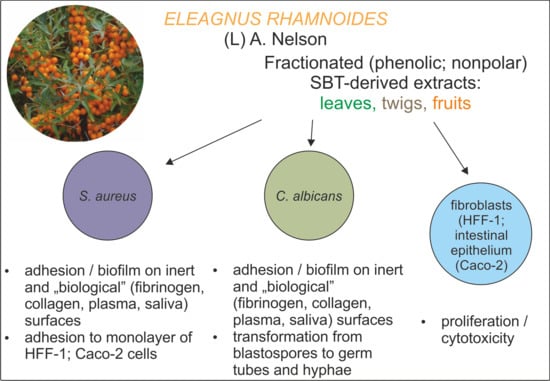
Phenolic and Nonpolar Fractions of Elaeagnus rhamnoides (L.) A. Nelson Extracts as Virulence Modulators—In Vitro Study on Bacteria, Fungi, and Epithelial Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Direct Antimicrobial Activity of Fractionated SBT Extracts
2.2. Antivirulence Properties of Fractionated SBT Extracts
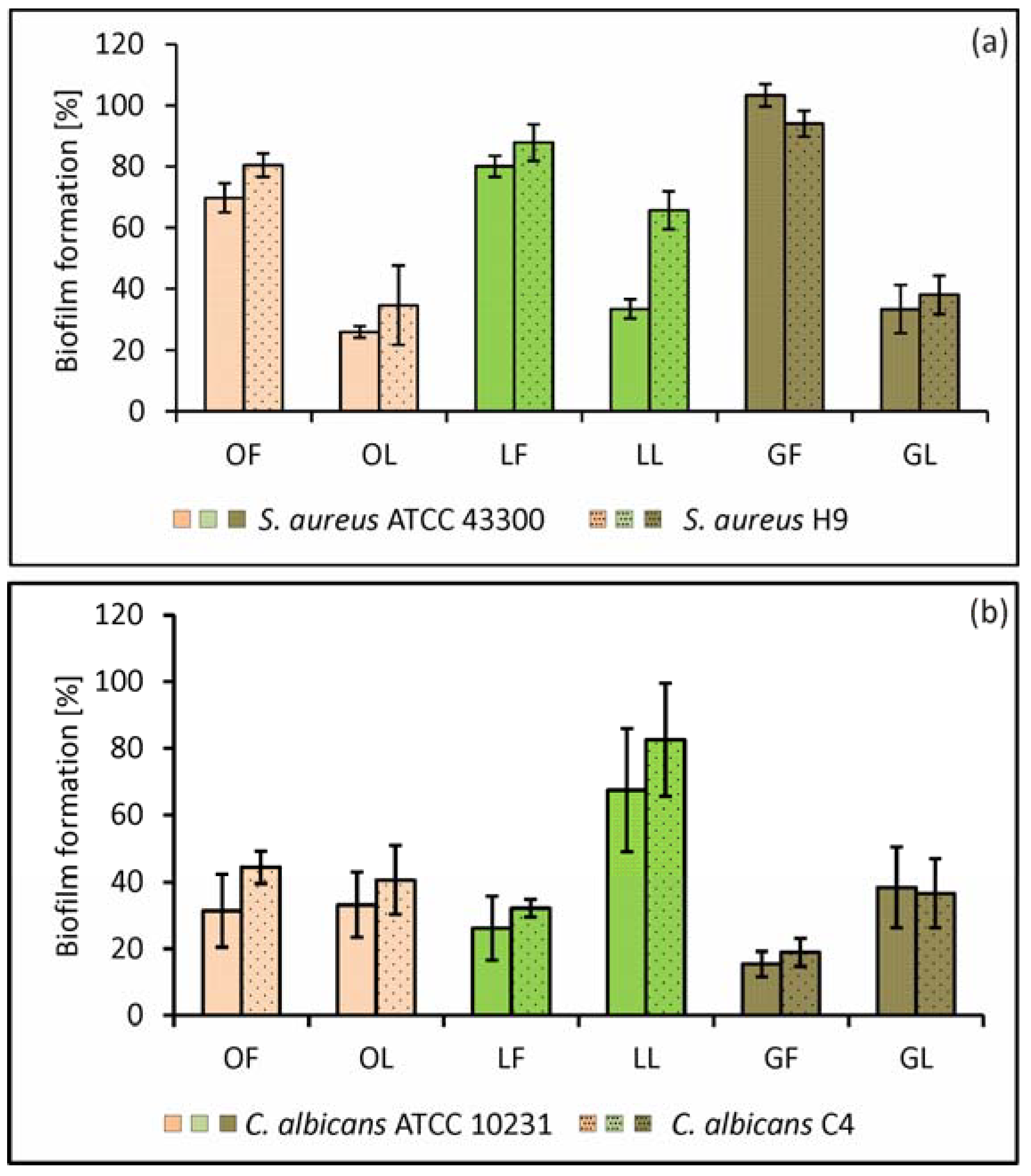
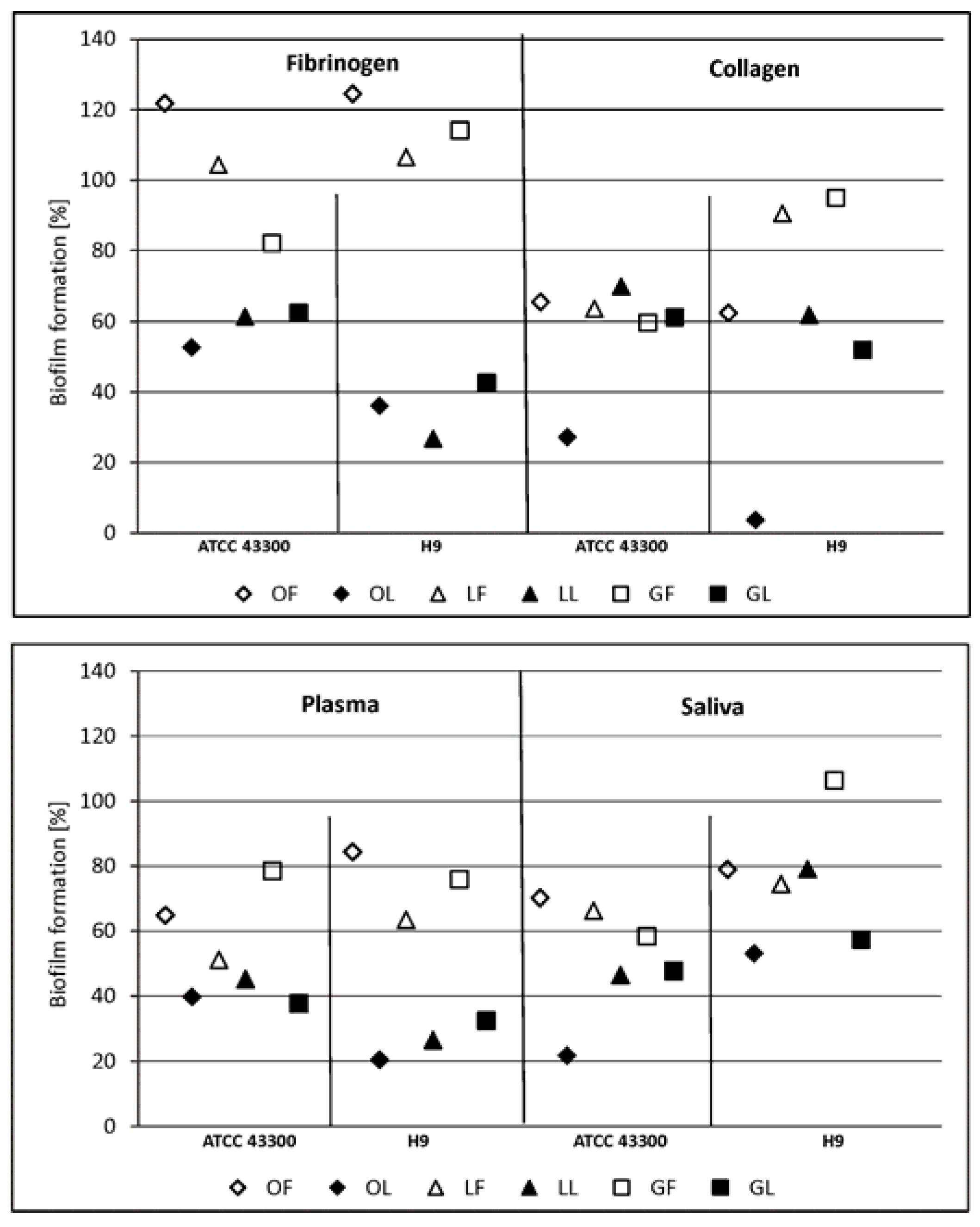
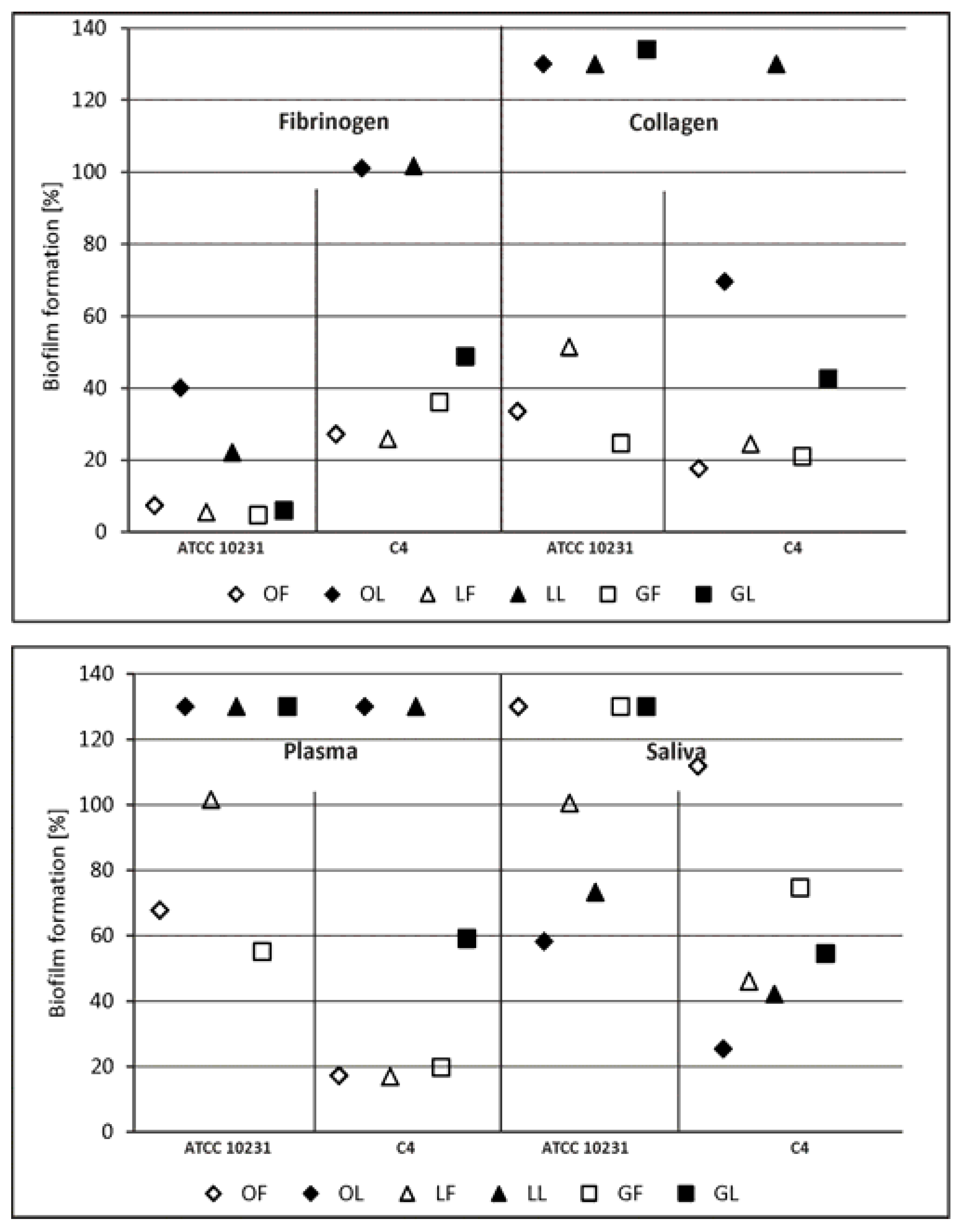
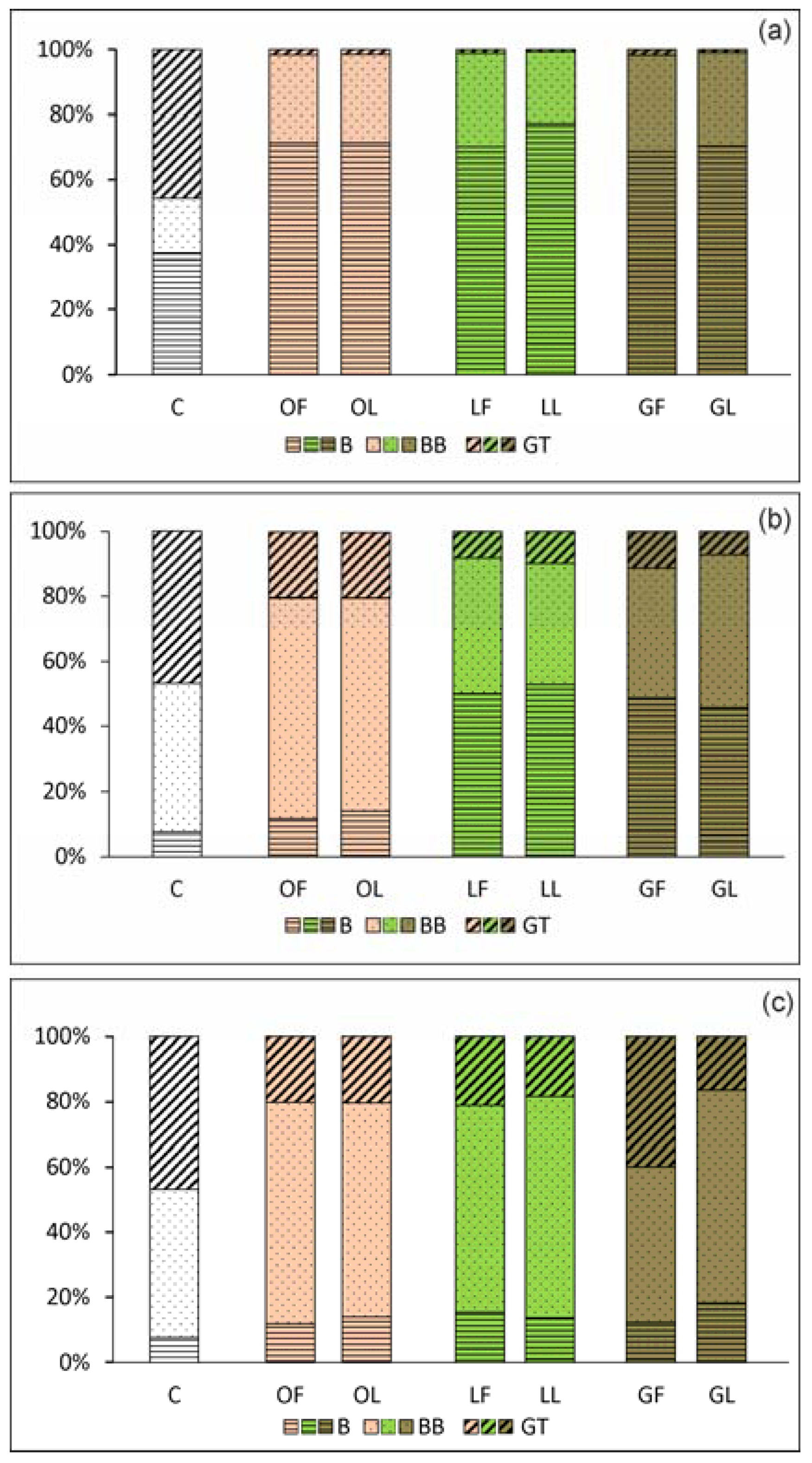
2.2.1. Adhesion and Biofilm Formation
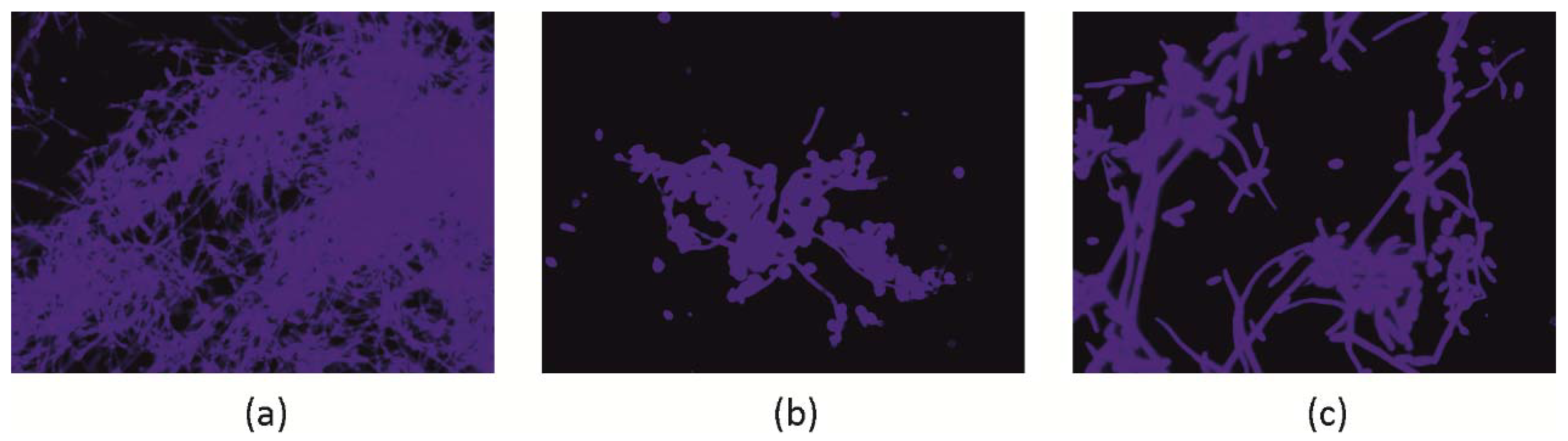
2.2.2. C. albicans Invasive Properties—Morphological Transformation
