In Silico Screening of Isocitrate Lyase for Novel Anti-Buruli Ulcer Natural Products Originating from Africa
Abstract
1. Introduction
2. Results and Discussion
2.1. Homology Modeling
2.2. Structure Validation and Quality Prediction
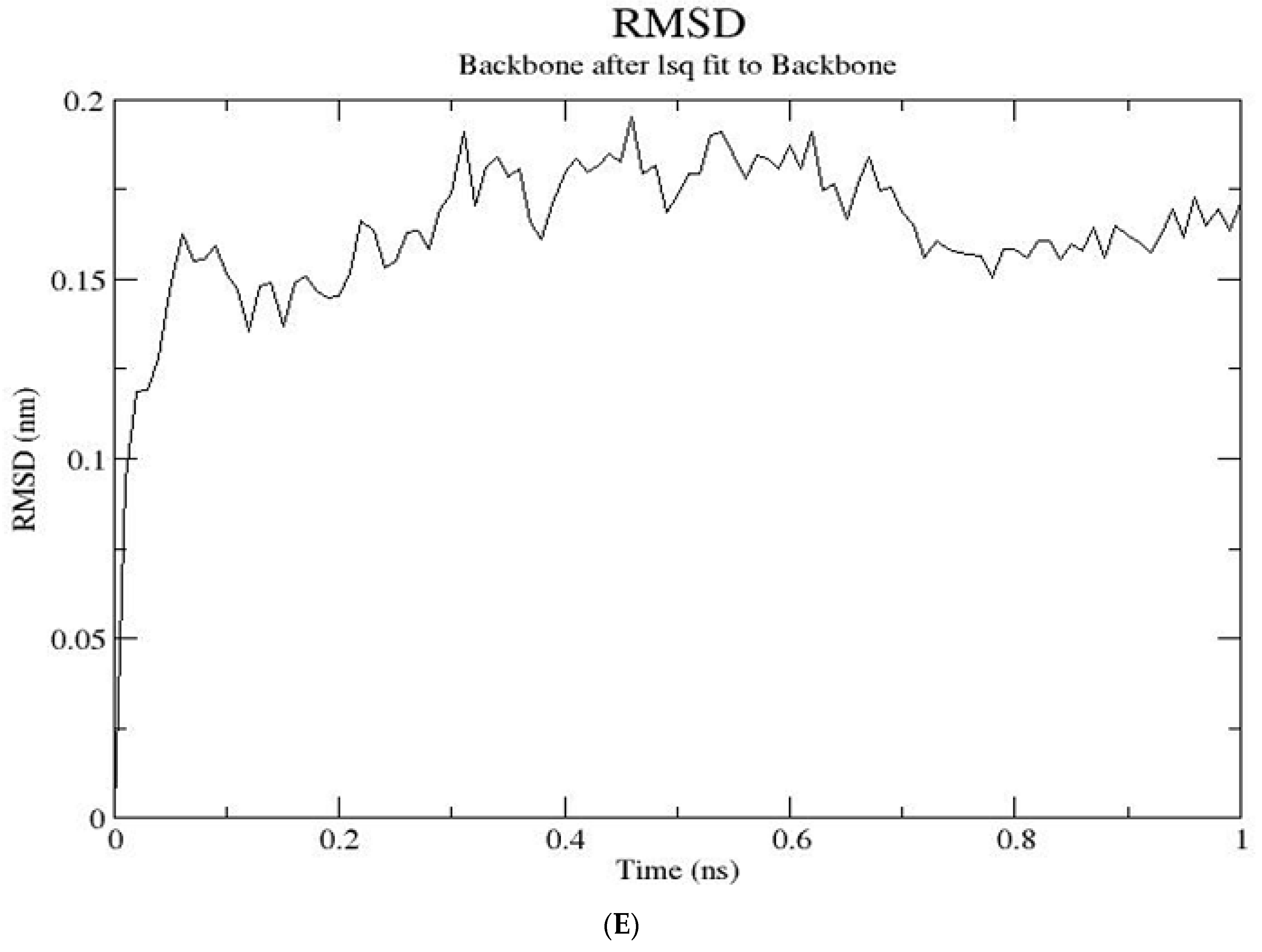
2.3. Molecular Dynamics Simulations
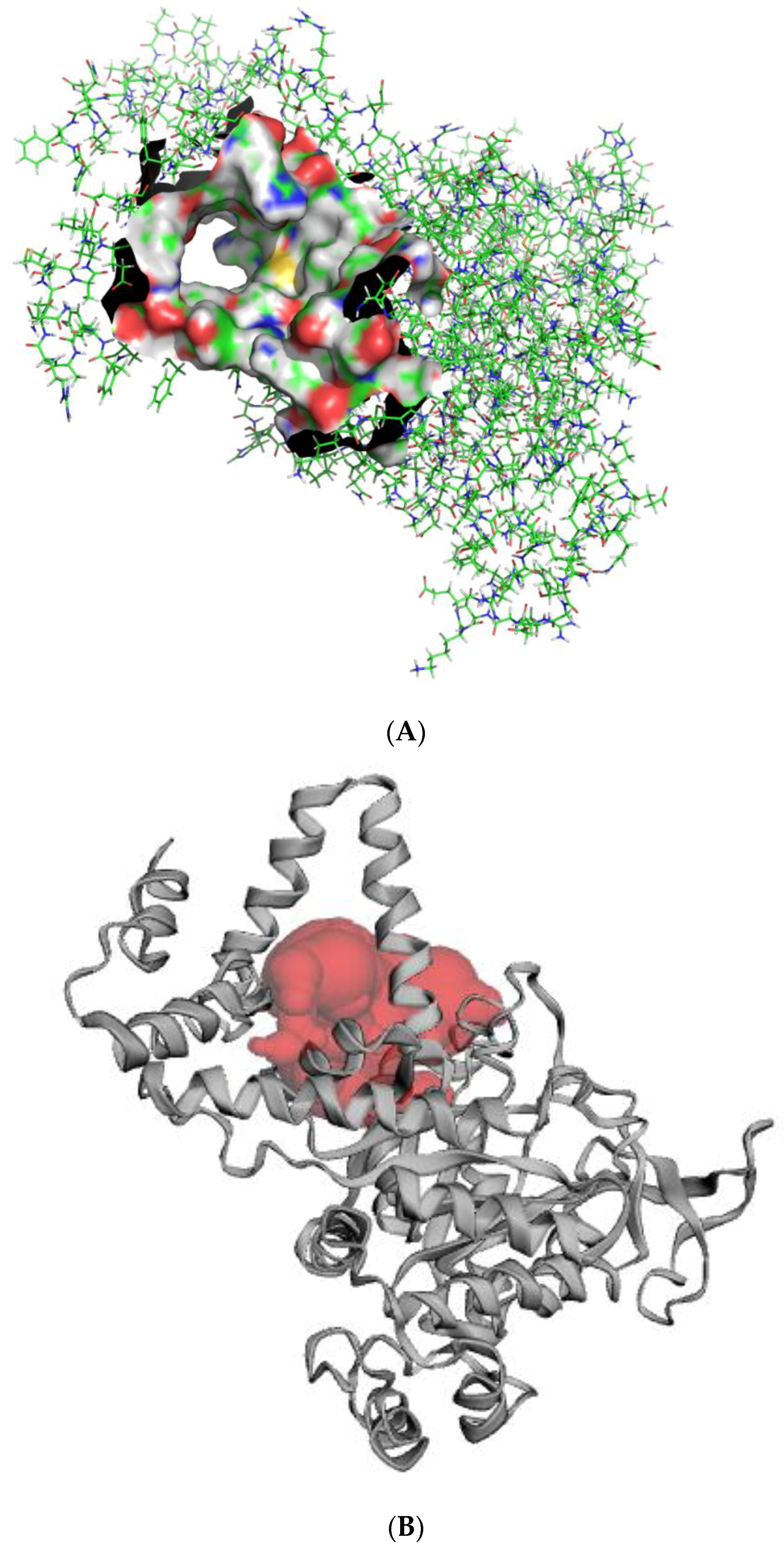
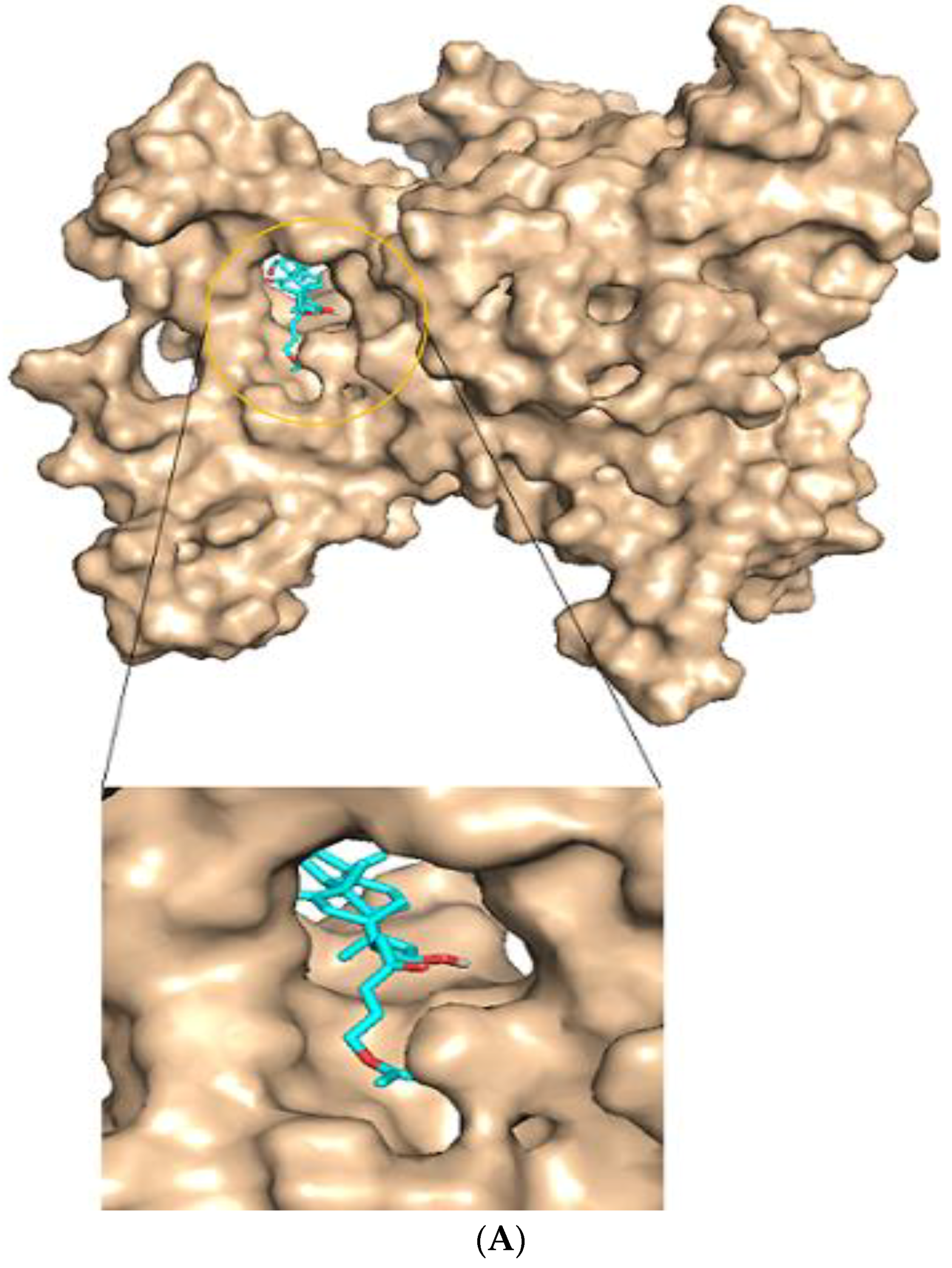
2.4. Active Site Detection
2.5. Virtual Screening Library of Natural Products
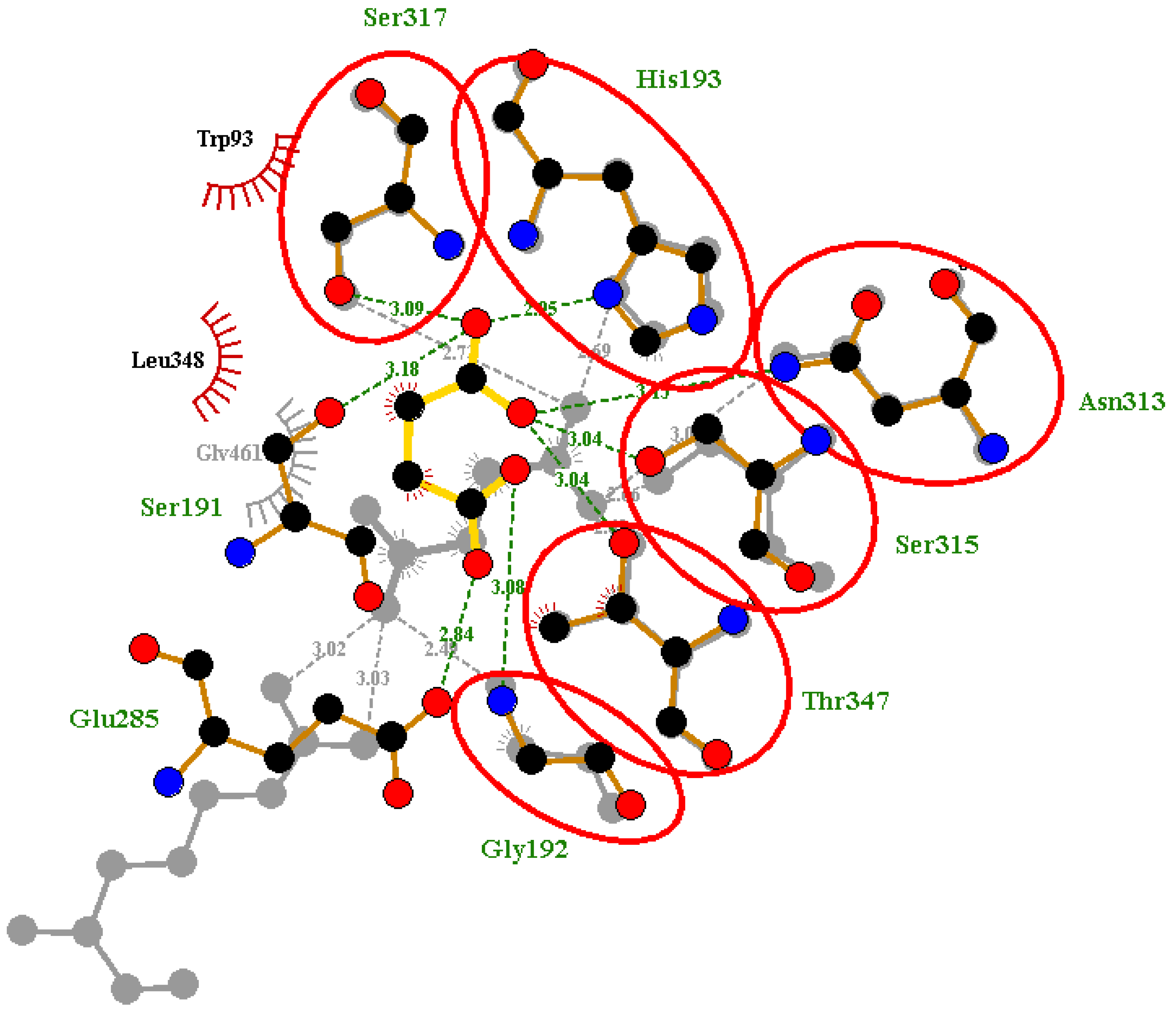
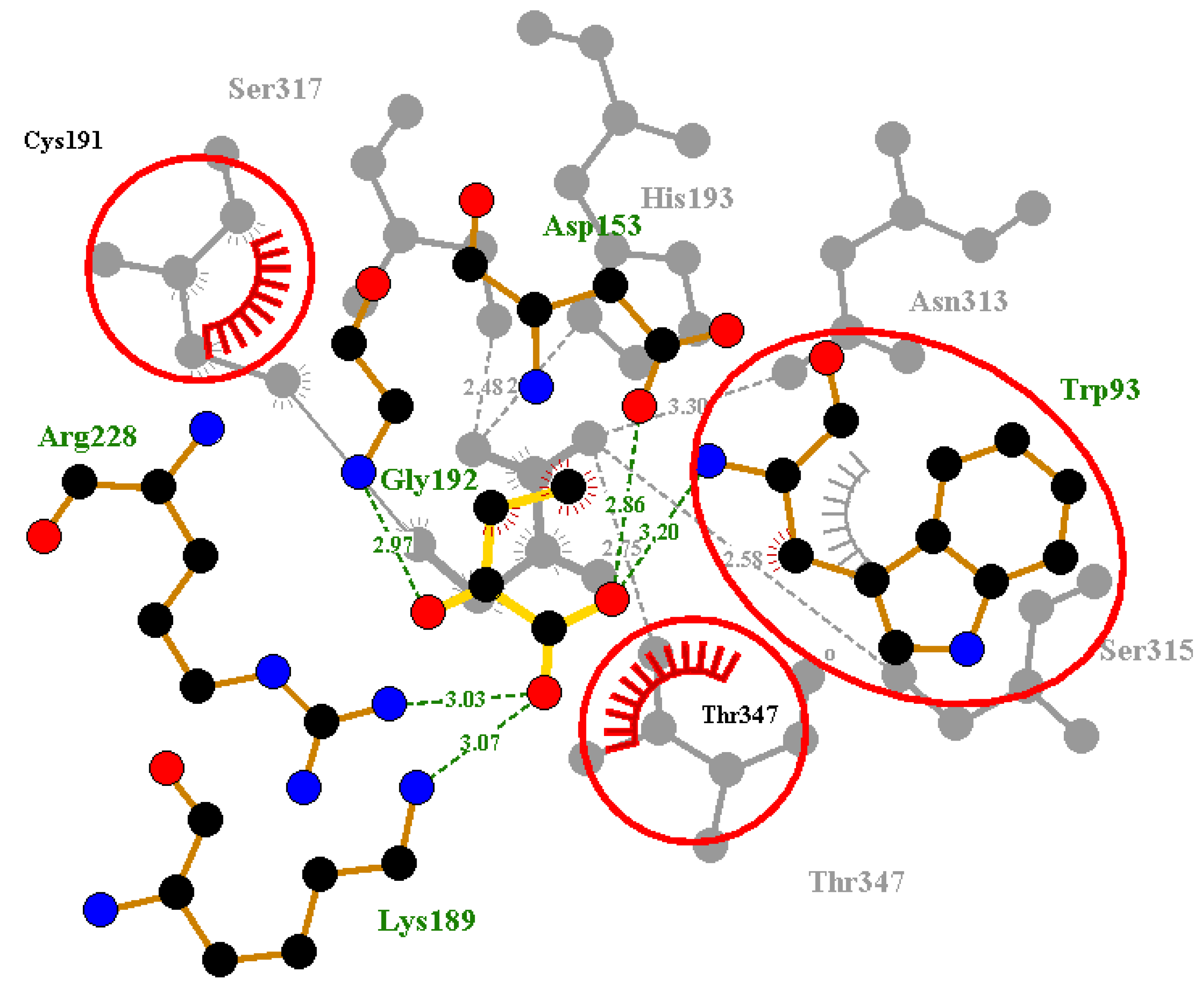
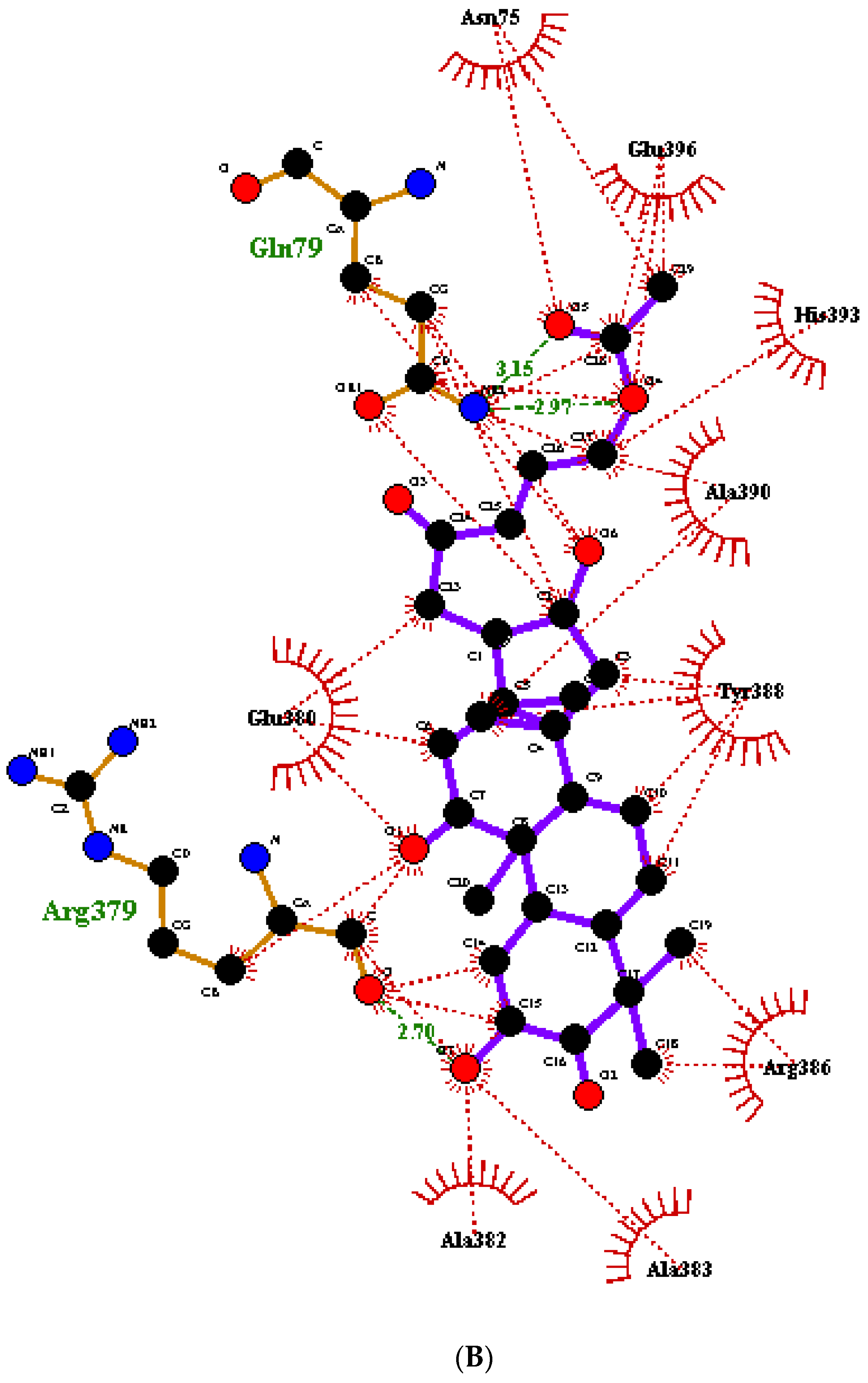
2.6. Protein-Ligand Interactions
2.7. Docking Protocol Validation
2.7.1. Superimposition and Alignment
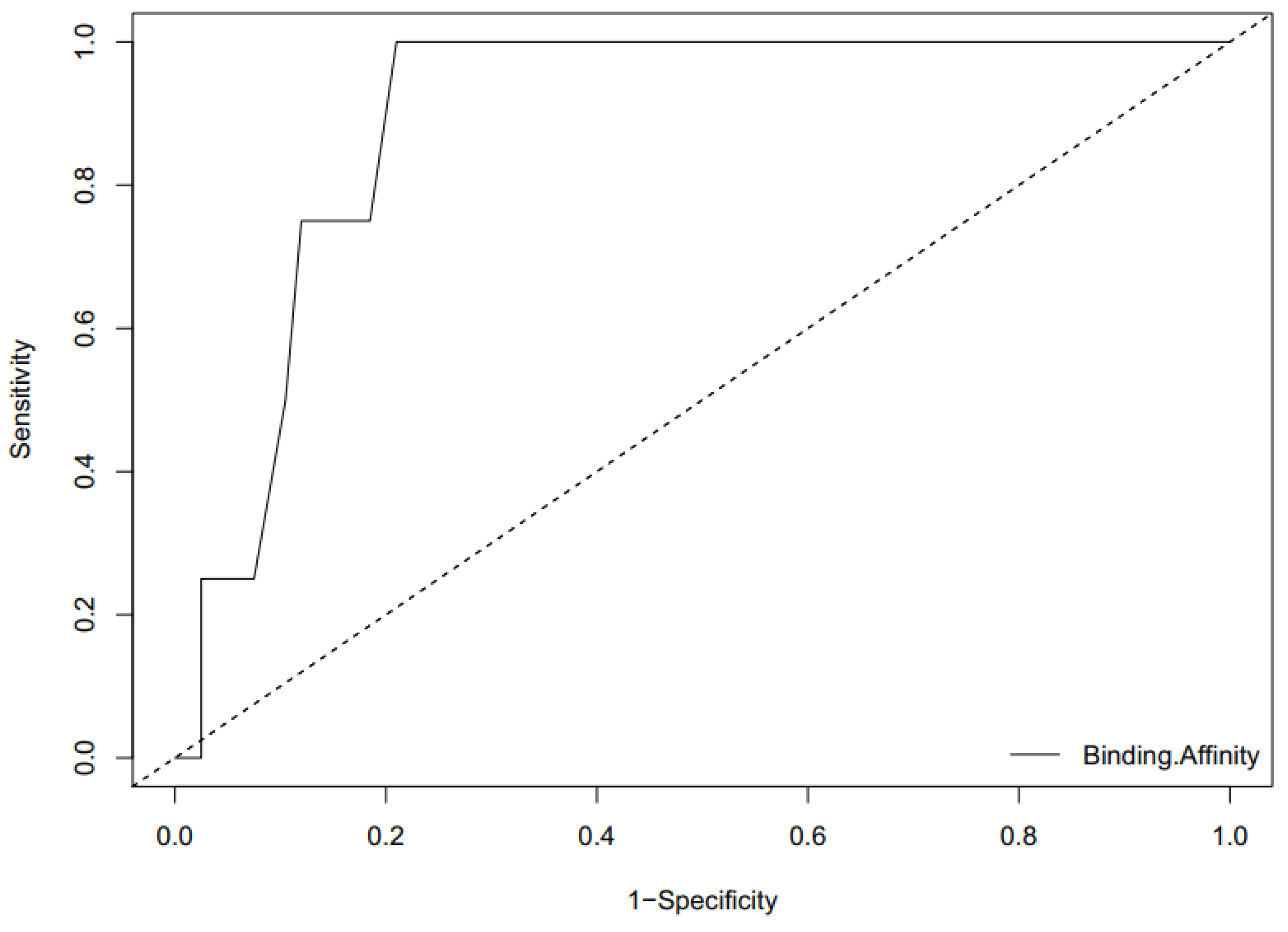
