3. Experimental
α-Amyrin (1)
Procedure 1: Compound 13 (300 mg, 0.55 mmol) was dissolved in 1 m tetrabutylammonium fluoride in THF (10 mL). After stirring for 14 h at room temperature, the reaction mixture was diluted with diethyl ether (20 mL) and washed with water (10 mL) and brine (10 mL). The organic phase was dried over magnesium sulfate, filtrated, and the solvent was removed under reduced pressure. The crude product was purified by column chromatography (silica gel, hexane/EtOAc, 9:1) to yield 1 as a colorless crystalline solid (220 mg, 94%).
Procedure 2: Compound
13 (100 mg, 0.18 mmol) was dissolved in methanol (50 mL), and iodine (23 mg, 0.18 mmol) was added. The reaction mixture was heated under reflux for 3 h. After cooling to room temperature, the reaction was quenched by adding a solution of sodium thiosulfate and water. The aqueous solution was extracted with diethyl ether (3 × 20 mL), and the combined organic extracts were washed with brine and dried over magnesium sulfate. Purification by column chromatography (silica gel, hexane/EtOAc, 9:1) gave
1 as a colorless crystalline solid (53 mg, 69%). m.p. = 178–181 °C (lit.: 180–181 °C) [
23];
= +84.8° (
c 0.315, CHCl
3) (lit.: +83.5° (
c 1.000, CHCl
3)) [
24]; R
f = 0.24 (silica gel, hexane/EtOAc, 9:1); IR (KBr): 3442
br, 2947
s, 2931
s, 2873
m, 2853
m, 1637
w, 1458
m, 1378
m, 1081w, 1036
m, 996
s, 666
m cm
−1;
1H NMR (500 MHz, CDCl
3): δ = 5.13 (
dd,
J = 3.6 Hz, 3.6 Hz, 1H, H-12), 3.22 (
dd,
J = 11.0, 5.2 Hz, 1H, H-3), 2.05–1.95 (
m, 1H, H-16a), 1.95–1.89 (
m, 2H, H-11a + H-11b), 1.88–1.79 (
m, 1H, H-15a), 1.70–1.58 (
m, 3H, H-1a + H-2a + H-2b), 1.58–1.49 (
m, 3H, H-7a + H-6a + H-9), 1.46–1.18 (
m, 8H, H-22a + H-6b + H-21a + H-7b + H-19 + H-18 + H-22b + H-21b), 1.07 (
s, 3H, H-27), 1.06–0.95 (
m, 2H, H-1b + H-15b), 1.01 (
s, 3H, H-23), 1.00 (
s, 3H, H-24), 0.96 (
s, 3H, H-25), 0.86 (
d,
J = 5.5 Hz, 3H, H-30), 0.89–0.83 (
m, 2H, H-16b + H-20), 0.80 (
s, 3H, H-28), 0.79 (
s, 3H, H-26), 0.79 (
d,
J = 6.1 Hz, 3H, H-29), 0.76–0.71 (
m, 1H, H-5) ppm;
13C NMR (125 MHz, CDCl
3): δ = 139.7 (C-13), 124.6 (C-12), 79.2 (C-3), 59.2 (C-18), 55.4 (C-5), 47.9 (C-9), 42.3 (C-14), 41.7 (C-22), 40.2 (C-8), 39.8 (C-19), 39.8 (C-20), 39.0 (C-1), 38.9 (C-10), 37.1 (C-4), 33.9 (C-17), 33.1 (C-7), 31.4 (C-21), 28.9 (C-28), 28.3 (C-24), 28.3 (C-16), 27.5 (C-2), 26.8 (C-15), 23.5 (C-11), 23.4 (C-27), 21.6 (C-30), 18.5 (C-6), 17.6 (C-29), 17.0 (C-23), 15.8 (C-25), 15.8 (C-26) ppm; MS (ASAP):
m/
z (%) = 409.2 ([M-H
2O + H]
+, 100), 427.2 ([M − H]
+, 16).
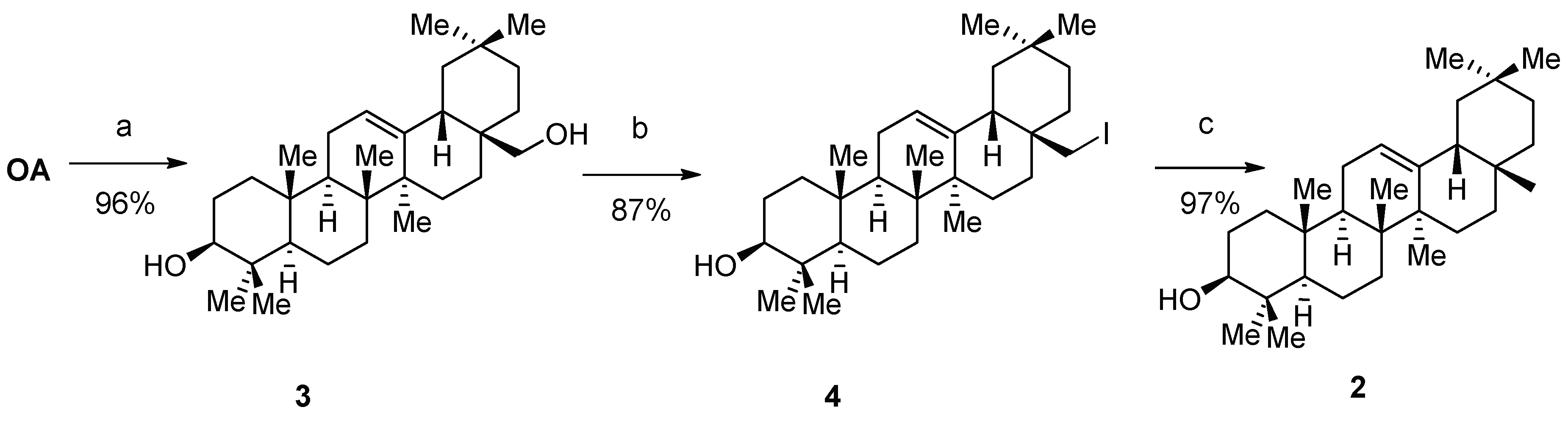
β-Amyrin; (3β) Olean-12-en-3-ol (2)
Procedure 1: Compound 14 (300 mg, 0.55 mmol) was dissolved in 1 m tetrabutylammonium fluoride in THF (10 mL). After 14 h of stirring at room temperature, the reaction mixture was diluted with diethyl ether (20 mL) and washed with water (10 mL) and brine (10 mL). The organic phase was dried over magnesium sulfate, filtrated, and the solvent was removed under reduced pressure. The crude product was purified by column chromatography (silica gel, hexane/EtOAc, 8:2) to yield 2 as a colorless crystalline solid (225 mg, 96%).
