Recent Advances in Applications of Ionic Liquids in Miniaturized Microextraction Techniques
Abstract
1. Introduction
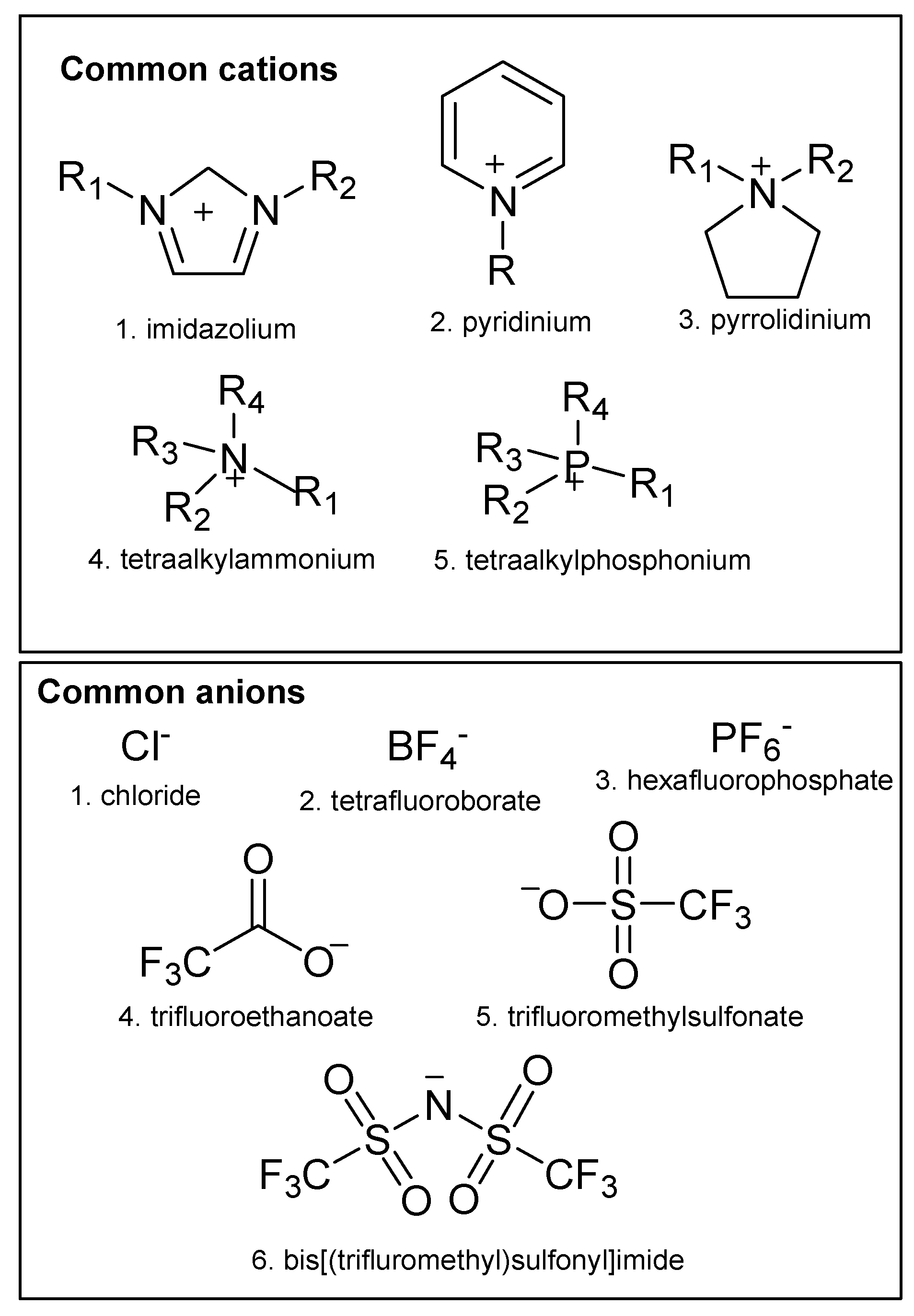
2. Ionic Liquids in Miniaturized Microextraction Techniques
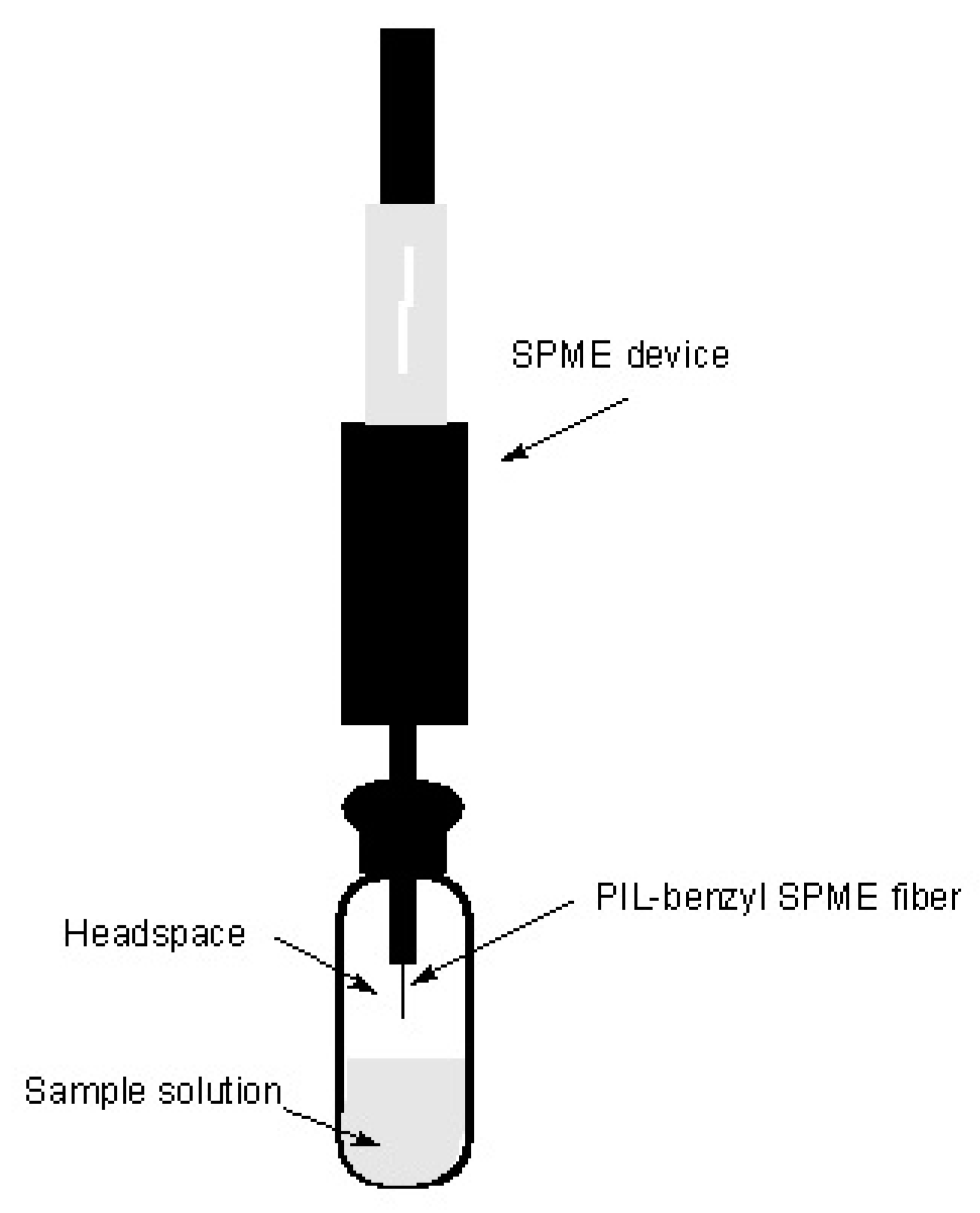
2.1. Solid-Phase Microextraction (SPME)
2.2. Dispersive Liquid-Liquid Microextraction (DLLME)
2.3. Stir Bar Sorptive Extraction (SBSE)
2.4. Single-Drop Microextraction (SDME)
2.5. Stir-Cake Sorptive Extraction (SCSE)
3. Conclusions
Conflicts of Interest
References
- Tobiszewski, M.; Mechlińska, A.; Namieśnik, J. Green analytical chemistry—Theory and practice. Chem. Soc. Rev. 2010, 39, 2869–2878. [Google Scholar] [CrossRef] [PubMed]
- Spietelun, A.; Marcinkowski, Ł.; De La Guardia, M.; Namieśnik, J. Green aspects, developments and perspectives of liquid phase microextraction techniques. Talanta 2014, 119, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Tobiszewski, M.; Mechlińska, A.; Zygmunt, B.; Namieśnik, J. Green analytical chemistry in sample preparation for determination of trace organic pollutants. TrAC-Trends Anal. Chem. 2009, 28, 943–951. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Miniaturized solid-phase extraction techniques. TrAC-Trends Anal. Chem. 2015, 73, 19–38. [Google Scholar] [CrossRef]
- Poole, C.F.; Lenca, N. Green sample-preparation methods using room-temperature ionic liquids for the chromatographic analysis of organic compounds. TrAC-Trends Anal. Chem. 2015, 71, 144–156. [Google Scholar] [CrossRef]
- Vičkačkaite, V.; Padarauskas, A. Ionic liquids in microextraction techniques. Cent. Eur. J. Chem. 2012, 10, 652–674. [Google Scholar] [CrossRef]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Walden, P. Ueber die Molekulargrösse und elektrische Leitfähigkeit einiger geschmolzener Salze (Molecular weights and electrical conductivity of several fused salts). Bull. Acad. Imp. Sci. 1914, 8, 405–422. [Google Scholar]
- Wilkes, J.S.; Zaworotko, M.J. Air and Water Stable I-Ethyl-3-methylimidazolium Based Ionic Liquids. Chem. Commun. 1992, 13, 965–967. [Google Scholar] [CrossRef]
- Shishov, A.; Bulatov, A.; Locatelli, M.; Carradori, S.; Andruch, V. Application of deep eutectic solvents in analytical chemistry: A review. Microchem. J. 2017, 135, 33–38. [Google Scholar] [CrossRef]