2.7.2. ROC Curve Analysis
2.8. Pharmacological Studies for Discovery of Leads
2.9. Prediction of Lead Compounds
2.10. Induced Fit Docking
3. Materials and Methods
3.1. Sequence Retrieval and Homology Modeling
3.2. Protein Structure Refinement
3.3. Molecular Dynamics Simulation of Protein Structure
3.4. Protein Validation and Active Site Prediction
3.5. Molecular Docking and Mechanisms of Binding
3.6. Validation of Docking Protocol
3.7. Pharmacological Profiling
3.8. Prediction of Activity Spectra for Substances (PASS) for Leads
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oliveira, M.S.; Fraga, A.G.; Torrado, E.; Castro, A.G.; Pereira, J.P.; Filho, A.L.; Milanezi, F.; Schmitt, F.C.; Meyers, W.M.; Portaels, F.; et al. Infection with Mycobacterium ulcerans Induces Persistent Inflammatory Responses in Mice. Infect. Immun. 2005, 73, 6299–6310. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.D.; Boakye, D.A.; Mosi, L.; Asiedu, K. In the case of transmission of Mycobacterium ulcerans in buruli ulcer disease Acanthamoeba species stand accused. Ghana Med. J. 2011, 45, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Leão, S.C.; Romano, M.I.; Jesus, M. Tuberculosis, Leprosy, and Other Mycobacterioses. In Bioinformatics in Tropical Disease Research: A Practical and Case-Study Approach; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2007. Available online: https://www.ncbi.nlm.nih.gov/books/NBK6820/ (accessed on 16 May 2018).