Procedure 2: Compound
4 (4 g, 7.24 mmol) was dissolved in acetic acid (1 L) at 40 °C. Zinc (2.5 g, 38.2 mmol) was added, and the reaction was stirred for 4 h at room temperature. The zinc was filtered off, and the filtrate was concentrated under reduced pressure at 40 °C. As soon as β-amyrin began to crystalize, the product was precipitated by adding water (1 L). The precipitate was filtered off and washed thoroughly with water. The white crystalline solid was dried to furnish
2 as a white crystalline solid (2.99 g, 97%). m.p. = 195–197 °C (lit.: 197–198 °C) [
25];
= +89° (
c 0.315, CHCl
3) (lit.: +89° (
c 0.5, CHCl
3)) [
26]; R
f = 0.33 (silica gel, hexane/EtOAc, 8:2); IR (KBr): ν = 3430
br, 2968
s, 2946
s, 2926
s, 2872
m, 2854
m, 1632
m, 1464
m, 1384
m, 1362
w, 1096
w, 1078
w, 1032
w, 996
w cm
−1;
1H NMR (500 MHz, CDCl
3): δ = 5.18 (
dd,
J = 3.6, 3.6 Hz, 1H, H-12), 3.26–3.17 (
m, 1H, H-3), 2.03–1.81 (m, 4H, H-16a + H-18 + H-11a + H-11b), 1.81–1.72 (
m, 1H, H-15a), 1.72–1.47 (
m, 7H, H-19a + H-1a + H-2a + H-2b + H-9 + H-6a + H-7a), 1.47–1.38 (
m, 2H, H-22a + H-6b), 1.38–1.28 (
m, 2H, H-21a + H-7b), 1.28–1.15 (
m, 1H, H-22b), 1.13 (
s, 3H, H-27), 1.15–0.91 (
m, 4H, H-21b + H-15b + H-2b + H-19b), 1.00 (
s, 3H, H-24), 0.97 (
s, 3H, H-26), 0.94 (
s, 3H, H-25), 0.87 (
s, 6H, H-30 + H-29), 0.90–0.76 (
m, 1H, H-16b), 0.83 (
s, 3H, H-28), 0.79 (
s, 3H, H-23), 0.74–0.70 (
m, 1H, H-5) ppm;
13C NMR (125 MHz, CDCl
3): δ = 145.3 (C-13), 121.9 (C-12), 79.2 (C-3), 55.4 (C-5), 47.8 (C-9), 47.4 (C-18), 47.0 (C-19), 41.9 (C-14), 40.0 (C-8), 38.9 (C-4), 38.8 (C-1), 37.3 (C-22), 37.1 (C-10), 34.9 (C-21), 33.5 (C-29), 32.8 (C-17), 32.7 (C-7), 31.2 (C-20), 28.6 (C-28), 28.3 (C-24), 27.4 (C-2), 27.1 (C-16), 26.3 (C-15), 26.2 (C-27), 23.9 (C-30), 23.7 (C-11), 18.6 (C-6), 17.0 (C-26), 15.8 (C-25), 15.7 (C-23) ppm; MS (ASAP):
m/
z (%) = 409.6 ([M − H
2O + H]
+, 100), 427.6 ([M + H]
+, 22).
(3β) Olean-12-en-3,28-diol, (+)-erythrodiol (3)
Lithium aluminum hydride (1.17 g, 30.6 mmol) was dissolved in THF (100 mL), and a solution of
OA (2.00 g, 4,379 mmol) in THF (70 mL) was added slowly at 0 °C. The reaction was heated under reflux until TLC showed the complete consumption of
OA. Surplus lithium aluminum hydride was quenched by adding THF/water (1:1) at 0 °C. The mixture was acidified with 2 M hydrochloric acid and extracted with diethyl ether (3 × 50 mL). The combined organic phases were washed with brine and dried over magnesium sulfate. The solvent was removed to afford
3 as a colorless crystalline solid (1.86 g, 96%). m.p. = 226–228 °C (lit.: 225–229 °C) [
27];
= +75° (
c 0.325, CHCl
3) (lit.: +70°) [
27]; R
f = 0.24 (silica gel, chloroform/hexane/EtOAc, 10:8:2); IR (KBr): ν = 3444
br, 2946
w, 2866
w, 1636
m, 1460
w, 1384
w, 1044
w, 1004
w cm
−1;
1H NMR (500MHz, CDCl
3): δ = 5.19 (
dd,
J = 3.6, 3.6 Hz, 1H, H-12), 3.55 (
d,
J = 11.0 Hz, 1H, H-28a), 3.21 (
d,
J = 11.0 Hz, 1H, H-28b), 3.28–3.15 (
m, 1H, H-3), 1.98 (
dd,
J = 13.5, 4.6 Hz, 1H, H-18), 1.94–1.79 (
m, 3H, H-16a + H-2a + H-2b), 1.77–1.66 (
m, 2H, H-15a + H-19a), 1.65–1.46 (
m, 7H, H-1a + H-11a + H-11b + H-6a + H-9 + H-22a + H-7a), 1.46–1.37 (
m, 1H, H-6b), 1.37–1.25 (
m, 3H, H-22b + H-7b + H-21a), 1.22–1.13 (
m, 2H, H-16b + H-21b), 1.16 (s, 3H, H-27), 1.10–1.03 (
m, 1H, H-19b), 1.03–0.91 (
m, 2H, H-15b + H-1b), 0.99 (
s, 3H, H-23), 0.94 (
s, 3H, H-26), 0.93 (
s, 3H, H-24), 0.88 (
s, 3H, H-29), 0.87 (
s, 3H, H-30), 0.79 (
s, 3H, H-25), 0.75–0.71 (
m, 1H, H-5) ppm;
13C NMR (125 MHz, CDCl
3): δ = 144.4 (C-13), 122.5 (C-12), 79.2 (C-3), 69.8 (C-28), 55.3 (C-5), 47.7 (C-9), 46.6 (C-19), 42.5 (C-18), 41.9 (C-14), 39.9 (C-8), 38.9 (C-4), 38.8 (C-1), 37.1 (C-10), 34.3 (C-21), 33.3 (C-29), 32.7 (C-7), 31.2 (C-22), 31.2 (C-20), 31.1 (C-17), 28.3 (C-23), 27.4 (C-11), 26.1 (C-27), 25.7 (C-15), 23.7 (C-2), 23.7 (C-30), 22.2 (C-16), 18.5 (C-6), 16.9 (C-26), 15.7 (C-24), 15.7 (C-25) ppm; MS (ASAP):
m/
z (%) = 425.6 ([M-H
2O + H]
+, 100), 443.6 ([M + H]
+, 48).