- Aguilera-Herrador, E.; Lucena, R.; Cárdenas, S.; Valcárcel, M. The roles of ionic liquids in sorptive microextraction techniques. TrAC-Trends Anal. Chem. 2010, 29, 602–616. [Google Scholar] [CrossRef]
- Sun, P.; Armstrong, D.W. Ionic liquids in analytical chemistry. Anal. Chim. Acta 2010, 661, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ghandi, K. A Review of Ionic Liquids, Their Limits and Applications. Green Sustain. Chem. 2014, 4, 44–53. [Google Scholar] [CrossRef]
- Prado, R.; Weber, C.C. Applications of Ionic Liquids. In Application, Purification and Recovery of Ionic Liquids; Elsevier: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Berthod, A.; Ruiz-Ángel, M.J.; Carda-Broch, S. Recent advances on ionic liquid uses in separation techniques. J. Chromatogr. A 2018, 1559, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, J.; Huang, Y.; Zhou, Q.; Zhang, X.; Zhang, S. Toxicity of ionic liquids: Database and prediction via quantitative structure-activity relationship method. J. Hazard. Mater. 2014, 278, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Alfonsi, K.; Colberg, J.; Dunn, P.J.; Fevig, T.; Jennings, S.; Johnson, T.A.; Kleine, H.P.; Knight, C.; Nagy, M.A.; Perry, D.A.; et al. Green chemistry tools to influence a medicinal chemistry and research chemistry based organisation. Green Chem. 2008, 10, 31–36. [Google Scholar] [CrossRef]
- Pawliszyn, J. (Ed.) Solid Phase Microextraction: Theory and Practice; Wiley-VCH, Inc.: New York, NY, USA, 1997. [Google Scholar]
- Silva, E.A.S.; Risticevic, S.; Pawliszyn, J. Recent trends in SPME concerning sorbent materials, configurations and in vivo applications. Trends Anal. Chem. 2013, 43, 24–36. [Google Scholar] [CrossRef]
- Tang, Z.; Duan, Y. Fabrication of porous ionic liquid polymer as solid-phase microextraction coating for analysis of organic acids by gas chromatography—Mass spectrometry. Talanta 2017, 172, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Gionfriddo, E.; Souza-Silva, E.; Pawliszyn, J. Headspace versus Direct Immersion Solid Phase Microextraction in Complex Matrixes: Investigation of Analyte Behavior in Multicomponent Mixtures. Anal. Chem. 2015, 87, 8448–8456. [Google Scholar] [CrossRef] [PubMed]