- Marsollier, L.; Robert, R.; Aubry, J.; Saint André, J.-P.; Kouakou, H.; Legras, P.; Manceau, A.-L.; Mahaza, C.; Carbonnelle, B. Aquatic insects as a vector for Mycobacterium ulcerans. Appl. Environ. Microbiol. 2002, 68, 4623–4628. [Google Scholar] [CrossRef] [PubMed]
- Marsollier, L.; Deniaux, E.; Brodin, P.; Marot, A.; Wondje, C.M.; Saint-André, J.-P.; Chauty, A.; Johnson, C.; Tekaia, F.; Yeramian, E.; et al. Protection against Mycobacterium ulcerans Lesion Development by Exposure to Aquatic Insect Saliva. PLoS Med. 2007, 4, e64. [Google Scholar] [CrossRef] [PubMed]
- Merritt, R.W.; Walker, E.D.; Small, P.L.C.; Wallace, J.R.; Johnson, P.D.R.; Benbow, M.E.; Boakye, D.A. Ecology and transmission of Buruli ulcer disease: A systematic review. PLoS Negl. Trop. Dis. 2010, 4, e911. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Treatment of Mycobacterium Ulcerans Disease (Buruli Ulcer); WHO: Geneva, Switzerland, 2012; pp. 1–73. [Google Scholar]
- Azumah, B.K.; Addo, P.G.; Dodoo, A.; Awandare, G.; Mosi, L.; Boakye, D.A.; Wilson, M.D. Experimental demonstration of the possible role of Acanthamoeba polyphaga in the infection and disease progression in Buruli Ulcer (BU) using ICR mice. PLoS ONE 2017, 12, e0172843. [Google Scholar] [CrossRef] [PubMed]
- WHO. Buruli Ulcer; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Kumar, S.; Basu, S.; Bhartiya, S.K.; Shukla, V.K. The Buruli Ulcer. Int. J. Low. Extrem. Wounds 2015, 14, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Klis, S.; Stienstra, Y.; Phillips, R.O.; Abass, K.M.; Tuah, W.; van der Werf, T.S. Long Term Streptomycin Toxicity in the Treatment of Buruli Ulcer: Follow-up of Participants in the BURULICO Drug Trial. PLoS Negl. Trop. Dis. 2014, 8, e2739. [Google Scholar] [CrossRef] [PubMed]
- Yeboah-Manu, D.; Kpeli, G.S.; Ruf, M.-T.; Asan-Ampah, K.; Quenin-Fosu, K.; Owusu-Mireku, E.; Paintsil, A.; Lamptey, I.; Anku, B.; Kwakye-Maclean, C.; et al. Secondary bacterial infections of buruli ulcer lesions before and after chemotherapy with streptomycin and rifampicin. PLoS Negl. Trop. Dis. 2013, 7, e2191. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.L.; Buntine, J.A.; Hayman, J.A.; Lavender, C.J.; Fyfe, J.A.M.; Hosking, P.; Starr, M.; Johnson, P.D.R. All-Oral Antibiotic Treatment for Buruli Ulcer: A Report of Four Patients. PLoS Negl. Trop. Dis. 2010, 4, e770. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Lefrancois, S.; Robert, J.; Chauffour, A.; Truffot, C.; Jarlier, V. In Vitro and In Vivo Activities of Rifampin, Streptomycin, Amikacin, Moxifloxacin, R207910, Linezolid, and PA-824 against Mycobacterium ulcerans. Antimicrob. Agents Chemother. 2006, 50, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Chauffour, A.; Robert, J.; Lefrançois, S.; Jarlier, V. Orally administered combined regimens for treatment of Mycobacterium ulcerans infection in mice. Antimicrob. Agents Chemother. 2007, 51, 3737–3739. [Google Scholar] [CrossRef] [PubMed]
- Scherr, N.; Pluschke, G.; Panda, M. Comparative Study of Activities of a Diverse Set of Antimycobacterial Agents against Mycobacterium tuberculosis and Mycobacterium ulcerans. Antimicrob. Agents Chemother. 2016, 60, 3132–3137. [Google Scholar] [CrossRef] [PubMed]
- Tsouh, P.V.F.; Addo, P.; Yeboah-Manu, D.; Boyom, F.F. Methods used in preclinical assessment of anti-Buruli ulcer agents: A global perspective. J. Pharmacol. Toxicol. Methods 2015, 73, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Buruli Ulcer (Mycobacterium ulcerans Infection). Available online: http://www.who.int/en/news-room/fact-sheets/detail/buruli-ulcer-(mycobacterium-ulcerans-infection (accessed on 15 June 2018).
- Johnson, P.D.R.; Stinear, T.; Small, P.L.C.; Pluschke, G.; Merritt, R.W.; Portaels, F.; Huygen, K.; Hayman, J.A.; Asiedu, K. Buruli Ulcer (M. ulcerans Infection): New Insights, New Hope for Disease Control. PLoS Med. 2005, 2, e108. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, C.; Gupta, A.; Kanjilal, S.; Katiyar, S. Drug discovery from plant sources: An integrated approach. Ayu 2012, 33, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Talele, T.; Khedkar, S.; Rigby, A. Successful Applications of Computer Aided Drug Discovery: Moving Drugs from Concept to the Clinic. Curr. Top. Med. Chem. 2010, 10, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Guo, S.; Cui, H.; Qi, J. Virtual Screening of Small Molecular Inhibitors against DprE1. Molecules 2018, 23, 524. [Google Scholar] [CrossRef] [PubMed]
- Billones, J.; Carrillo, M.; Organo, V.; Macalino, S.J.; Emnacen, I.; Bernadette, J. Virtual Screening against Mycobacterium tuberculosis Lipoate Protein Ligase B (MtbLipB) and In Silico ADMET Evaluation of Top Hits. Orient. J. Chem. 2013, 29, 1457–1468. [Google Scholar] [CrossRef]
- Kumar, A.; Siddiqi, M.I. Virtual screening against Mycobacterium tuberculosis dihydrofolate reductase: Suggested workflow for compound prioritization using structure interaction fingerprints. J. Mol. Graph. Model. 2008, 27, 476–488. [Google Scholar] [CrossRef] [PubMed]
- McKinney, J.D.; zu Bentrup, K.H.; Muñoz-Elías, E.J.; Miczak, A.; Chen, B.; Chan, W.-T.; Swenson, D.; Sacchettini, J.C.; Jacobs, W.R.; Russell, D.G. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 2000, 406, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Dunn, M.F.; Ramirez-Trujillo, J.A.; Hernandez-Lucas, I. Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. Microbiology 2009, 155, 3166–3175. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-V.; Wahab, H.A.; Choong, Y.S. Potential inhibitors for isocitrate lyase of Mycobacterium tuberculosis and non-M. tuberculosis: A summary. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.; Lee, S.; Park, J.; Lee, D. Predicting unintended effects of drugs based on off-target tissue effects. Biochem. Biophys. Res. Commun. 2016, 469, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Chartier, M.; Morency, L.-P.; Zylber, M.I.; Najmanovich, R.J. Large-scale detection of drug off-targets: Hypotheses for drug repurposing and understanding side-effects. BMC Pharmacol. Toxicol. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.L.; Xie, L.; Xie, L.; Bourne, P.E.; Palsson, B.Ø. Drug off-target effects predicted using structural analysis in the context of a metabolic network model. PLoS Comput. Biol. 2010, 6. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.M.; Nasrullah, I.; Tahir, S.; Tong, Y. Comparative Genomics Analysis of Mycobacterium ulcerans for the Identification of Putative Essential Genes and Therapeutic Candidates. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Ntie-Kang, F.; Zofou, D.; Babiaka, S.B.; Meudom, R.; Scharfe, M.; Lifongo, L.L.; Mbah, J.A.; Mbaze, L.M.; Sippl, W.; Efange, S.M.N. AfroDb: A Select Highly Potent and Diverse Natural Product Library from African Medicinal Plants. PLoS ONE 2013, 8, e78085. [Google Scholar] [CrossRef] [PubMed]
- Alvin, A.; Miller, K.I.; Neilan, B.A. Exploring the potential of endophytes from medicinal plants as sources of antimycobacterial compounds. Microbiol. Res. 2014, 169, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Dankwa, B.; Kwofie, K.S. In Silico Prediction of Potential Natural Product-Derived Lead Compounds for the Treatment of Buruli Ulcer. In Proceedings of the Waccbip Research Conference, Legon, Accra, 6–7 July 2017; p. 106. [Google Scholar]
- Benson, D.A.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2015, 43, D30–D35. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.W.; Prlić, A.; Altunkaya, A.; Bi, C.; Bradley, A.R.; Christie, C.H.; Di Costanzo, L.; Duarte, J.M.; Dutta, S.; Feng, Z.; et al. The RCSB protein data bank: Integrative view of protein, gene and 3D structural information. Nucleic Acids Res. 2017, 45, D271–D281. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.W.; Prlić, A.; Bi, C.; Bluhm, W.F.; Christie, C.H.; Dutta, S.; Green, R.K.; Goodsell, D.S.; Westbrook, J.D.; Woo, J.; et al. The RCSB Protein Data Bank: Views of structural biology for basic and applied research and education. Nucleic Acids Res. 2015, 43, D345–D356. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef] [PubMed]