(3β) Olean-12-en-28-iodo-3-ol (4)
Compound 3 (4.0 g, 9.0 mmol) was dissolved in THF (50 mL) and heated under reflux. Imidazole (2.4 g, 35.3 mmol), triphenylphosphane (4.8 g, 18.3 mmol) and iodine (2.0 g, 15.8 mmol) were added one after another. The reaction was heated for 10 min, cooled, and excess iodine was quenched by adding a saturated sodium thiosulfate solution. The resulting mixture was extracted with diethyl ether (3 × 100 mL), the combined organic phases were washed with brine and dried over magnesium sulfate, and the solvent was removed under reduced pressure. Purification by column chromatography (silica gel, chloroform/hexane/EtOAc, 10:8:2) gave 4 as a colorless crystalline solid (4.3 g, 87%). m.p. = 185–188 °C (decomp); = +66° (c 0.335, CHCl3); Rf = 0.31 (silica gel, hexane/EtOAc, 8:2); IR (KBr): ν = 3442br, 2948s, 2926m, 2864m, 1636w, 1462w, 1384w, 1364w, 1180w, 1096w, 1044w, 1030w, 996w, 604w cm−1; 1H NMR (500 MHz, CDCl3): δ = 5.24 (dd, J = 3.6, 3.6 Hz, 1H, H-12), 3.35 (d, J = 9.9 Hz, 1H, H-28a), 3.25–3.18 (m, 1H, H-3), 3.04 (d, J = 9.9, 1H, H-28b), 2.15 (dd, J = 13.5, 3.7 Hz, 1H, H-18), 1.96 (m, 1H, H-16a), 1.92–1.80 (m, 2H, H-11a + H-11b), 1.73 (m, 1H, H-19a), 1.65–1.23 (m, 12H, H-1a + H-9 + H-15a + H-15b + H-2a + H-6a + H-7a + H-6b + H-21a + H-7b + H-22a + H-22b), 1.16 (s, 3H, H-27), 1.21–1.12 (m, 2H, H-21b + H-16b), 1.09 (m, 1H, H-19b), 1.01–0.91 (m, 2H, H-2b + H-1b), 1.00 (s, 3H, H-24), 0.93 (s, 3H, H-26), 0.93 (s, 3H, H-25), 0.90 (s, 3H, H-29), 0.87 (s, 3H, H-30), 0.79 (s, 3H, H-23), 0.75–0.71 (m, 1H, H-5) ppm; 13C NMR (125 MHz, CDCl3): δ = 143.6 (C-13), 123.3 (C-12), 79.1 (C-3), 55.3 (C-5), 47.7 (C-9), 47.6 (C-19), 45.0 (C-18), 41.7 (C-14), 39.9 (C-8), 38.9 (C-4), 38.8 (C-1), 37.1 (C-10), 35.2 (C-17), 34.7 (C-21), 34.7 (C-22), 33.0 (C-29), 32.5 (C-7), 31.3 (C-20), 28.2 (C-24), 27.4 (C-15), 26.3 (C-27), 26.1 (C-2), 25.7 (C-28), 24.6 (C-16), 23.7 (C-11), 23.7 (C-30), 18.5 (C-6), 16.5 (C-26), 15.7 (C-25), 15.7 (C-23) ppm; MS (ASAP): m/z (%) = 535.6 ([M-H2O + H]+, 100), 553.6 ([M + H]+, 18).
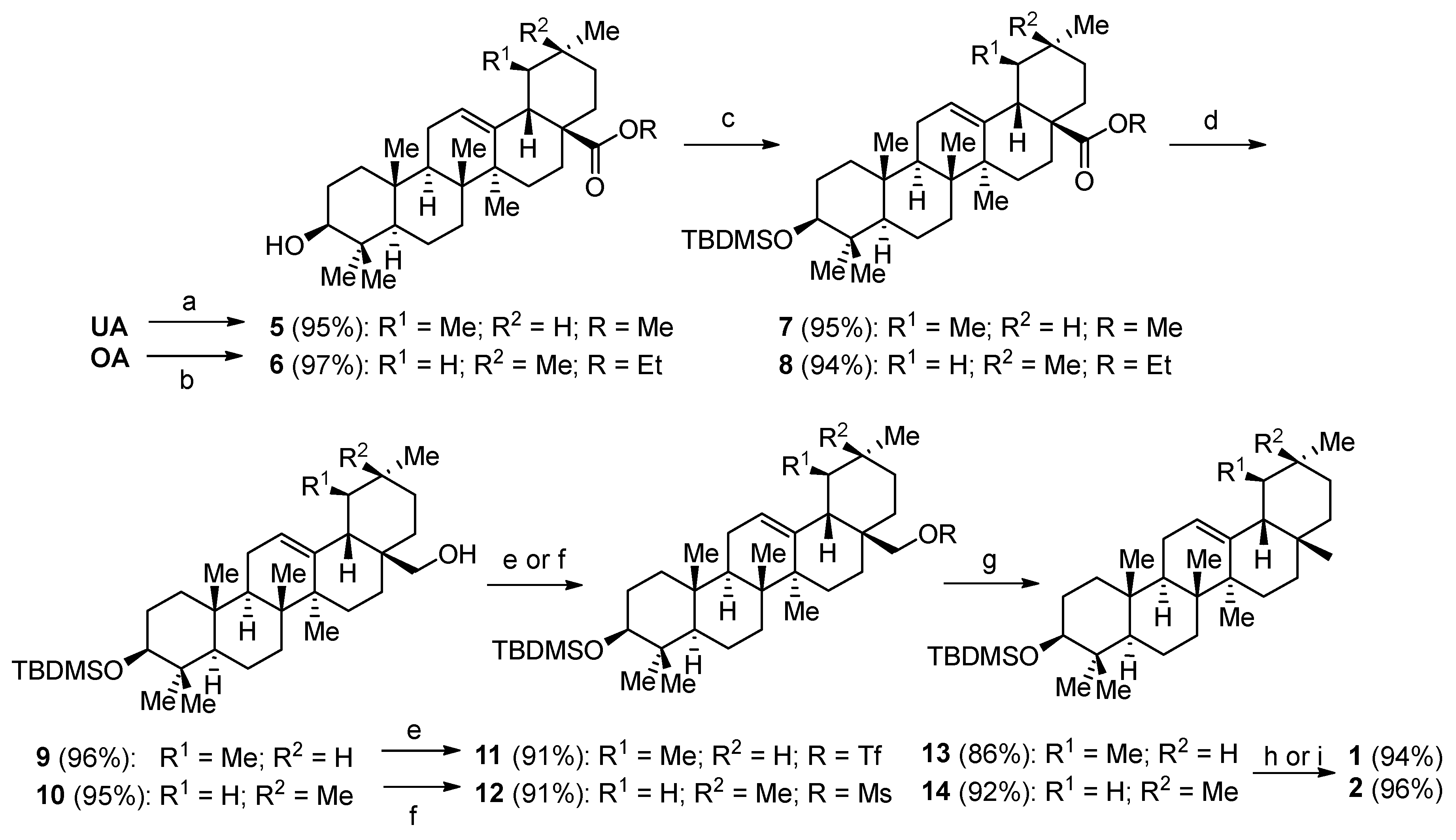
(3β) Methyl 3-hydroxyurs-12-en-28-oate (5)
Ursolic acid (20.0 g, 0.044 mol) was dissolved in DMF (250 mL) and potassium carbonate (30.0 g, 0.220 mol) was added. The mixture was stirred for 30 min, and methyl iodide (5 mL, 0.080 mol) was added. After stirring for 12 h at room temperature, the product was precipitated from the mixture by adding water (1 L). The solid was filtered off and washed with 2 M hydrochloric acid (2 × 20 mL), water (2 × 20 mL), and the resulting product was dried in a desiccator yielding
5 as a colorless crystal solid (19.7 g, 95%). m.p. = 172–174 °C (lit.: 172–173 °C) [
28];
= +62.3° (
c 0.385, CHCl
3) (lit.: +49.8° (
c 1.000, CHCl
3)) [
28]; R
f = 0.25 (silica gel, hexane/EtOAc 8:2); IR (KBr): ν = 3446
br, 2946
m, 2926
m, 2870
w, 1724
w, 1636
m, 1456
w, 1384
w, 1232
w, 1200
w, 1166
w, 1144
w, 1112
w, 1092
w, 1044
m, 1032
m, 756
m cm
−1;
1H NMR (500 MHz, CHCl
3) δ = 5.24 (
dd,
J = 3.6, 3.6 Hz, 1H, H-12), 3.60 (
s, 3H, H-31), 3.21 (
dd,
J = 11.1, 4.9 Hz, 1H, H-3), 2.22 (