- Merdivan, M.; Pino, V.; Anderson, J.L. Determination of volatile polycyclic aromatic hydrocarbons in waters using headspace solid-phase microextraction with a benzyl-functionalized crosslinked polymeric ionic liquid coating. Environ. Technol. 2017, 38, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Feng, J.; Bu, Y.; Luo, C. Ionic liquid coated copper wires and tubes for fiber-in-tube solid-phase microextraction. J. Chromatogr. A 2016, 1458, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.; Assadi, Y.; Milani Hosseini, M.R.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of organic compounds in water using dispersive liquid-liquid microextraction. J. Chromatogr. A 2006, 1116, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Rodríguez, M.J.; Rocío-Bautista, P.; Pino, V.; Afonso, A.M. Ionic liquids in dispersive liquid-liquid microextraction. TrAC-Trends Anal. Chem. 2013, 51, 87–106. [Google Scholar] [CrossRef]
- Mansour, F.R.; Khairy, M.A. Pharmaceutical and biomedical applications of dispersive liquid-liquid microextraction. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1061–1062, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lu, W.; Chen, L. Recent Advances in Dispersive Liquid-Liquid Microextraction for Organic Compounds Analysis in Environmental Water: A Review. Curr. Anal. Chem. 2012, 8, 78–90. [Google Scholar] [CrossRef]
- Zhou, Q.; Bai, H.; Xie, G.; Xiao, J. Trace determination of organophosphorus pesticides in environmental samples by temperature-controlled ionic liquid dispersive liquid-phase microextraction. J. Chromatogr. A 2008, 1188, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, E.; Zhu, W.; Gao, H.; Zhou, Z. Determination of four heterocyclic insecticides by ionic liquid dispersive liquid-liquid microextraction in water samples. J. Chromatogr. A 2009, 1216, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Baltussen, E.; Sandra, P.; David, F.; Cramers, C. Stir bar sorptive extraction (SBSE), a novel extraction technique for aqueous samples: Theory and principles. J. Microcolumn Sep. 1999, 11, 737–747. [Google Scholar] [CrossRef]
- Camino-Sánchez, F.J.; Rodríguez-Gómez, R.; Zafra-Gómez, A.; Santos-Fandila, A.; Vílchez, J.L. Stir bar sorptive extraction: Recent applications, limitations and future trends. Talanta 2014, 130, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Mao, X.; He, M.; Chen, B.; Hu, B. Development of novel sol-gel coatings by chemically bonded ionic liquids for stir bar sorptive extraction—Application for the determination of NSAIDS in real samples. Anal. Bioanal. Chem. 2014, 406, 7261–7273. [Google Scholar] [CrossRef] [PubMed]