- Eswar, N.; Webb, B.; Marti-Renom, M.A.; Madhusudhan, M.S.; Eramian, D.; Shen, M.; Pieper, U.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Protein Sci. 2007, 50. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.-Y.; Sali, A. Statistical potential for assessment and prediction of protein structures. Protein Sci. 2006, 15, 2507–2524. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.; Sali, A. Comparative protein structure modeling using MODELLER. In Current Protocols in Bioinformatics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; Volume 2016. [Google Scholar]
- Fiser, A. Template-Based Protein Structure Modeling. In Methods in Molecular Biology; Springer: New York, NY, USA, 2010; Volume 673, pp. 73–94. [Google Scholar]
- Hasan, M.A.; Alauddin, S.M.; Al Amin, M.; Nur, S.M.; Mannan, A. In silico molecular characterization of cysteine protease YopT from Yersinia pestis by homology modeling and binding site identification. Drug Target Insights 2014. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhang, Y. Improving the Physical Realism and Structural Accuracy of Protein Models by a Two-Step Atomic-Level Energy Minimization. Biophys. J. 2011, 101, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Bekker, H.; Berendsen, H.; Dijkstra, E.; Achterop, S.; Vondrumen, R.; Vanderspoel, D.; Sijbers, A.; Keegstra, H.; Renardus, M. Gromacs—A Parallel Computer for Molecular-Dynamics Simulations; World Scientific Publishing: Singapore, 1993. [Google Scholar]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Fiser, A.; Sali, A. ModLoop: Automated modeling of loops in protein structures. Bioinformatics 2003, 19, 2500–2501. [Google Scholar] [CrossRef] [PubMed]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef] [PubMed]
- Yakubu, A.; De Donato, M.; Imumorin, I.G. Modelling functional and structural impact of non-synonymous single nucleotide polymorphisms of the DQA1 gene of three Nigerian goat breeds. S. Afr. J. Anim. Sci. 2017, 47. [Google Scholar] [CrossRef]
- Cristobal, S.; Zemla, A.; Fischer, D.; Rychlewski, L.; Elofsson, A. A study of quality measures for protein threading models. BMC Bioinform. 2001, 2. [Google Scholar] [CrossRef]
- Wallner, B.; Elofsson, A. Can correct protein models be identified? Protein Sci. 2003, 12, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Meng, X.-Y.; Zhang, H.-X.; Mezei, M.; Cui, M. Molecular docking: A powerful approach for structure-based drug discovery. Curr. Comput. Aided. Drug Des. 2011, 7, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Das, S.; Stanley, A.; Yadav, L.; Sudhakar, A.; Varma, A.K. Optimized Hydrophobic Interactions and Hydrogen Bonding at the Target-Ligand Interface Leads the Pathways of Drug-Designing. PLoS ONE 2010, 5, e12029. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.L.E.; dos Santos, G.B.; Franco, M.S.F.; Federico, L.B.; da Silva, C.H.T.P.; Santos, C.B.R. Molecular modeling and statistical analysis in the design of derivatives of human dipeptidyl peptidase IV. J. Biomol. Struct. Dyn. 2018, 36, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Heifets, A.; Lilien, R.H. LigAlign: Flexible ligand-based active site alignment and analysis. J. Mol. Graph. Model. 2010, 29, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.J.; Froufe, H.J.C.; Costa, A.F.T.; Santos, A.F.; Oliveira, L.G.; Osório, S.R.M.; Abreu, R.M.V.; Pintado, M.; Ferreira, I.C.F.R. Docking studies in target proteins involved in antibacterial action mechanisms: Extending the knowledge on standard antibiotics to antimicrobial mushroom compounds. Molecules 2014, 19, 1672–1684. [Google Scholar] [CrossRef] [PubMed]
- Triballeau, N.; Acher, F.; Brabet, I.; Pin, J.-P.; Bertrand, H.-O. Virtual screening workflow development guided by the “receiver operating characteristic” curve approach. Application to high-throughput docking on metabotropic glutamate receptor subtype 4. J. Med. Chem. 2005, 48, 2534–2547. [Google Scholar] [CrossRef] [PubMed]
- Shamsara, J. Correlation between Virtual Screening Performance and Binding Site Descriptors of Protein Targets. Int. J. Med. Chem. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Goksuluk, D.; Korkmaz, S.; Zararsiz, G.; Karaagaoglu, A.E. EasyROC: An interactive web-tool for ROC curve analysis using R language environment. R J. 2016, 8, 213–230. [Google Scholar]
- Cruz, J.V.; Neto, M.F.A.; Silva, L.B.; da Ramos, R.; da Costa, J.; Brasil, D.S.B.; Lobato, C.C.; da Costa, G.V.; Bittencourt, J.A.H.M.; da Silva, C.H.T.P.; et al. Identification of Novel Protein Kinase Receptor Type 2 Inhibitors Using Pharmacophore and Structure-Based Virtual Screening. Molecules 2018, 23, 453. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [PubMed]
- El Khouli, R.H.; Macura, K.J.; Barker, P.B.; Habba, M.R.; Jacobs, M.A.; Bluemke, D.A. Relationship of temporal resolution to diagnostic performance for dynamic contrast enhanced MRI of the breast. J. Magn. Reson. Imaging 2009, 30, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Sriram, D.; Yogeeswari, P.; Methuku, S.; Vyas, D.R.K.; Senthilkumar, P.; Alvala, M.; Jeankumar, V.U. Synthesis of various 3-nitropropionamides as Mycobacterium tuberculosis isocitrate lyase inhibitor. Bioorg. Med. Chem. Lett. 2011, 21, 5149–5154. [Google Scholar] [CrossRef] [PubMed]
- Gilson, M.K.; Liu, T.; Baitaluk, M.; Nicola, G.; Hwang, L.; Chong, J. BindingDB in 2015: A public database for medicinal chemistry, computational chemistry and systems pharmacology. Nucleic Acids Res. 2016, 44, D1045–D1053. [Google Scholar] [CrossRef] [PubMed]
- Mujovo, S.F.; Hussein, A.A.; Meyer, J.J.M.; Fourie, B.; Muthivhi, T.; Lall, N. Bioactive compounds from Lippia javanica and Hoslundia opposita. Nat. Prod. Res. 2008, 22, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Lagunin, A.; Stepanchikova, A.; Filimonov, D.; Poroikov, V. PASS: Prediction of activity spectra for biologically active substances. Bioinformatics 2000, 16, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Jamkhande, P.G.; Pathan, S.K.; Wadher, S.J. In silico PASS analysis and determination of antimycobacterial, antifungal, and antioxidant efficacies of maslinic acid in an extract rich in pentacyclic triterpenoids. Int. J. Mycobacteriol. 2016, 5, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Addo, P.; Quartey, M.; Abbas, M.; Adu-Addai, B.; Owusu, E.; Okang, I.; Dodoo, A.; De Souza, D.; Ankrah, N.; Ofori-Adjei, D. In-Vitro Susceptibility of Mycobacterium Ulcerans to Herbal Preparations. Internet J. Trop. Med. 2007, 4, 1–11. [Google Scholar]
- Xu, M.; Lill, M.A. Induced fit docking, and the use of QM/MM methods in docking. Drug Discov. Today Technol. 2013, 10, e411–e418. [Google Scholar] [CrossRef] [PubMed]
- Carlson, H.A. Protein flexibility and drug design: How to hit a moving target. Curr. Opin. Chem. Biol. 2002, 6, 447–452. [Google Scholar] [CrossRef]
- Farid, R.; Day, T.; Friesner, R.A.; Pearlstein, R.A. New insights about HERG blockade obtained from protein modeling, potential energy mapping, and docking studies. Bioorg. Med. Chem. 2006, 14, 3160–3173. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.; Day, T.; Jacobson, M.P.; Friesner, R.A.; Farid, R. Novel procedure for modeling ligand/receptor induced fit effects. J. Med. Chem. 2006, 49, 534–553. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein-Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed]
- Kumar Deokar, H.; Barch, H.P.; Buolamwini, J.K. Homology Modeling of Human Concentrative Nucleoside Transporters (hCNTs) and Validation by Virtual Screening and Experimental Testing to Identify Novel hCNT1 Inhibitors. Drug Des. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Tran, L.M.; Stang, J.L. Induced-fit docking studies of the active and inactive states of protein tyrosine kinases. J. Mol. Graph. Model. 2009, 28, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Medina-Franco, J.L.; Méndez-Lucio, O.; Yoo, J. Rationalization of activity cliffs of a sulfonamide inhibitor of DNA methyltransferases with induced-fit docking. Int. J. Mol. Sci. 2014, 15, 3253–3261. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.J.; Wang, J.Z.; Huang, N.Y.; Deng, W.Q.; Zou, K. Induced-fit docking and virtual screening for 8-hydroxy-3-methoxy- 5H-pyrido [2,1-c] pyrazin-5-one derivatives as inducible nitric oxide synthase inhibitors. J. Chem. Pharm. Res. 2014, 6, 1187–1194. [Google Scholar]
- Sherman, W.; Beard, H.S.; Farid, R. Use of an Induced Fit Receptor Structure in Virtual Screening. Chem. Biol. Drug Des. 2006, 67, 83–84. [Google Scholar] [CrossRef] [PubMed]
- How Is the IFD Score Calculated and What Is Its Units? Schrödinger. Available online: https://www.schrodinger.com/kb/307 (accessed on 13 June 2018).