d,
J = 11.0 Hz, 1H, H-18), 2.03–1.95 (
m, 1H, H-16a), 1.90 (
dd,
J = 8.9, 3.6 Hz, 2H, H-11a + H-11b), 1.99 (
td,
J = 13.4, 4.5 Hz, 1H, H-2a), 1.70–1.56 (
m, 7H, H-16b + H-22a + H-1a + H-1b + H-15a + H-22b + H-15b), 1.57–1.41 (
m, 4H, H-6a + H-9 + H-21a + H-7a), 1.40–1.23 (
m, 4H, H-6b + H-20 + H-21b + H-7b), 1.07 (
s, 3H, H-27), 1.06–0.98 (
m, 2H, H-2b + H-19), 0.98 (
s, 3H, H-24), 0.93 (
d,
J = 6.1 Hz, 3H, H-30), 0.91 (
s, 3H, H-25), 0.85 (
d,
J = 6.5 Hz, 3H, H-29), 0.77 (
s, 3H, H-23), 0.74 (
s, 3H, H-26), 0.73–0.69 (
m, 1H, H-5 ppm;
13C NMR (125 MHz, CDCl
3) δ = 178.2 (C-28), 138.3 (C-13), 125.7 (C-12), 79.2 (C-3), 55.4 (C- 5), 53.0 (C-18), 51.6 (C-31), 48.2 (C-17), 47.7 (C-9), 42.1 (C-14), 39.6 (C-8), 39.2 (C-20), 39.0 (C-19), 38.9 (C-1), 38.8 (C-4), 37.1 (C-10), 36.8 (C-22), 33.1 (C-7), 30.8 (C-21), 28.3 (C-24), 28.2 (C-2), 27.4 (C-15), 24.4 (C-16), 23.8 (C-27), 23.5 (C-11), 21.3 (C-30), 18.5 (C-6), 17.2 (C-29), 17.1 (C-26), 15.8 (C-23), 15.6 (C-25) ppm; MS (ASAP):
m/
z (%) = 453.6 ([M − H
2O + H]
+, 100), 471.7 ([M + H]
+, 86).
(3β) Ethyl 3-hydroxyolean-12-en-28-oate (6)
Oleanolic acid (10.0 g, 0.022 mol) was dissolved in DMF (200 mL) and potassium carbonate (15.0 g, 0.110 mol) was added. The mixture was stirred for 30 min, and ethyl iodide (3.5 mL, 0.044 mol) was added. After stirring for 12 h at room temperature, the product was precipitated by adding water (800 mL). The product was filtered off and washed with 2
M hydrochloric acid (2 × 20 mL), water (2 × 20 mL); drying in a desiccator gave
6 as a colorless crystalline solid (10.3 g, 97%). m.p. = 175–178 °C (Lit.: 217.5–218.0 °C) [
29];
= +73.4° (
c 0.34, CHCl
3) (lit.: +78.9°) [
29]; R
f = 0.68 (silica gel, hexane/EtOAc, 8:2); IR (KBr): ν= 3446
br, 2946
m, 2868
w, 1722
m, 1636
w, 1462
w, 1386
w, 1364
w, 1260
w, 1178
m, 1162
m, 1124
w, 1094
w, 1064
w, 1036
m cm
−1;
1H NMR (500 MHz, CDCl
3): δ = 5.28 (
dd,
J = 3.6, 3.6 Hz, 1H, H-12), 4.08 (
qq,
J = 10.8, 7.1 Hz, 2H, H-31), 3.20 (
m, 1H, H-3), 2.86 (
dd,
J = 13.9, 4.3 Hz, 1H, H-18), 2.00–1.91 (
m, 1H, H-16a), 1.90–1.80 (
m, 2H, H-11a + H-11b), 1.73–1.55 (
m, 7H, H-22a + H-19a + H-1a + H-15a + H-2a + H-2b + H-16b), 1.54–1.40 (
m, 4H, H-6a + H-22b + H-9 + H-7a), 1.40–1.24 (
m, 3H, H-6b + H-21a + H-7b), 1.22 (
t,
J = 7.1 Hz, 3H, H-32), 1.20–1.10 (
m, 2H, H-21b + H-19b), 1.13 (
s, 3H, H-27), 1.10–1.00 (
m, 1H, H-15b), 0.98 (
s, 3H, H-24), 0.98–0.93 (
m, 1H, H-1b), 0.92 (
s, 3H, H-30), 0.90 (
s, 3H, H-25), 0.89 (
s, 3H, H-29), 0.77 (
s, 3H, H-23), 0.74 (
s, 3H, H-26), 0.71 (
m, 1H, H-5) ppm;
13C NMR (125 MHz, CDCl
3): δ = 177.8 (C-28), 144.0 (C-13), 122.4 (C-12), 79.2 (C-3), 60.2 (C-31), 55.4 (C-5), 47.79 (C-9), 46.7 (C-17), 46.1 (C-19), 41.9 (C-14), 41.5 (C-18), 39.5 (C-8), 38.9 (C-1), 38.6 (C-4), 37.2 (C-10), 34.1 (C-21), 33.3 (C-29), 32.9, (C-7), 32.6 (C-22), 30.8 (C-20), 28.3 (C-24), 27.8 (C-15), 27.4 (C-2), 26.0 (C-27), 23.8 (C-30), 23.6(C-11), 23.2 (C-16), 18.5 (C-6), 17.1 (C-26), 15.7 (C-23), 15.5 (C-25), 14.4 (C-32) ppm; MS (ESI, MeOH):
m/
z (%) = 467.3 ([M − H
2O + H]
+, 70), 480.2 ([M + H]
+, 74), 991.5 ([2M + Na]
+, 100).