2.2.3. S. aureus Invasive Properties—Adhesion to Monolayers of Eukaryotic Cells
2.3. Considerations on the Antimicrobial Activity of Fractionated SBT Extracts, in Relation to Their Composition
3. Materials and Methods
3.1. Plant Material and Chemical Analysis of the Fractionated SBT-Derived Extracts
3.2. Preparation of Solutions of Fractionated SBT-Derived Extracts and Reference Compounds
3.3. Cytotoxic Activity of Fractionated SBT-Derived Extracts
3.4. Microorganisms and Culture Conditions
3.5. Minimal Inhibitory/Bactericidal/Fungicidal Concentration (MIC/MBC/MFC)
3.6. S. aureus and C. albicans Adhesion and Biofilm Formation on the Abiotic (Polystyrene) Surface
3.7. S. aureus and C. albicans Adhesion and Biofilm Formation on the Surface Conditioned with Host-Derived Proteins/Body Fluids
3.8. C. albicans Invasive Properties—Evaluation of Morphogenesis Potential
3.9. S. aureus Invasive Properties—Evaluation of Adhesion to Eukaryotic Cell Monolayers
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm activity of plant polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef] [PubMed]
- Sadekuzzaman, M.; Yang, S.; Mizan, M.F.R.; Ha, S.D. Current and recent advanced strategies for combating biofilms. Compr. Rev. Food Sci. Food Saf. 2015, 14, 491–509. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Abreu, A.C.; Dias, C.; Saavedra, M.J.; Borges, F.; Simões, M. New perspectives on the