl. Spectrosc. Rev. 2013, 48, 439–457. [Google Scholar] [CrossRef]
- Rajput, A.H.; Uitti, R.J.; Stern, W.; Laverty, W.; O’Donnell, K.; O'Donnell, D.; Yuen, W.K.; Dua, A. Geography, drinking water chemistry, pesticides and herbicides and the etiology of Parkinson's disease. Can. J. Neurol. Sci. 2016, 14, 414–418. [Google Scholar] [CrossRef]
- Barres, B.; Micoud, A.; Corio-Costet, M.-F.; Debieu, D.; Fillinger-David, S.; Walker, A.-S.; Délye, C.; Grosman, J.; Siegwart, M. Trends and challenges in pesticide resistance detection. Trends Plant Sci. 2016, 21, 834–853. [Google Scholar]
- Cen, H.; Weng, H.; Yao, J.; He, M.; Lv, J.; Hua, S.; Li, H.; He, Y. Chlorophyll fluorescence imaging uncovers photosynthetic fingerprint of citrus huanglongbing. Front. Plant Sci. 2017, 8, 1509. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Zhang, C.; Huang, W.; Liu, F.; He, Y. Application of hyperspectral imaging to detect sclerotinia sclerotiorum on oilseed rape stems. Sensors 2018, 18, 123. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, S.; Wang, N.; Nie, P.; He, Y.; Sun, D.W.; Yao, J. Application of time series hyperspectral imaging (TS-HSI) for determining water distribution within beef and spectral kinetic analysis during dehydration. Food Bioprocess Technol. 2013, 6, 2943–2958. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, H.; Liu, F.; He, Y. Application of near-infrared hyperspectral imaging with variable selection methods to determine and visualize caffeine content of coffee beans. Food Bioprocess Technol. 2016, 10, 1–9. [Google Scholar] [CrossRef]
- Hua, Y.; Zhang, H. Qualitative and quantitative detection of pesticides with terahertz time-domain spectroscopy. IEEE Trans. Microw. Theory Tech. 2010, 58, 2064–2070. [Google Scholar]
- Lee, D.K.; Kim, G.; Kim, C.; Jhon, Y.M.; Kim, J.H.; Lee, T.; Son, J.H.; Seo, M. Ultrasensitive detection of residual pesticides using THz near-field enhancement. IEEE Trans. Terahertz Sci. Technol. 2016, 6, 389–395. [Google Scholar] [CrossRef]
- Authority, E.F.S. Conclusion on the peer review of the pesticide risk assessment of confirmatory data submitted for the active substance haloxyfop-P. Efsa J. 2016, 12, 9–14. [Google Scholar]
- Bauerschmidt, S.; Preu, S.; Malzer, S.; Döhler, G.H.; Wang, L.J.; Lu, H.; Gossard, A.C. Continuous wave Terahertz emitter arrays for spectroscopy and imaging applications. In Proceedings of the Terahertz Physics, Devices, and Systems IV: Advanced Applications in Industry and Defense, Orlando, FL, USA, 26 April 2010; SPIE: Bellingham, WA, USA, 2010; Volume 7671. [Google Scholar] [CrossRef]
- Kitamura, S. Terahertz Spectrometry Device and Method, and Nonlinear Optical Crystal Inspection Device and Method. U.S. Patent 9,091,665, 28 July 2015. [Google Scholar]
- Fan, X.; Xie, W.; Jiang, W.; Yi, L.; Huang, X. An improved threshold function method for power quality disturbance signal de-noising based on stationary wavelet transform. Trans. China Electrotech. Soc. 2016, 31, 219–318. [Google Scholar]
- Ling, Z.B.; Wang, P.Y.; Wan, Y.X.; Wang, Y.Z.; Cheng, D.F.; Tong-Lin, L.I. A combined wavelet transform algorithm used for de-noising magnetotellurics data in the strong human noise. Chin. J. Geophys. 2016, 59, 3436–3447. [Google Scholar]
- El B’charri, O.; Latif, R.; Elmansouri, K.; Abenaou, A.; Jenkal, W. ECG signal performance de-noising assessment based on threshold tuning of dual-tree wavelet transform. Biomed. Eng.Online 2017, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Parrott, E.P.J.; Ung, S.Y.; Pickwell-Macpherson, E. A Robust baseline and reference modification and acquisition algorithm for accurate THz imaging. IEEE Trans. Terahertz Sci. Technol. 2017, 7, 493–501. [Google Scholar] [CrossRef]
- Ipatov, A.; Cordova, F.; Doriol, L.J.; Casida, M.E. Excited-state spin-contamination in time-dependent density-functional theory for molecules with open-shell ground states. J. Mol. Struct. 2017, 914, 60–73. [Google Scholar] [CrossRef]
- Huang, K.; Li, Y.; Tao, S.; Wei, G.; Huang, Y.; Chen, D.; Wu, C. Purification, characterization and biological activity of polysaccharides from dendrobium officinale. Molecules 2016, 21, 701. [Google Scholar] [CrossRef] [PubMed]