(3β) Methyl 3-{[(1,1-Dimethylethyl)dimethylsilyl]oxy}-urs-12-en-28-oate (7)
Compound
5 (19.0 g, 0.040 mol) was dissolved in dry DMF (300 mL), and imidazole (7.0 g, 0.103 mol) and TBDMSCl (6.8 g, 0.044 mol) were added. The reaction mixture was stirred for 24 h at 50 °C. The product was precipitated by adding water; it was filtered off and washed with 2
M hydrochloric acid (2 × 20 mL) and water (2 × 50 mL). The resulting solid was dried in a desiccator to yield
7 as a colorless crystalline solid (22.1 g, 95%) [
30] m.p. = 153 °C;
= +57.4° (
c 0.305, CHCl
3); R
f = 0.34 (silica gel, hexane/EtOAc, 9:1); IR (KBr): ν = 3446
br, 2948
m, 2930
m, 2856
w, 1728
w, 1636
m, 1460
w, 1386
w, 1360
w, 1252
w, 1166
w, 1142
w, 1108
m, 1100
m, 1032
w, 1004
w, 836
m cm
−1;
1H NMR (500 MHz, CDCl
3): δ = 5.24 (
dd,
J = 3.4, 3.4 Hz, 1H, H-12), 3.60 (
s, 3H, H-32), 3.18 (
dd,
J = 11.3, 4.6 Hz, 1H, H-3), 2.22 (
d,
J = 11.3 Hz, 1H, H-18), 2.04–1.94 (
m, 1H, H-16a), 1.93–1.87 (
m, 2H, H-11a + H-11b), 1.82–1.71 (
m, 1H, H-2a), 1.70–1.42 (
m, 10H, H-22a + H-1a + H-21a + H-15a + H-22b + H-1b + H-6a + H-15b + H-7a + H-9), 1.42–1.24 (
m, 4H, H-19 + H-7b + H-21b + H-6b), 1.07 (
s, 3H, H-27), 1.06–1.00 (
m, 2H, H-2b + H-20), 0.94 (
d,
J = 6.1 Hz, 3H, H-30), 0.91 (
s, 3H, H-25), 0.90 (
s, 3H, H-24), 0.88 (
s, 9H, H-34), 0.87–0.85 (
m, 1H, H-16b), 0.86 (
s, 3H, H-23), 0.85 (
s, 3H, H-26), 0.74 (
d,
J = 6.3 Hz, 3H, H-29), 0.72–0.67 (
m, 1H, H-5), 0.03 (
s, 6H, H-31) ppm;
13C NMR (125 MHz, CDCl
3): δ = 178.2 (C-28), 138.3 (C-13), 125.8 (C-12), 79.7 (C-3), 55.5 (C-5), 53.1 (C-18), 51.6 (C-32), 48.3 (C-17), 47.8 (C-9), 42.2 (C-14), 39.7 (C-4), 39.5 (C-8), 39.2 (C-19), 39.0 (C-20), 38.8 (C-1), 37.0 (C-10), 36.8 (C-22), 33.2 (C-7), 30.8 (C-21), 28.7 (C-24), 28.2 (C-2), 27.8 (C-15), 26.1 (C-34), 24.4 (C-16), 23.8 (C-27), 23.5 (C-11), 21.3 (C-30), 18.7 (C-6), 18.3 (C-33), 17.2 (C-29), 17.1 (C-23), 16.3 (C-26), 15.6 (C-25), −3.6 (C-31a), −4.7 (C-31b) ppm; MS (ESI, MeOH):
m/
z (%) = 585.2 ([M + H]
+, 100), 607.4 ([M + Na]
+, 16), 1192.5 ([2M + Na]
+, 62).
(3β) Ethyl 3-{[(1,1-Dimethylethyl)dimethylsilyl]oxy}-olean-12-en-28-oate (8)
Compound
6 (10.0 g, 0.021 mol) was dissolved in DMF (100 mL), imidazole (3.5 g, 0.520 mol) and TBDMSCl (3.5 g, 0.045 mol) were added. The reaction mixture was stirred for 24 h at 50 °C. The product was precipitated by adding water, which was filtrated off and washed with 2
m hydrochloric acid (2 × 20 mL) and water (2 × 50 mL), and dried in a desiccator to yield
8 as a colorless crystalline solid (11.83 g, 94%) [
30]. m.p. = 87 °C;
= +61.3° (
c 0.345, CHCl
3); R
f = 0.75 (silica gel, hexane/EtOAc, 9:1); IR (KBr): ν = 3444
br, 2950
s, 2932
s, 2858
s, 1636
w, 1464
m, 1388
m, 1362
w, 1254
m, 1146
w, 1102
s, 1076
m, 1064
m, 1050
m, 1006
m, 836
m, 774
m cm
−1;
1H NMR (500 MHz, CDCl
3): δ = 5.28 (
dd,
J = 3.7, 3.7 Hz, 1H, H-12), 4.16–3.99 (
m, 2H, H-31), 3.18 (
dd,
J = 11.1, 4.6 Hz, 1H, H-3), 2.86 (
dd,
J = 14.0, 4.5 Hz, 1H, H-18), 2.02–1.78 (
m, 3H, H-16a + H-11a + H-11b), 1.77–1.48 (
m, 9H, H-22a + H-19a + H-2a + H-16b + H-15a + H-1a + H-9 + H-22b + H-6a), 1.48–1.38 (
m, 2H, H-15b + H-7a), 1.38–1.27 (
m, 3H, H-6b + H-21b + H-7b), 1.22 (
t,
J = 7.1 Hz, 3H, H-32), 1.20–1.14 (
m, 2H, H-21b + H-19b), 1.13 (
s, 3H, H-27), 1.10–0.99 (
m, 1H, H-2b), 0.93–0.89 (
m, 1H, H-1b), 0.92 (
s, 3H, H-30), 0.90 (
s, 3H, H-23), 0.90 (
s, 3H, H-24), 0.90 (
s, 3H, H-29), 0.88 (
s, 9H, H-35), 0.74 (
s, 3H, H-25), 0.73 (
s, 3H, H-26), 0.72–0.67 (
m, 1H, H-5), 0.03 (
s, 6H, H-33) ppm;
13C NMR (125 MHz, CDCl
3)
: δ = 177.9 (C-28), 144.0 (C-13), 122.5 (C-12), 79.7 (C-3), 60.2 (C-31), 55.5 (C-5), 47.84 (C-9), 46.7 (C-17), 46.1 (C-19), 41.9 (C-14), 41.5 (C-18), 39.6 (C-4), 39.5 (C-10), 38.6 (C-1), 37.1 (C-8), 34.1 (C-21), 33.3 (C-29), 33.0 (C-7), 32.6 (C-22), 30.9 (C-20), 28.7 (C-24), 27.8 (C-15), 27.8 (C-2), 26.1 (C-32), 26.0 (C-27), 23.8 (C-30), 23.6 (C-11), 23.2 (C-16), 18.7 (C-6), 18.3 (C-34), 17.1 (C-26), 16.3 (C-25), 15.5 (C-23), 14.4 (C-35), −3.6 (C-33b), −4.7 (C-33a) ppm; MS (ASAP):
m/
z (%) = 467.2 ([M-Me
2BuSiOH + H]
+, 100), 599.3 ([M + H]
+, 16).