use of phytochemicals as an emergent strategy to control bacterial infections including biofilms. Molecules 2016, 21, 877. [Google Scholar] [CrossRef] [PubMed]
- Kim Ta, C.A.; Arnason, J.T. Mini review of phytochemicals and plant taxa with activity as microbial biofilm and quorum sensing inhibitors. Molecules 2016, 21, 29. [Google Scholar] [CrossRef]
- Sadowska, B.; Budzyńska, A.; Stochmal, A.; Żuchowski, J.; Różalska, B. Novel properties of Hippophae rhamnoides L. twig and leaf extracts—Anti-virulence action and synergy with antifungals studied in vitro on Candida spp. model. Microb. Pathog. 2017, 107, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Destandau, E.; Le Floch, G.; Lucchesi, M.E.; Elfakir, C. Antimicrobial, antioxidant and phytochemical investigations of sea buckthorn (Hippophaë rhamnoides L.) leaf, stem, root and seed. Food Chem. 2012, 131, 754–760. [Google Scholar] [CrossRef]
- Rafalska, A.; Abramowicz, K.; Krauze, M. Sea buckthorn (Hippophae rhamnoides L.) as a plant for universal application. World Sci. News 2017, WSN 72, 123–140. [Google Scholar]
- Suryakumar, G.; Gupta, A. Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L.). J. Ethnopharmacol. 2011, 138, 268–278. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. A Service of the U.S. National Institutes of Health. 2018. Available online: http://clinicaltrials.gov (accessed on 5 June 2018).