- Vriend, G. WHAT IF: A molecular modeling and drug design program. J. Mol. Graph. 1990, 8, 52–56. [Google Scholar] [CrossRef]
- Arnold, K.; Bordoli, L.; Kopp, J.; Schwede, T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics 2006, 22, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-Pdb Viewer: An environment for comparative protein modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Binkowski, T.A.; Naghibzadeh, S.; Liang, J. CASTp: Computed Atlas of Surface Topography of proteins. Nucleic Acids Res. 2003, 31, 3352–3355. [Google Scholar] [CrossRef] [PubMed]
- Dundas, J.; Ouyang, Z.; Tseng, J.; Binkowski, A.; Turpaz, Y.; Liang, J. CASTp: Computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic Acids Res. 2006, 34, W116–W118. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.K.; Ray, D.; Guchhait, N. Unraveling the binding interaction and kinetics of a prospective anti-HIV drug with a model transport protein: Results and challenges. Phys. Chem. Chem. Phys. 2013, 15, 1275–1287. [Google Scholar] [CrossRef] [PubMed]
- Padilha, E.C.; Serafim, R.B.; Sarmiento, D.Y.R.; Santos, C.F.; Santos, C.B.R.; Silva, C.H.T.P. New PPARα/γ/δ Optimal Activator Rationally Designed by Computational Methods. J. Braz. Chem. Soc. 2016, 27, 1636–1647. [Google Scholar] [CrossRef]
- Khan, M.F.; Nahar, N.; Bin Rashid, R.; Chowdhury, A.; Rashid, M.A. Computational investigations of physicochemical, pharmacokinetic, toxicological properties and molecular docking of betulinic acid, a constituent of Corypha taliera (Roxb.) with Phospholipase A2 (PLA2). BMC Complement. Altern. Med. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Irwin, J.J.; Sterling, T.; Mysinger, M.M.; Bolstad, E.S.; Coleman, R.G. ZINC: A free tool to discover chemistry for biology. J. Chem. Inf. Model. 2012, 52, 1757–1768. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open chemical toolbox. J. Cheminform. 2011, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, A.M.; Bajorath, J. BindingDB and ChEMBL: Online compound databases for drug discovery. Expert Opin. Drug Discov. 2011, 6, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Costa, J.; da Silva Lopes Costa, K.; Cruz, J.V.; da Silva Ramos, R.; Silva, L.B.; Do Socorro Barros Brasil, D.; de Paula da Silva, C.H.T.; Dos Santos, C.B.R.; da Cruz Macedo, W.J. Virtual Screening and Statistical Analysis in the Design of New Caffeine Analogues Molecules with Potential Epithelial Anticancer Activity. Curr. Pharm. Des. 2018, 24, 576–594. [Google Scholar] [CrossRef] [PubMed]
- Gey van Pittius, N.C.; Sampson, S.L.; Lee, H.; Kim, Y.; van Helden, P.D.; Warren, R.M. Evolution and expansion of the Mycobacterium tuberculosis PE and PPE multigene families and their association with the duplication of the ESAT-6 (esx) gene cluster regions. BMC Evol. Biol. 2006, 6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mysinger, M.M.; Carchia, M.; Irwin, J.J.; Shoichet, B.K. Directory of useful decoys, enhanced (DUD-E): Better ligands and decoys for better benchmarking. J. Med. Chem. 2012, 55, 6582–6594. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
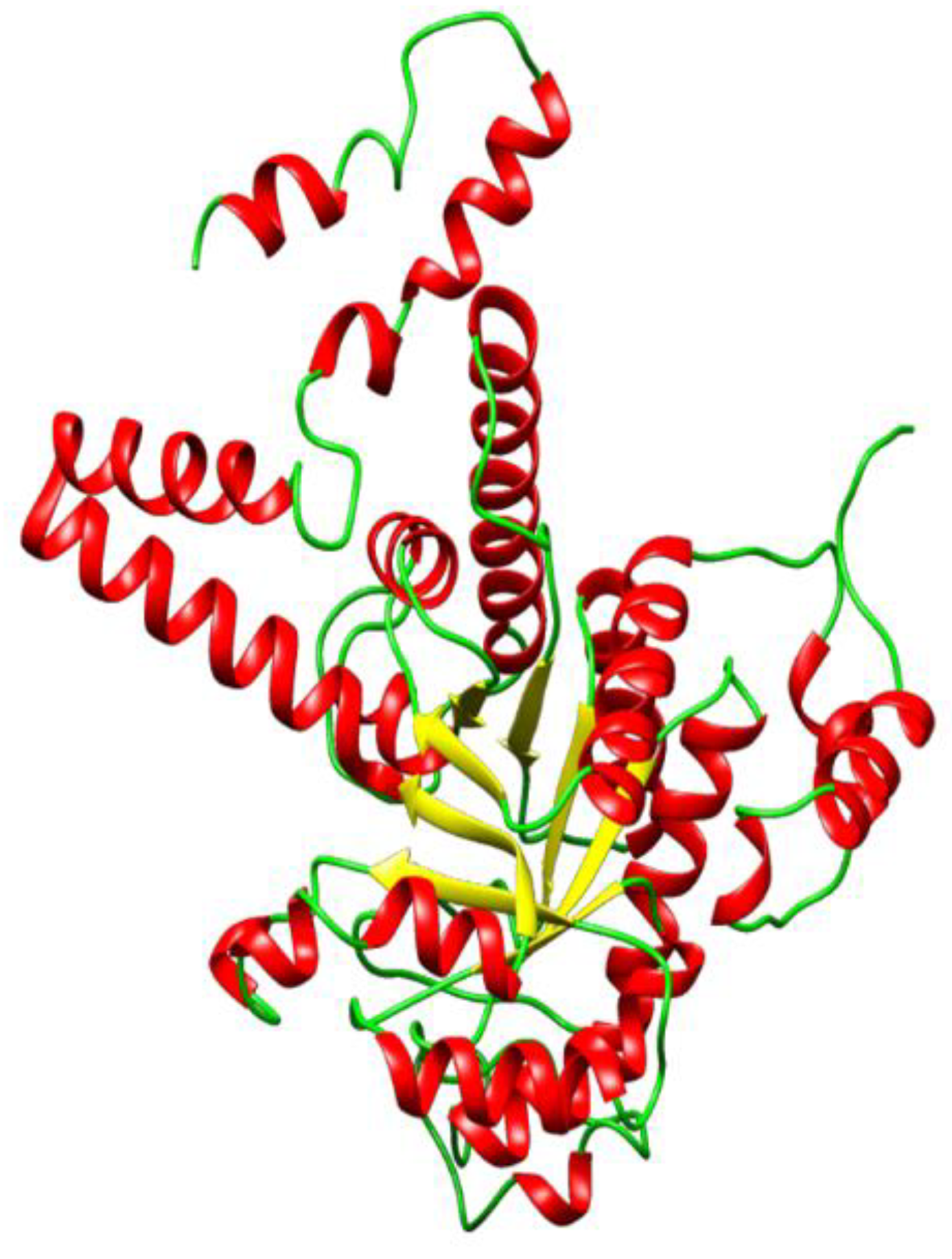
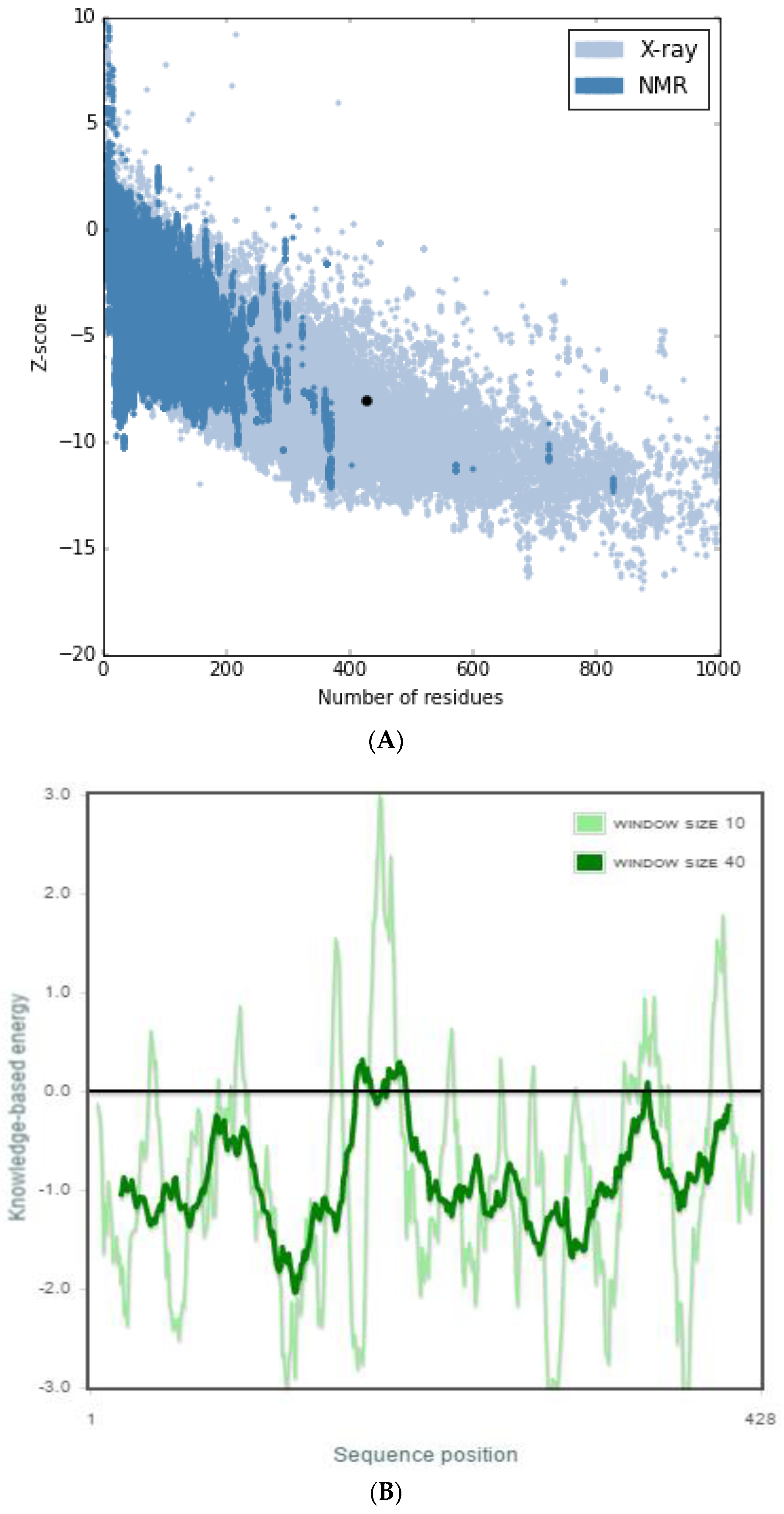
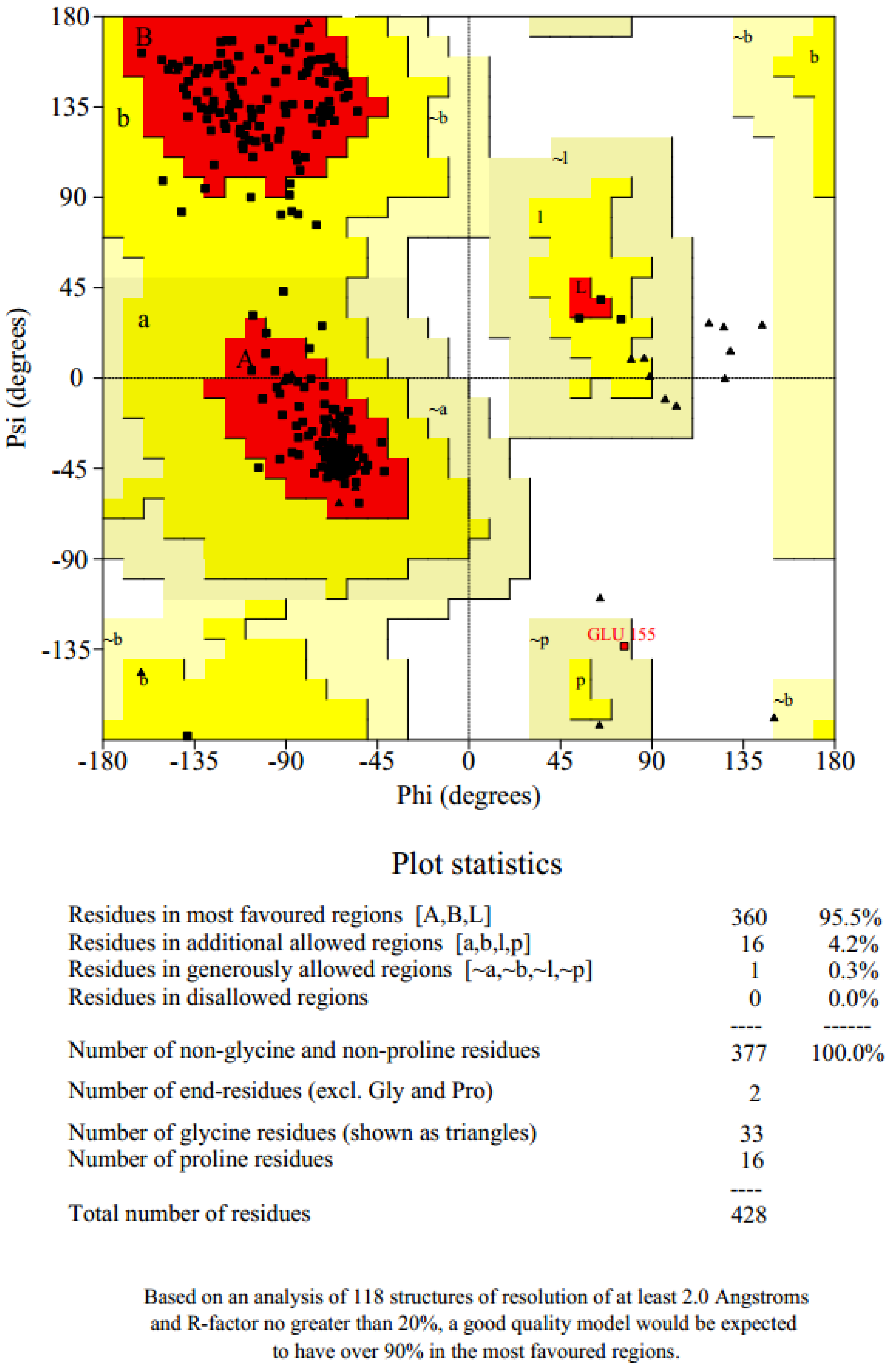