(3β) 3-{[(1,1-Dimethylethyl)dimethylsilyl]oxy}-urs-12-en-28-ol (9)
A solution of 7 (20 g, 0.03 mol) dissolved in dry THF (150 mL) was added to a solution of lithium aluminum hydride (5 g, 0.13 mol) in dry THF (300 mL) at 0 °C. The reaction mixture was allowed to warm to room temperature and finally heated for 3 h under reflux. Surplus lithium aluminum hydride was deactivated by adding THF/water (1:1) at 0 °C. An aqueous solution of sodium hydroxide (20 mL) was added, and the mixture was stirred for 15 min. The precipitate was filtered off and washed with diethyl ether (2 × 100 mL). The aqueous phase was extracted with diethyl ether (2 × 50 mL) and the combined organic extracts were washed with brine and dried over magnesium sulfate. After filtration, the solvent was removed under reduced pressure to yield crude 9. Further purification with column chromatography afforded (silica gel, hexane/EtOAc 9:1) 9 as a colorless crystalline solid (18.2 g, 96%). m.p. = 113–116 °C; = +59.8° (c 0.325, CHCl3); Rf = 0.25 (silica gel, hexane/EtOAc, 9:1); IR (KBr): 3448br, 2929s, 2856s, 1636w, 1461s, 1388m, 1254m, 1101s, 1069s, 1003m, 883m, 836s, 774m cm−1; 1H NMR (500 MHz, CDCl3): δ = 5.13 (dd, J = 3.4, 3.4 Hz, 1H, H-12), 3.52 (d, J = 10.9 Hz, 1H, H-28a), 3.22–3.16 (m, 2H, H-28b + H-3), 1.98–1.86 (m, 3H, H-16a + H-11a + H-11b), 1.83–1.72 (m, 1H, H-15a), 1.65–1.44 (m, 8H, H-2a + H-1a + H-22a + H-7a + H-6a + H-9 + H-21a + H-2b), 1.44–1.30 (m, 5H, H-19 + H-22b + H-6b + H-18 + H-7b), 1.28–1.15 (m, 2H, H-16b + H-21b), 1.10 (s, 3H, H-27), 0.98 (s, 3H, H-26), 1.03–0.93 (m, 2H, H-15b + H-1b), 0.94 (s, 3H, H-25), 0.94 (d, J = 6.7 Hz, 3H, H-30), 0.91 (s, 3H, H-24), 0.89 (s, 9H, H-33), 0.89 (m, 1H, H-20), 0.81 (d, J = 5.6 Hz, 3H, H-29), 0.75 (s, 3H, H-23), 0.74–0.67 (m, 1H, H-5), 0.03 (s, 6H, H-31) ppm; 13C NMR (125 MHz, CDCl3): δ = 138.8 (C-13), 125.3 (C-12), 79.6 (C-3), 70.1 (C-28), 55.4 (C-5), 54.2 (C-18), 47.9 (C-9), 42.2 (C-14), 40.2 (C-8), 39.6 (C-19), 39.5 (C-20), 39.5 (C-4), 39.0 (C-1), 38.2 (C-17), 36.9 (C-10), 35.3 (C-22), 33.1 (C-7), 30.8 (C-21), 28.7 (C-24), 27.8 (C-2), 26.2 (C-15), 26.1 (C-33), 23.6 (C-11), 23.5 (C-16), 23.5 (C-27), 21.5 (C-30), 18.7 (C-6), 18.3 (C-32), 17.5 (C-29), 16.9 (C-26), 16.3 (C-23), 15.9 (C-25), −3.6 (C-31a), −4.7 (C-31b) ppm; MS (ASAP): m/z (%) = 425.6 ([M-Me2BuSiOH + H]+, 100), 557.8 ([M + H]+, 20).
(3β) 3-{[(1,1-Dimethylethyl)dimethylsilyl]oxy}-olean-12-en-28-ol (10)
A solution of
8 (10.0 g, 0.017 mol) in dry THF (100 mL) was added slowly to a solution of lithium aluminum hydride (3.0 g, 0.080 mol) in dry THF (150 mL) at 0 °C. The solution was heated under reflux until TLC showed the reaction to be complete. The mixture was cooled to 0 °C and surplus of LiAlH
4 was deactivated by adding THF/water (1:1). An aqueous solution (2
M) of sodium hydroxide (10 mL) was added, and the mixture was stirred for 15 min. The solid was filtered off, washed with diethyl ether (2 × 50 mL), and the organic phase was washed with brine (25 mL). The aqueous phase was washed with diethyl ether (2 × 50 mL). The combined organic phases were dried over magnesium sulfate and concentrated under reduced pressure. After column chromatography (silica gel, hexane/EtOAc 9:1) compound
10 was obtained as a colorless crystalline solid (8.7 g, 95%). m.p. = 106–108 °C (lit.: 110–111 °C) [
15];
= +64.3° (
c 0.345, CHCl
3) (lit.: +61.0° (
c 0.5, CHCl
3)) [
15]; R
f = 0.28 (silica gel, hexane/EtOAc, 9:1); IR (KBr): 3444
br, 2950
s, 2932
s, 2858
s, 1636
w, 1464
m, 1388
m, 1362
w, 1254
m, 1102
s, 1076
m, 1064
m, 1050
m, 1006
m, 836
m, 774
m cm
−1;
1H NMR (400 MHz, CDCl
3): δ = 5.19 (
dd,
J = 3.6, 3.6 Hz, 1H, H-12), 3.55 (
d,
J = 10.5 Hz, 1H, H-28a), 3.22 (
d,
J = 10.6 Hz, 1H, H-28b), 3.21–3.16 (
m, 1H, H-3), 2.06–1.95 (
m, 1 H, H-18), 1.94–1.77 (
m, 3 H, H-16a + H-11a + H-11b), 1.78–1.65 (
m, 2H, H-19a + H-2a), 1.65–1.24 (
m, 11H, H-15a + H-1a + H-6a + H-7a + H-9 + H-22a + H-15b + H-6b + H-7b + H-22b + H-21a), 1.24–1.12 (
m, 2H, H-16b + H-21b), 1.16 (
s, 3H, H-27), 1.12–0.96 (
m, 2H, H-19b + H-2b), 0.95–0.87 (
m, 1H, H-1b), 0.93 (
s, 3H, H-26), 0.93 (
s, 3H, H-23), 0.91 (
s, 3H, H-30), 0.89 (
s, 9H, H-33), 0.89 (
s, 3H, H-24), 0.89 (
s, 3H, H-29), 0.75 (
s, 3H, H-25), 0.71 (
m, 1H, H-5), 0.03 (
s, 6H, H-31) ppm;
13C NMR (100 MHz, CDCl
3): δ = 144.3 (C-13), 122.6 (C-12), 79.6 (C-3), 69.9 (C-28), 55.4 (C-5), 47.8 (C-9), 46.6 (C-19), 42.5 (C-18), 41.9 (C-14), 40.0 (C-8), 39.5 (C-4), 38.8 (C-1), 37.1 (C-17), 37.0 (C-10), 34.3 (C-21), 33.4 (C-29), 32.8 (C-22), 31.2 (C-20), 31.1 (C-7), 28.7 (C-30), 27.8 (C-15), 26.1 (C-33), 26.1 (C-24), 25.7 (C-2), 23.8 (C-27), 23.7 (C-11), 22.2 (C-16), 18.7 (C-6), 18.3 (C-32), 16.9 (C-26), 16.3 (C-25), 15.7 (C-23), −3.6 (C-31a), −4.7 (C-31b) ppm; MS (ASAP):
m/
z (%) = 425.5 ([M − Me
2BuSiOH + H]
+, 100), 557.6 ([M + H]
+, 24).