- Olas, B.; Żuchowski, J.; Lis, B.; Skalski, B.; Kontek, B.; Grabarczyk, Ł.; Stochmal, A. Comparative chemical composition, antioxidant and anticoagulant properties of phenolic fraction (a rich in non-acylated and acylated flavonoids and non-polar compounds) and non-polar fraction from Elaeagnus rhamnoides (L.) A. Nelson fruits. Food Chem. 2018, 247, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, N.K.; Kumar, R.; Siddiqui, M.S.; Gupta, A. Mechanism of wound-healing activity of Hippophae rhamnoides L. leaf extract in experimental burns. Evid.-Based Complement. Altern. Med. 2011, 2011, 659705. [Google Scholar] [CrossRef] [PubMed]
- Wani, T.A.; Wani, S.M.; Ahmad, M.; Ahmad, M.; Gani, A.; Masoodi, F.A. Bioactive profile, health benefits and safety evaluation of sea buckthorn (Hippophae rhamnoides L.): A review. Cogent Food Agric. 2016, 2, 1–9. [Google Scholar] [CrossRef]
- Upadhyay, A.; Upadhyay, I.; Kollanoor-Johny, A.; Venkitanarayanan, K. Combating pathogenic microorganisms using plant-derived antimicrobials: A minireview of the mechanistic basis. Biomed. Res. Int. 2014, 2014, 761741. [Google Scholar] [CrossRef] [PubMed]
- Verma, H.; Sharma, M.; Chahota, R.; Palial, A. Assessment of antimycotic activity of sea buckthorn (Hippophae rhamnoides) leaf exacts against common fungi associated with skin dermatitis. Vet. World 2013, 6, 205–208. [Google Scholar] [CrossRef]
- Arora, R.; Mundra, S.; Yadav, A.; Srivastava, R.B.; Stobdan, T. Antimicrobial activity of seed, pomace and leaf extracts of sea buckthorn (Hippophae rhamnoides L.) against foodborne and food spoilage pathogens. Afr. J. Biotechnol. 2012, 11, 10424–10430. [Google Scholar] [CrossRef]
- Zakynthios, G.; Varzakas, T. Hippophae rhamnoides: Safety and nutrition. Curr. Res. Nutr. Food Sci. 2015, 3, 89–97. [Google Scholar] [CrossRef]
- Kumar, M.Y.; Tirpude, R.J.; Maheshwari, D.T.; Bansal, A.; Misra, K. Antioxidant and antimicrobial properties of phenolic rich fraction of sea buckthorn (Hippophae rhamnoides L.) leaves in vitro. Food Chem. 2013, 141, 3443–3450. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Lee, H.K. The role of skin and orogenital microbiota in protective immunity and chronic immune-mediated inflammatory disease. Front. Immunol. 2018, 8, 1955. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune, responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Höök, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Sardi, J.C.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Robertson, S.N.; Williams, C. Strength in numbers: Antifungal strategies against fungal biofilms. Int. J. Antimicrob. Agents 2013, 43, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Heras, B.; Scanlon, M.J.; Martin, J.L. Targeting virulence not viability in the search for future antibacterials. Br. J. Clin. Pharmacol. 2015, 79, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Duperthuy, M.; Wai, S.N. Sub-optimal treatment of bacterial biofilms. Antibiotics 2016, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Crosby, H.A.; Kwieciński, J.; Horswill, A.R. Staphylococcus aureus aggregation and coagulation mechanisms, and their function in host-pathogen interactions. Adv. Appl. Microbiol. 2016, 96, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Josse, J.; Laurent, F.; Diot, A. Staphylococcal adhesion and host cell invasion: Fibronectin-binding and other mechanisms. Front. Immunol. 2017, 8, 2433. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, I.D.; Wilson, D.; Wächtler, B.; Brunke, S.; Naglik, J.R.; Hube, B. Candida albicans dimorphism as a therapeutic target. Expert Rev. Anti-Infect. Ther. 2012, 10, 85–93. [Google Scholar] [CrossRef] [PubMed]
- De Groot, P.E.J.; Bader, O.; de Boer, A.D.; Weig, M.; Chauhan, N. Adhesins in human fungal pathogens: Glue with plenty of stick. Eukaryot. Cell 2013, 12, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.L.S.; de Lima Perez, A.L.A.; Rocha, I.M.; Pinheiro, A.; de Castro, R.D.; Carlo, H.L.; de Oliveira, E.; Castellano, L.R. Does scientific evidence for the use of natural products in the treatment of oral candidiasis exist? A systematic review. Evid.-Based Complement. Altern. Med. 2015, 2015, 147804. [Google Scholar] [CrossRef]