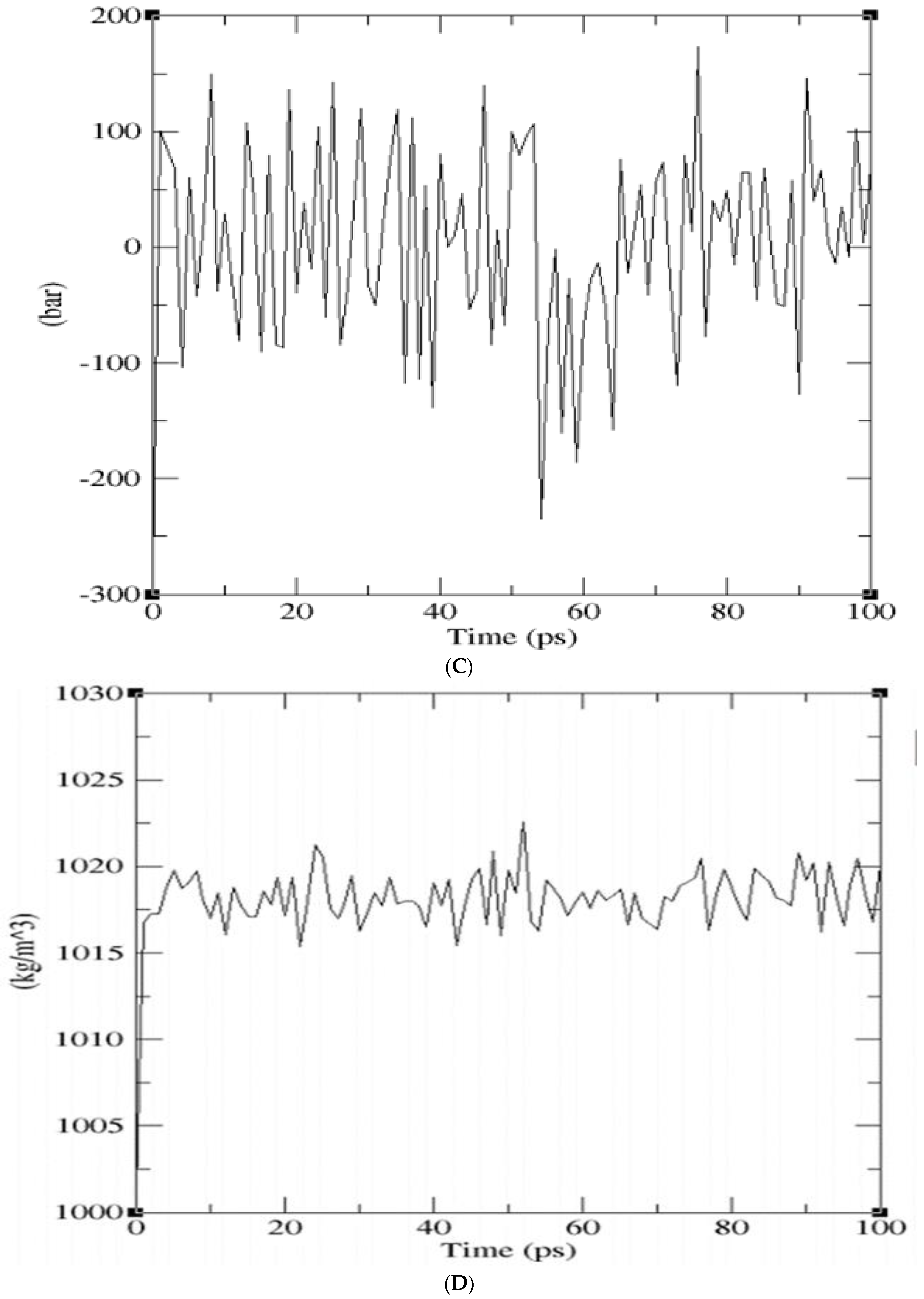


| Models | DOPE Score |
|---|---|
| Model 1 | −47200.15625 |
| Model 2 | −47099.78906 |
| Model 3 | −47185.48047 |
| Model 4 | −47193.96484 |
| Model 5 | −47291.21875 |
| Predicted Ligands | Binding Energy/(Kcal/mol) | Hydrogen Bond Interacting Residues | Hydrophobic Bond Interacting Residues |
|---|---|---|---|
| ZINC95486006 | −9.5 | Asn75, Ser357, Glu380, Ala390 | Met76, Gln79, Ala353, Leu354, Met358, Leu361, Ala362, Tyr365, Tyr373, Leu376, His393, Glu396 |
| ZINC95486007 | −8.7 | Glu380, Asn75, Ala390 | Met76, Gln79, Leu354, Ser357, Met358, Leu361, Ala362, Tyr365, Tyr373, Leu376, His393, Glu396 |
| ZINC38143792 | −8.6 | Glu380, Arg379, Ser357 | Gln79, Ala382, Ala383, Arg386, Tyr388, Ala390 |
| ZINC95485880 | −8.6 | Glu380, Arg386, His393 | Asn75, Met76, Gln79, Ala383, Tyr388 |
| ZINC95486305 | −8.8 | Gln79, Arg379 | Asn75, Glu396, His393, Ala390, Glu380, Tyr388, Arg386, Ala382, Ala383 |
| ZINC95486303 | −8.7 | Asn319, Lys321 | Asn67, Leu69, Gln79, Gln80, Ala83, Leu85, Pro316, Trp320, Ile329, Ile346, Ala349, Ala353, Tyr388 |
| ZINC95485905 | −8.5 | Glu380, His393 | Asn75, Gln79, Leu376, Arg379, Ala383, Tyr388, Ala390 |
| ZINC95486183 | −10.0 | Glu380 | Leu69, Met76, Asn75, Gln79, Pro316, Trp320, Ile346, Ala349, Ala353, Leu376, Trp388, Ala390, His393, Glu396, Val397 |
| ZINC95486184 | −9.6 | Ala349 | Leu69, Asn75, Met76, Gln79, Pro316, Trp320, Ile346, Gly350, His352, Ala353, Leu354, Ser357, Tyr388 |
| ZINC95486142 | −9.4 | Pro316 | Ser315, Ser317, Asn319, Trp320, Lys321, Ile346, Ala349, His352, Asn355 |
| Compound ZINC ID/Name | Number of Lipinski’s Rules Violated | MW (g/mol) | No. HA | No. HD | xLogP | Water Solubility (mg/mL) | Log S | Bio. Sc |
|---|---|---|---|---|---|---|---|---|
| ZINC95486006 | 3 | 666.805 | 12 | 7 | 0.86 | Moderately soluble | −4.46 | 0.17 |
| ZINC95486007 | 3 | 668.821 | 12 | 7 | 1.02 | Moderately soluble | −4.86 | 0.17 |
| ZINC38143792 | 0 | 487.701 | 5 | 3 | 4.93 | Moderately soluble | −5.92 | 0.56 |
| ZINC95485880 | 0 | 416.561 | 3 | 2 | 3.79 | Moderately soluble | −5.03 | 0.55 |
| ZINC95486305 | 1 | 500.362 | 7 | 2 | 2.49 | Soluble | −3.72 | 0.55 |
| RIFAMPICIN | 3 | 822.94 | 14 | 6 | 3.07 | Poorly soluble | −8.18 | 0.17 |
| STREPTOMYCIN | 3 | 581.57 | 15 | 11 | −5.83 | Soluble | 1.80 | 0.17 |
| CLARITHROMYCIN | 2 | 747.95 | 14 | 4 | 2.13 | Moderately soluble | −5.94 | 0.17 |
| MOXIFLOXACIN | 0 | 401.43 | 6 | 3 | 1.85 | Soluble | −2.70 | 0.55 |
| AMIKACIN | 3 | 585.60 | 17 | 13 | −5.91 | Highly Soluble | 2.23 | 0.17 |
| Compound ZINC ID | GI Absorption | BBB Permeant | P-gp Substrate | CYP1A2 Inhibitor | CYP2C19 Inhibitor | CYP2C9 Inhibitor | CYP2D6 Inhibitor | CYP3A4 Inhibitor |
|---|---|---|---|---|---|---|---|---|
| ZINC95486006 | Low | No | Yes | No | No | No | No | No |
| ZINC95486007 | Low | No | Yes | No | No | No | No | No |
| ZINC38143792 | High | No | Yes | No | No | No | No | No |
| ZINC95485880 | High | Yes | Yes | No | No | No | No | No |
| ZINC95486305 | High | No | Yes | No | No | No | No | Yes |
| ZINC95486303 | Low | No | Yes | No | No | No | No | Yes |
| ZINC95485905 | Low | No | No | No | No | Yes | No | No |
| ZINC95486183 | Low | No | Yes | No | No | No | No | No |
| ZINC95486184 | Low | No | Yes | No | No | No | No | No |