(3β) 3-{[(1,1-Dimethylethyl)dimethylsilyl]oxy}-28-{[(trifluoromethyl)sulfonyl]oxy}-urs-12-en (11)
Compound 9 (1.0 g, 1.8 mmol) was dissolved in DCM (50 mL). At 0 °C, pyridine (0.3 mL, 3.71 mmol) and trifluoromethanesulfonic anhydride (0.45 mL, 2.7 mmol) were added slowly. The reaction mixture was stirred for 15 min at 0 °C. Then NaHCO3 (20 mL, aq, satd.) was added, and the mixture was washed with 2 M hydrochloric acid. The organic phase was dried over magnesium sulfate, filtered, and the solvent was removed under reduced pressure to yield 11 as a colorless amorphous solid (1.13 g, 91%). Rf = 0.46 (silica gel, hexane) 1H NMR (500 MHz, CDCl3): δ = 5.17 (dd, J = 3.5, 3.5 Hz, 1H, H-12), 4.19 (d, J = 9.2 Hz, 1H, H-28a), 3.72 (d, J = 9.3 Hz, 1H, H-28b), 3.18 (dd, J = 11.2, 4.6 Hz, 1H, H-3), 2.06–1.94 (m, 1H, H-16a), 1.94–1.89 (m, 2H, H-11a + H-11b), 1.89–1.69 (m, 1H, H-15a), 1.69–1.62 (m, 1H, H-22a), 1.63–1.31 (m, 7H, H-2a + H-1a + H-7a + H-6a + H-9 + H-21a + H-2b), 1.31–1.15 (m, 7H, H-19 + H-18 + H-22b + H-6b + H-7b + H16b + H-21b), 1.11 (s, 3H, H-27), 1.09–1.02 (m, 1H, H-15b), 1.00 (s, 3H, H-26), 0.99–0.94 (m, 1H, H-1b), 0.94 (s, 3H, H-23), 0.94 (s, 3H, H-30), 0.91 (s, 3H, H-24), 0.88 (s, 9H, H-33), 0.88 (m, 1H, H-20), 0.81 (d, J = 5.4 Hz, 3H, H-29), 0.75 (s, 3H, H-25), 0.73–0.68 (m, 1H, H-5), 0.03 (s, 6H, H-31) ppm; 13C NMR (125 MHz, CDCl3): δ = 137.2 (C-13), 127.0 (C-12), 118.8 (q, J = 319.8 Hz, C-34), 84.3 (C-3), 79.6 (C-28), 55.4 (C-5), 53.7 (C-18), 47.8 (C-9), 42.1 (C-14), 40.2 (C-8), 39.5 (C-4), 39.4 (C-19), 39.3 (C-20), 39.0 (C-1), 37.9 (C-17), 36.9 (C-10), 35.2 (C-22), 32.9 (C-7), 30.2 (C-21), 28.7 (C-24), 27.8 (C-2), 26.1 (C-33), 25.8 (C-15), 23.6 (C-11), 23.5 (C-27), 23.0 (C-16), 21.3 (C-30), 18.6 (C-6), 18.3 (C-32), 17.2 (C-29), 16.6 (C-26), 16.3 (C-23), 15.9 (C-25), −3.6 (C-31a), −4.7 (C-31b) ppm; MS (ASAP): m/z (%) = 557.5.2 ([M-Me2BuSiOH + H]+, 14), 407.5 ([M-HOTf-Me2BuSiOH + H]+, 100).
(3β) 3-{[(1,1-Dimethylethyl)dimethylsilyl]oxy}-olean-12-en-28-methansulfonate (12)
Compound 10 (5 g, 9.0 mmol) was dissolved in DCM (200 mL). At 0 °C triethylamine (4 mL, 28.7 mmol) and methanesulfonyl chloride (0.76 mL, 9.87 mmol) were added. The reaction mixture was stirred for an hour at room temperature and quenched by adding water (50 mL) and 2 M hydrochloric acid (20 mL). The organic phase was separated. The aqueous phase was extracted with DCM (50 mL). The combined organic extracts were washed with brine and dried over magnesium sulfate. Purification by column chromatography (silica gel, hexane/EtOAc, 9:1) afforded 12 as a colorless crystalline solid (5.31 g, 91%). m.p. = 71–74 °C; = +66.4° (c 0.320, CHCl3); Rf = 0.25 (silica gel, hexane/EtOAc, 9:1); IR (KBr): 3444br, 2952s, 2930s, 2856s, 1638w, 1460m, 1386m, 1358s, 1254m, 1176s, 1100m, 1072m, 1004m, 954s, 838s, 776m, 530m cm−1; 1H NMR (500 MHz, CDCl3): δ = 5.23 (dd, J = 3.5, 3.5 Hz, 1H, H-12), 4.18 (d, J = 9.3 Hz, 1H, H-28a), 3.78 (d, J = 9.3 Hz, 1H, H-28b), 3.18 (dd, J = 11.1, 4.5 Hz, 1H, H-3), 2.98 (s, 3H, H-34), 2.06–1.84 (m, 3H, H-16a + H-11a + H-11b), 1.85–1.74 (m, 1H, H-2a), 1.70–1.43 (m, 11H, H-1a + H-15a + H-19a + H-21a + H-6a + H-15b + H-7a + H-22a + H-9+ H-21b + H-6b), 1.42–1.33 (m, 4H, H-16a + H-19b + H-18 + H-22b), 1.33– 1.18 (m, 2H, H-16b + H-7b), 1.17 (s, 3H, H-27), 1.13–1.07 (m, 1H, H-2b), 1.07–0.99 (m, 1H, H-1b), 0.96 (s, 3H, H-26), 0.92 (s, 3H, H-24), 0.91 (s, 3H, H-23), 0.90 (s, 3H, H-30), 0.89 (s, 9H, H-33), 0.88 (s, 3H, H-29), 0.75 (s, 3H, H-25), 0.73–0.67 (m, 1H, H-5), 0.03 (s, 6H, H-32) ppm; 13C NMR (125 MHz, CDCl3): δ = 143.2 (C-13), 123.7 (C-12), 79.6 (C-3), 76.7 (C-28), 55.4 (C-5), 47.7 (C-9), 46.2 (C-19), 42.4 (C-18), 41.8 (C-14), 40.0 (C-8), 39.5 (C-4), 38.8 (C-1), 37.1 (C-34), 37.0 (C-17), 36.5 (C-10), 33.9 (C-21), 33.2 (C-29), 32.7 (C-22), 31.4 (C-20), 31.0 (C-7), 28.7 (C-30), 27.8 (C-15), 26.2 (C-24), 26.1 (C-33), 25.5 (C-2), 23.8 (C-11), 23.7 (C-27), 21.9 (C-16), 18.6 (C-6), 18.3 (C-32), 16.8 (C-26), 16.3 (C-25), 15.7 (C-23), −3.6 (C-31a), −4.7 (C-31b) ppm; MS (ASAP): m/z (%) = 407.3 ([M-Me2BuSiOH-MeSO3H + H]+, 10), 503.3 ([M − Me2BuSiOH + H]+, 60).