- Chisvert, A.; Benedé, J.L.; Anderson, J.L.; Pierson, S.A.; Salvador, A. Introducing a new and rapid microextraction approach based on magnetic ionic liquids: Stir bar dispersive liquid microextraction. Anal. Chim. Acta 2017, 983, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Benedé, J.L.; Anderson, J.L.; Chisvert, A. Trace determination of volatile polycyclic aromatic hydrocarbons in natural waters by magnetic ionic liquid-based stir bar dispersive liquid microextraction. Talanta 2018, 176, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Dasgupta, P.-K. Liquid Droplet. A Renewable Gas Sampling Interface. Anal. Chem. 1995, 67, 2042–2049. [Google Scholar] [CrossRef]
- An, J.; Rahn, K.L.; Anderson, J.L. Headspace single drop microextraction versus dispersive liquid-liquid microextraction using magnetic ionic liquid extraction solvents. Talanta 2017, 167, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wei, S.; Li, X.; Zhao, Y.; Shao, M.; Zhang, H.; Yu, A. Ultrasonic nebulization headspace ionic liquid-based single drop microextraction of flavour compounds in fruit juices. Talanta 2013, 106, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, L.; Lin, F.; Yuan, D. Novel extraction approach for liquid samples stir cake sorptive extraction using monolith. J. Sep. Sci. 2011, 34, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, F.; Jiang, Y. An improved ionic liquid-based headspace single-drop microextraction-liquid chromatography method for the analysis of camphor and trans-anethole in compound liquorice tablets. J. Chromatogr. Sci. 2012, 50, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Huang, X.; Yuan, D. Preparation of stir cake sorptive extraction based on polymeric ionic liquid for the enrichment of benzimidazole anthelmintics in water, honey and milk samples. Anal. Chim. Acta 2014, 840, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mei, M.; Ouyang, T.; Huang, X. Talanta Preparation of a new polymeric ionic liquid-based sorbent for stir cake sorptive extraction of trace antimony in environmental water samples. Talanta 2016, 161, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, X. Analytica Chimica Acta Preparation of a polymeric ionic liquid-based adsorbent