- Hudson, D.A.; Sciascia, Q.L.; Sanserds, R.J.; Norris, G.E.; Edwards, P.J.B.; Sullivan, P.A.; Farley, P.C. Identification of the dialysable serum inducer of germ-tube formation in Candida albicans. Microbiology 2004, 150, 3041–3049. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, Z.; Zhang, J.; Yang, L. Antifungal compounds against Candida infections from traditional Chinese medicine. BioMed Res. Int. 2017, 2017, 4614183. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morad, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81 (Suppl. 1), 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81 (Suppl. 1), 243S–255S. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Cao, Y.; Huang, Q. Edible nanoencapsulation vehicles for oral delivery of phytochemicals: A perspective paper. J. Agric. Food Chem. 2017, 65, 6727–6735. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Uperti, D.K.; Singh, B.R.; Pandey, G.; Verma, S.; Roy, S.; Naqvi, A.H.; Rawat, A.K.S. Quercetin sensitized fluconazole-resistant Candida albicans to induce apoptotic cell death by modulating quorum sensing. Antimicrob. Agents Chemother. 2015, 59, 2153–2168. [Google Scholar] [CrossRef] [PubMed]
- Ming, D.; Wang, D.; Cao, F.; Xiang, H.; Mu, D.; Cao, J.; Li, B.; Zhong, L.; Dong, X.; Zhong, X.; et al. Kaempferol inhibits the primary attachment phase of biofilm formation in Staphylococcus aureus. Front. Microbiol. 2017, 8, 2263. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, J.H.; Cho, H.S.; Joo, S.W.; Cho, M.H.; Lee, J. Anti-biofilm activities of quercetin and tannic acid against Staphylococcus aureus. Biofouling 2013, 29, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, H.; Wang, L.; Song, Z.; Shi, L.; Li, W.; Deng, X.; Wang, J. Isorhamnetin attenuates Staphylococcus aureus-induced lung cell injury by inhibiting alpha-hemolysin expression. J. Microbiol. Biotechnol. 2016, 26, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Duarte, S.; Murata, R.M.; Scott-Anne, K.; Gregoire, S.; Watson, G.E.; Singh, A.P.; Vorsa, N. Influence of cranberry proanthocyanidins on formation of biofilms by Streptococcus mutans on saliva-coated apatitic surface and on dental caries development in vivo. Caries Res. 2010, 44, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Rane, H.S.; Bernardo, S.M.; Howell, A.B.; Lee, S.A. Cranberry-derived proanthocyanidins prevent formation of Candida albicans biofilms in artificial urine through biofilm- and adherence-specific mechanisms. J. Antimicrob. Chemother. 2014, 69, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Alshami, I.; Alharbi, A.E. Hibiscus sabdariffa extract inhibits in vitro biofilm formation capacity of Candida albicans isolated from recurrent urinary tract infections. Asian Pac. J. Trop. Biomed. 2014, 4, 104–108. [Google Scholar] [CrossRef]
- Luiz, R.L.; Vila, T.V.; de Mello, J.C.; Nakamura, C.V.; Rozental, S.; Ishida, K. Proanthocyanidins polymeric tannin from Stryphnodendron adstringens are active against Candida albicans biofilms. BMC Complement. Altern. Med. 2015, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Laaksonen, O.; Kallio, H.; Yang, B. Proanthocyanidins in sea buckthorn (Hippophaë rhamnoides L.) Berries of different origins with special reference to the influence of genetic background and growth location. J. Agric. Food Chem. 2016, 64, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Netala, V.R.; Ghosh, S.B.; Bobbu, P.; Anitha, D.; Tartte, V. Triterpenoid saponis: A review on biosynthesis, applications and mechanism of their action. Int. J. Pharm. Pharm. Sci. 2015, 7, 24–28. [Google Scholar]
- Quave, C.L.; Lyles, J.T.; Kavanaugh, J.S.; Nelson, K.; Parlet, C.P.; Crosby, H.A.; Heilmann, K.P.; Horswill, A.R. Castanea sativa (European Chestnut) leaf extract rich in ursine and oleanene derivatives block Staphylococcus aureus virulence and pathogenesis without detectable resistance. PLoS ONE 2015, 10, e0136486. [Google Scholar] [CrossRef] [PubMed]
- Bunel, V.; Ouedraogo, M.; Stevigny, C.; Duez, P. Methods applied to the in vitro primary toxicology testing of natural products: State of the art, strenghts, and limits. Planta Med. 2014, 80, 1210–1226. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing—EUCAST. 2012. Available online: http://www.eucast.org (accessed on 5 June 2018).