(3β) 3-{[(1,1-Dimethylethyl)dimethylsilyl]oxy}-urs-12-en (13)
A solution of 11 (1.0 g, 1.45 mmol) in dry THF (10 mL) was added at 0 °C to a solution of lithium aluminum hydride (0.2 g, 5.27 mmol) in dry THF (20 mL). The reaction mixture was stirred for four hours at room temperature. Work-up as described above, followed by column chromatography (silica gel, hexane), gave 13 as a colorless crystalline solid (0.68 g, 86%). m.p. = 179–182 °C; = +69.4° (c 0.305, CHCl3); Rf = 0.92 (silica gel, hexane); IR (KBr): 3448br, 2926s, 2855s, 1636w, 1458m, 1386m, 1253m, 1101s, 1069m, 1004w, 835m, 774m cm−1; 1H NMR (500 MHz, CDCl3): δ = 5.13 (dd, J = 3.6, 3.6 Hz, 1H, H-12), 3.20 (dd, J = 11.2, 4.6 Hz, 1H, H-3), 2.05–1.95 (m, 1H, H-16a), 1.95–1.79 (m, 3H, H-11a + H-11b + H-15a), 1.66–1.58 (m, 2H, H-2a + H-1a), 1.58–1.21 (m, 12H, H-6a + H-7a + H-9 + H-2b + H-22a + H-6b + H-21a + H-7b + H-18 + H-19 + H-22b + H-21b), 1.07 (s, 3H, H-27), 1.01 (s, 3H, H-26), 1.00–0.96 (m, 2H, H-15b + H-1b), 0.95 (s, 3H, H-23), 0.92 (d, J = 6.0 Hz, 3H, H-30), 0.91 (s, 3H, H-24), 0.89 (s, 9H, H-33), 0.89–0.83 (m, 2H, H-20 + H-16b), 0.80 (s, 3H, H-28), 0.79 (d, J = 6.0 Hz, 3H, H-29), 0.76 (s, 3H, H-25), 0.71 (m, 1H, H-5), 0.04 (s, 6H, H-31) ppm; 13C NMR (125 MHz, CDCl3): δ = 139.7 (C-13), 124.7 (C-12), 79.7 (C-3), 59.3 (C-18), 55.5 (C-5), 48.0 (C-9), 42.3 (C-14), 41.7 (C-22), 40.2 (C-8), 39.8 (C-19), 39.8 (C-20), 39.5 (C-4), 39.0 (C-1), 37.0 (C-10), 33.9 (C-17), 33.2 (C-7), 31.5 (C-21), 28.9 (C-28), 28.7 (C-24), 28.3 (C-16), 27.9 (C-2), 26.8 (C-15), 26.1 (C-33), 23.6 (C-11), 23.4 (C-27), 21.6 (C-30), 18.7 (C-6), 18.3 (C-32), 17.6 (C-29), 17.1 (C-26), 16.3 (C-25), 15.9 (C-23), −3.6 (C-32a), −4.7 (C-32b) ppm; MS (ASAP): m/z (%) = 409.2 ([M − Me2BuSiOH + H]+, 100), 541.3 ([M + H]+, 2).
(3β) 3-{[(1,1-Dimethylethyl)dimethylsilyl]oxy}-olean-12-en (14)
Reduction of
12 (4 g, 6.3 mmol) in THF (50 mL) at 0 °C with lithium aluminum hydride (1.2 g, 31.6 mmol) in THF (100 mL), as described above, followed by column chromatography (silica gel, hexane) gave
14 as a colorless crystalline solid (3.14 g, 92%). m.p. = 174–176 °C (lit.: 179.5–180.5 °C) [
15];
= +72.6° (
c 0.315, CHCl
3) (lit.: +78.6° (
c 0.21, CHCl
3)) [
15]; R
f = 0.94 (silica gel, hexane); IR (KBr): 3447
br, 2949
s, 2930
s, 2856
s, 1636
w, 1461
m, 1387
m, 1253
m, 1100
m, 1076
m, 884
m, 835
s, 771
m cm
−1;
1H NMR (500 MHz, CDCl
3): δ = 5.18 (
dd,
J = 3.5, 3.5 Hz, 1H, H-12), 3.19 (
dd,
J = 11.1, 4.6 Hz, 1H, H-3), 2.04–1.91 (
m, 2H, H-16a + H-18), 1.93–1.72 (
m, 3H, H-11a + H-11b + H-15a), 1.72–1.37 (
m, 9H, H-19a + H-2a + H-1a + H-6a + H-9 + H-7a + H-2b + H-22a + H-6b), 1.37–1.28 (
m, 2H, H-21a + H-7b), 1.29–1.17 (
m, 1H, H-22b), 1.13 (
s, 3H, H-27), 1.13–1.07 (
m, 1H, H-21b), 1.06–0.99 (
m, 1H, H-19b), 0.99–0.95 (
m, 1H, H-15b), 0.96 (
s, 3H, H-26), 0.94–0.90 (
m, 1H, H-1b), 0.94 (
s, 3H, H-25), 0.91 (
s, 3H, H-24), 0.89 (
s, 9H, H-33), 0.88 (
s, 3H, H-30), 0.87 (
s, 3H, H-29), 0.84 (
s, 3H, H-28), 0.83–0.77 (
m, 1H, H-16b), 0.76 (
s, 3H, H-23), 0.74–0.69 (
m, 1H, H-5), 0.04 (
s, 6H, H-31) ppm;
13C NMR (125 MHz, CDCl
3): δ = 145.3 (C-13), 122.0 (C-12), 79.7 (C-3), 55.5 (C-5), 47.9 (C-9), 47.4 (C-18), 47.0 (C-19), 41.9 (C-14), 40.00 (C-8), 39.5 (C-4), 38.8 (C-1), 37.3 (C-22), 37.0 (C-10), 34.9 (C-21), 33.5 (C-30), 32.9 (C-7), 32.7 (C-17), 31.3 (C-20), 28.7 (C-24), 28.6 (C-28), 27.8 (C-2), 27.1 (C-16), 26.3 (C-15), 26.2 (C-27), 26.1 (C-33), 23.9 (C-29), 23.7 (C-11), 18.8 (C-6), 18.3 (C-32), 17.0 (C-26), 16.3 (C-23), 15.7 (C-25), −3.6 (C-31a), −4.7 (C-31b) ppm; MS (ASAP):
m/
z (%) = 409.2 ([M-Me
2BuSiOH + H]
+, 100), 541.3 ([M + H]
+, 18).