for stir cake sorptive extraction of preservatives in orange juices and tea drinks. Anal. Chim. Acta 2016, 916, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mei, M.; Huang, X.; Yuan, D. Talanta Sensitive determination of estrogens in environmental waters treated with polymeric ionic liquid-based stir cake sorptive extraction and liquid chromatographic analysis. Talanta 2016, 152, 98–104. [Google Scholar] [CrossRef] [PubMed]
| Preferred | Usable | Undesirable |
|---|---|---|
| Water | Cyclohexane | Pentane |
| Acetone Ethanol | Heptane | Hexane(s) |
| 2-Propanol | Toluene | Di-isopropyl ether |
| 1-Propanol | Methylcyclohexane | Diethyl ether |
| Ethyl acetate | Methyl t-butyl ether | Dichloromethane |
| Isopropyl acetate | Isooctane | Dichloroethane |
| Methanol | Acetonitrile | Chloroform |
| Methyl ethyl ketone | 2-MethylTHF | Dimethyl formamide |
| 1-Butanol | Tetrahydrofuran | N-Methylpyrrolidinone |
| t-Butanol | Xylenes | Pyridine |
| Ils (nontoxic combinations of ions) | Dimethyl sulfoxide | Dimethyl acetate |
| Acetic acid | Dioxane | |
| Ethylene glycol | Dimethoxyethane | |
| Benzene | ||
| Carbon tetrachloride |
| SPME | DLLME | SBSE | SDME | SCSE | |||||
|---|---|---|---|---|---|---|---|---|---|
| Conventional Sorbents (PDMS, DVB, CAR, PEG, CW) | ILs | Conventional Organic Sorbents | ILs | Conventional Sorbents (PDMS, EG-silicone, PA) | ILs | Conventional Organic Sorbents | ILs | Conventional Sorbents (MIPs) | ILs |
|
|
|
|
|
|
|
|
|
|
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kissoudi, M.; Samanidou, V. Recent Advances in Applications of Ionic Liquids in Miniaturized Microextraction Techniques. Molecules 2018, 23, 1437. https://doi.org/10.3390/molecules23061437
Kissoudi M, Samanidou V. Recent Advances in Applications of Ionic Liquids in Miniaturized Microextraction Techniques. Molecules. 2018; 23(6):1437. https://doi.org/10.3390/molecules23061437
Chicago/Turabian StyleKissoudi, Maria, and Victoria Samanidou. 2018. "Recent Advances in Applications of Ionic Liquids in Miniaturized Microextraction Techniques" Molecules 23, no. 6: 1437. https://doi.org/10.3390/molecules23061437
APA StyleKissoudi, M., & Samanidou, V. (2018). Recent Advances in Applications of Ionic Liquids in Miniaturized Microextraction Techniques. Molecules, 23(6), 1437. https://doi.org/10.3390/molecules23061437