| ZINC95486142 | Low | No | No | No | No | No | No | No |
| ZINC86037206 | High | No | No | No | No | Yes | No | Yes |
| ZINC31761332 | Low | No | No | No | No | Yes | No | Yes |
| ZINC95486231 | High | No | Yes | No | No | No | No | No |
| ZINC03197457 | Low | No | No | No | No | No | No | No |
| ZINC95485943 | High | No | Yes | No | No | No | No | No |
| ZINC95486001 | High | No | Yes | No | No | Yes | No | No |
| ZINC40431237 | High | No | No | No | No | Yes | No | Yes |
| ZINC95486182 | Low | No | No | No | No | No | No | No |
| ZINC03941105 | High | No | No | No | No | Yes | No | No |
| ZINC95485882 | Low | No | No | No | No | No | No | No |
| RIFAMPICIN | Low | No | Yes | No | No | No | No | No |
| STREPTOMYCIN | Low | No | Yes | No | No | No | No | No |
| CLARITHROMYCIN | Low | No | Yes | No | No | No | No | No |
| MOXIFLOXACIN | High | No | Yes | No | No | No | Yes | No |
| AMIKACIN | Low | No | Yes | No | No | No | No | No |
| Compounds ZINC ID | Cardiac Toxicity | Mutagenicity |
|---|---|---|
| ZINC95486006 | No | Negative |
| ZINC95486007 | No | Negative |
| ZINC38143792 | No | Negative |
| ZINC95485880 | No | Negative |
| ZINC95486305 | No | Negative |
| ZINC95486303 | No | Negative |
| ZINC95485905 | No | Negative |
| ZINC95486183 | No | Negative |
| ZINC95486184 | No | Negative |
| ZINC95486142 | Yes | Negative |
| ZINC86037206 | No | Negative |
| ZINC31761332 | No | Negative |
| ZINC95486231 | No | Negative |
| ZINC03197457 | No | Negative |
| ZINC95485943 | No | Negative |
| ZINC95486001 | No | Negative |
| ZINC40431237 | No | Negative |
| ZINC95486182 | Yes | Negative |
| ZINC03941105 | No | Negative |
| ZINC95485882 | No | Negative |
| ZINC38143792 | 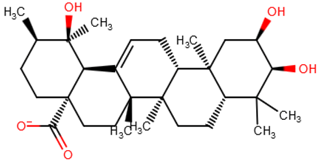 |
| ZINC95485880 | 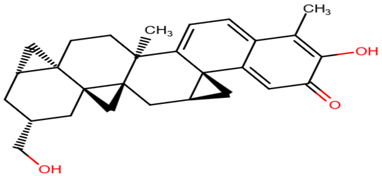 |
| ZINC95486305 | 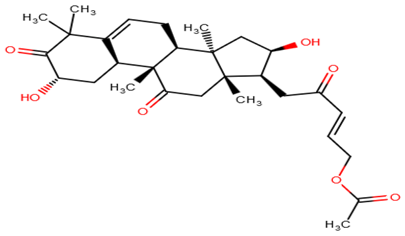 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwofie, S.K.; Dankwa, B.; Odame, E.A.; Agamah, F.E.; Doe, L.P.A.; Teye, J.; Agyapong, O.; Miller, W.A., III; Mosi, L.; Wilson, M.D. In Silico Screening of Isocitrate Lyase for Novel Anti-Buruli Ulcer Natural Products Originating from Africa. Molecules 2018, 23, 1550. https://doi.org/10.3390/molecules23071550
Kwofie SK, Dankwa B, Odame EA, Agamah FE, Doe LPA, Teye J, Agyapong O, Miller WA III, Mosi L, Wilson MD. In Silico Screening of Isocitrate Lyase for Novel Anti-Buruli Ulcer Natural Products Originating from Africa. Molecules. 2018; 23(7):1550. https://doi.org/10.3390/molecules23071550
Chicago/Turabian StyleKwofie, Samuel K., Bismark Dankwa, Emmanuel A. Odame, Francis E. Agamah, Lady P. A. Doe, Joshua Teye, Odame Agyapong, Whelton A. Miller, III, Lydia Mosi, and Michael D. Wilson. 2018. "In Silico Screening of Isocitrate Lyase for Novel Anti-Buruli Ulcer Natural Products Originating from Africa" Molecules 23, no. 7: 1550. https://doi.org/10.3390/molecules23071550
APA StyleKwofie, S. K., Dankwa, B., Odame, E. A., Agamah, F. E., Doe, L. P. A., Teye, J., Agyapong, O., Miller, W. A., III, Mosi, L., & Wilson, M. D. (2018). In Silico Screening of Isocitrate Lyase for Novel Anti-Buruli Ulcer Natural Products Originating from Africa. Molecules, 23(7), 1550. https://doi.org/10.3390/molecules23071550