- Sepahpour, S.; Selamat, J.; Abdul Manap, M.Y.; Khatib, A.; Abdull Razis, A.F. Comparative analysis of chemical composition, antioxidant activity and quantitative characterization of some phenolic compounds in selected herbs and spices in different solvent extraction systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Pesticides | Thresholds | Evaluations | |||||
|---|---|---|---|---|---|---|---|
| Level-1 | Level-2 | Level-3 | Level-4 | Level-5 | PSNR | RMSE | |
| Chlorpyrifos | 0.037 | 0.120 | 0.150 | 0.197 | 0.052 | 39.330 | 0.026 |
| Fipronil | 0.018 | 0.099 | 0.118 | 0.260 | 0.227 | 36.865 | 0.036 |
| Carbofuran | 0.048 | 0.432 | 0.692 | 0.472 | 1.548 | 35.821 | 0.107 |
| Dimethoate | 0.714 | 1.064 | 0.893 | 1.225 | 1.441 | 32.502 | 0.173 |
| Methomyl | 0.428 | 0.877 | 0.986 | 1.622 | 0.074 | 33.088 | 0.159 |
| Thidiazuron | 1.172 | 0.784 | 0.781 | 0.568 | 0.054 | 32.553 | 0.124 |
| DFT Simulation (THz) | THz Experiment (THz) | Shift (THz) | Vibration Modes |
|---|---|---|---|
| Chlorpyrifos | |||
| 1.47 | 1.47 | 0 | δ(C-C)oop |
| 1.90 | 1.93 | −0.03 | υ(P=O)ip + δ(C-C)oop |
| 2.74 | 2.73 | 0.01 | υ(P=O)ip |
| Fipronil | |||
| 0.82 | 0.76 | 0.06 | δ(C-N)ip |
| 1.18 | 1.23 | −0.05 | δ(C-N)ip |
| 1.36 | - | - | δ(C-N)ip + δ(C-S)oop |
| 1.67 | - | - | δ(C-N)ip + δ(C-S)oop |
| 2.31 | 2.31 | 0 | δ(C-N)ip |
| Carbofuran | |||
| 1.15 | - | - | δ(C-N)ip |
| 2.20 | - | - | δ(C-N)ip + υbreathe |
| 2.72 | 2.72 | 0 | δ(C-O)ip |
| 3.06 | 3.06 | 0 | δ(C-O)ip |
| Dimethoate | |||
| 0.72 | - | - | δ(C-C)ip |
| 1.03 | 1.05 | −0.02 | δ(C-C)ip |
| 1.93 | 1.89 | 0.04 | δ(C-N)ip + δ(C-O)oop |
| 2.64 | - | - | δ(C-S)ip + δ(C-O)oop |
| 2.90 | 2.92 | −0.02 | δ(C-S)ip + δ(C-O)oop |
| Methomyl | |||
| 1.01 | 1.01 | 0 | δ(C-N)ip |
| 1.59 | 1.65 | −0.06 | δ(C-N)ip |
| 2.01 | 1.91 | 0.10 | δ(C-N)ip + δ(C-S)oop |
| 2.68 | 2.72 | −0.04 | δ(C-H)ip |
| 3.09 | 3.20 | −0.11 | δ(C-O)ip |
| Thidiazuron | |||
| 0.98 | 0.99 | −0.01 | υbreathe |
| 1.58 | 1.57 | 0.01 | δ(C-N)ip |
| 2.21 | 2.17 | 0.04 | δ(C-O)oop |
| 2.63 | 2.66 | -0.03 | δ(N-C-N)oop |
| Pesticides | CAS Number | Molecular Formula | Molecular Mass | Molecular Structure |
|---|---|---|---|---|
| Chlorpyrifos | 2921-88-2 | C9H11Cl3NO3PS | 350.59 | 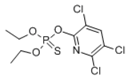 |
| Fipronil | 120068-37-3 | C12H4Cl2F6N4OS | 437.2 |  |
| Carbofuran | 1563-66-2 | C12H15NO3 | 221.25 | 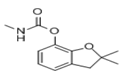 |
| Dimethoate | 60-51-5 | C5H12NO3PS2 | 229.12 |  |
| Methomyl | 16752-77-5 | C5H10N2O2S | 162.23 |  |
| Thidiazuron | 51707-55-2 | C9H8N4OS | 220.2 | 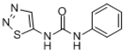 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, F.; Lin, L.; He, Y.; Nie, P.; Cai, C.; Dong, T.; Pan, Y.; Tang, Y.; Luo, S. Spectral Characterization and Molecular Dynamics Simulation of Pesticides Based on Terahertz Time-Domain Spectra Analyses and Density Functional Theory (DFT) Calculations. Molecules 2018, 23, 1607. https://doi.org/10.3390/molecules23071607
Qu F, Lin L, He Y, Nie P, Cai C, Dong T, Pan Y, Tang Y, Luo S. Spectral Characterization and Molecular Dynamics Simulation of Pesticides Based on Terahertz Time-Domain Spectra Analyses and Density Functional Theory (DFT) Calculations. Molecules. 2018; 23(7):1607. https://doi.org/10.3390/molecules23071607
Chicago/Turabian StyleQu, Fangfang, Lei Lin, Yong He, Pengcheng Nie, Chengyong Cai, Tao Dong, Yi Pan, Yu Tang, and Shaoming Luo. 2018. "Spectral Characterization and Molecular Dynamics Simulation of Pesticides Based on Terahertz Time-Domain Spectra Analyses and Density Functional Theory (DFT) Calculations" Molecules 23, no. 7: 1607. https://doi.org/10.3390/molecules23071607
APA StyleQu, F., Lin, L., He, Y., Nie, P., Cai, C., Dong, T., Pan, Y., Tang, Y., & Luo, S. (2018). Spectral Characterization and Molecular Dynamics Simulation of Pesticides Based on Terahertz Time-Domain Spectra Analyses and Density Functional Theory (DFT) Calculations. Molecules, 23(7), 1607. https://doi.org/10.3390/molecules23071607