- Sadowska, B.; Bonar, A.; von Eiff, C.; Proctor, R.A.; Chmiela, M.; Rudnicka, W.; Różalska, B. Characteristic of Staphylococcus aureus, isolated from airways of cystic fibrosis patients, and their small colony variants. Immunol. Med. Microbiol. 2002, 32, 191–197. [Google Scholar] [CrossRef]
Sample Availability: Samples of the investigated fractions of sea buckthorn extracts are not available from the authors. |
| Fraction | Compound Type | Relative Peak Area (%) | ||
|---|---|---|---|---|
| Leaf | Twig | Fruit * | ||
| Phenolic | Unidentified polar compounds | 15.8 | 35.7 | 20.9 |
| Hydrolysable tanins and ellagic acid | 31.3 | - | - | |
| Proanthocyanidins and catechin | - | 47.5 | - | |
| Isorhamnetin glycosides | 7.0 | - | 29.5 | |
| Triterpenoid saponins | 15.0 | - | - | |
| Triterpenoids | 5.8 | 5.4 | 8.0 | |
| Non-polar | Unidentified non-polar compound | 18.9 | 36.5 | 29.7 |
| Triterpenoid saponins | 30.5 | - | - | |
| Triterpenoids | 38.5 | 33.9 | 44.8 | |
| Acylated triterpenoids ** | 5.6 | 24.4 | 24.5 | |
| Microorganism | Phenolic Fraction (MIC) [mg∙mL−1] | |
|---|---|---|
| LF (Leaf Extract) | GF (Twig Extract) | |
| Gram-positive | ||
| Staphylococcus aureus ATCC 29213 | 0.5 | 1.0 |
| Staphylococcus aureus ATCC BAA-1708 | 0.5 | 0.5 |
| Staphylococcus aureus ATCC 43300 | 0.5 | 0.25 |
| Staphylococcus aureus H9 * | 0.5 | 0.5 |
| Streptococcus mutans ATCC 25175 | >1.0 | >1.0 |
| Bacillus cereus ATCC | 0.5 | 0.5 |
| Lactobacillus acidophilus ATCC 4356 | >1.0 | >1.0 |
| Gram-negative | ||
| Helicobacter pylori ATCC 700392 | 1.0 | 1.0 |
| Escherichia coli ATCC 25922 | >1.0 | >1.0 |
| Proteus vulgaris ATCC 8427 | 1.0 | 1.0 |
| Pseudomonas aeruginosa ATCC 25619 | >1.0 | >1.0 |
| Fungi | ||
| Candida albicans ATCC 10231 | 1.0 | 1.0 |
| Candida albicans ATCC 90028 | >1.0 | >1.0 |
| Candida albicans A4 * | >2.0 | >1.0 |
| Candida parapsilosis ATCC 22019 | 1.0 | 1.0 |
| Candida krusei ATCC 14243 | >1.0 | >1.0 |
| Candida glabrata G1 * | >1.0 | >1.0 |
| Microorganism | Compound (MIC) [mg∙mL−1] | |||
|---|---|---|---|---|
| Ellagic Acid | Epicatechin | Quercetin | Ursolic Acid | |
| Staphylococcus aureus ATCC 43300 | >1.0 | >1.0 | >1.0 | 0.25 |
| Staphylococcus aureus H9 * | >1.0 | 1.0 | >1.0 | 0.5 |
| Candida albicans ATCC 10231 | 2.0 | >2.0 | >2.0 | 0.031 |
| Candida albicans A4 * | >2.0 | >2.0 | >2.0 | 0.031 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Różalska, B.; Sadowska, B.; Żuchowski, J.; Więckowska-Szakiel, M.; Budzyńska, A.; Wójcik, U.; Stochmal, A. Phenolic and Nonpolar Fractions of Elaeagnus rhamnoides (L.) A. Nelson Extracts as Virulence Modulators—In Vitro Study on Bacteria, Fungi, and Epithelial Cells. Molecules 2018, 23, 1498. https://doi.org/10.3390/molecules23071498
Różalska B, Sadowska B, Żuchowski J, Więckowska-Szakiel M, Budzyńska A, Wójcik U, Stochmal A. Phenolic and Nonpolar Fractions of Elaeagnus rhamnoides (L.) A. Nelson Extracts as Virulence Modulators—In Vitro Study on Bacteria, Fungi, and Epithelial Cells. Molecules. 2018; 23(7):1498. https://doi.org/10.3390/molecules23071498
Chicago/Turabian StyleRóżalska, Barbara, Beata Sadowska, Jerzy Żuchowski, Marzena Więckowska-Szakiel, Aleksandra Budzyńska, Urszula Wójcik, and Anna Stochmal. 2018. "Phenolic and Nonpolar Fractions of Elaeagnus rhamnoides (L.) A. Nelson Extracts as Virulence Modulators—In Vitro Study on Bacteria, Fungi, and Epithelial Cells" Molecules 23, no. 7: 1498. https://doi.org/10.3390/molecules23071498
APA StyleRóżalska, B., Sadowska, B., Żuchowski, J., Więckowska-Szakiel, M., Budzyńska, A., Wójcik, U., & Stochmal, A. (2018). Phenolic and Nonpolar Fractions of Elaeagnus rhamnoides (L.) A. Nelson Extracts as Virulence Modulators—In Vitro Study on Bacteria, Fungi, and Epithelial Cells. Molecules, 23(7), 1498. https://doi.org/10.3390/molecules23071498