Cocoa Shell: A By-Product with Great Potential for Wide Application
Abstract
1. Introduction
2. Cocoa Shell Application
2.1. Use in Feedstuff
2.2. Use in Agriculture
2.3. Use in Biofuels
2.4. Use as an Adsorbent
2.5. Use as a Dye
2.6. Use in Food Products
3. Cocoa Shell Extracts
3.1. Polyphenol-Rich Cocoa Shell Extracts
3.2. Methylxanthine-Rich Cocoa Shell Extracts
3.3. Fiber-Rich Cocoa Shell Extracts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ravindran, R.; Jaiswal, A.K. Exploitation of Food Industry Waste for High-Value Products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef] [PubMed]
- ICCO-International Cocoa Organization Annual Report 2007/2008. Available online: https://www.icco.org/about-us/international-cocoa-agreements/cat_view/1-annual-report/23-icco-annual-report-in-english.html (accessed on 19 September 2017).
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of cocoa (Theobroma cacao L.) co-products. Food Res. Int. 2012, 49, 39–45. [Google Scholar] [CrossRef]
- Barazarte, H.; Sangronis, E.; Unai, E. La cascara de cacao (Theobroma cacao L.): Una posible fuente comercial de pectinas. Arch. Latinoam. Nutr. 2008, 58, 64–70. [Google Scholar] [PubMed]
- Sukha, D.A. Potential value added products from Trinidad and Tobago cocoa. In Proceedings of the Revitalisation of the Trinidad and Tobago cocoa industry—Targets, Problems and Options, St. Augustine, FL, USA, 20 September 2003; pp. 69–73. [Google Scholar]
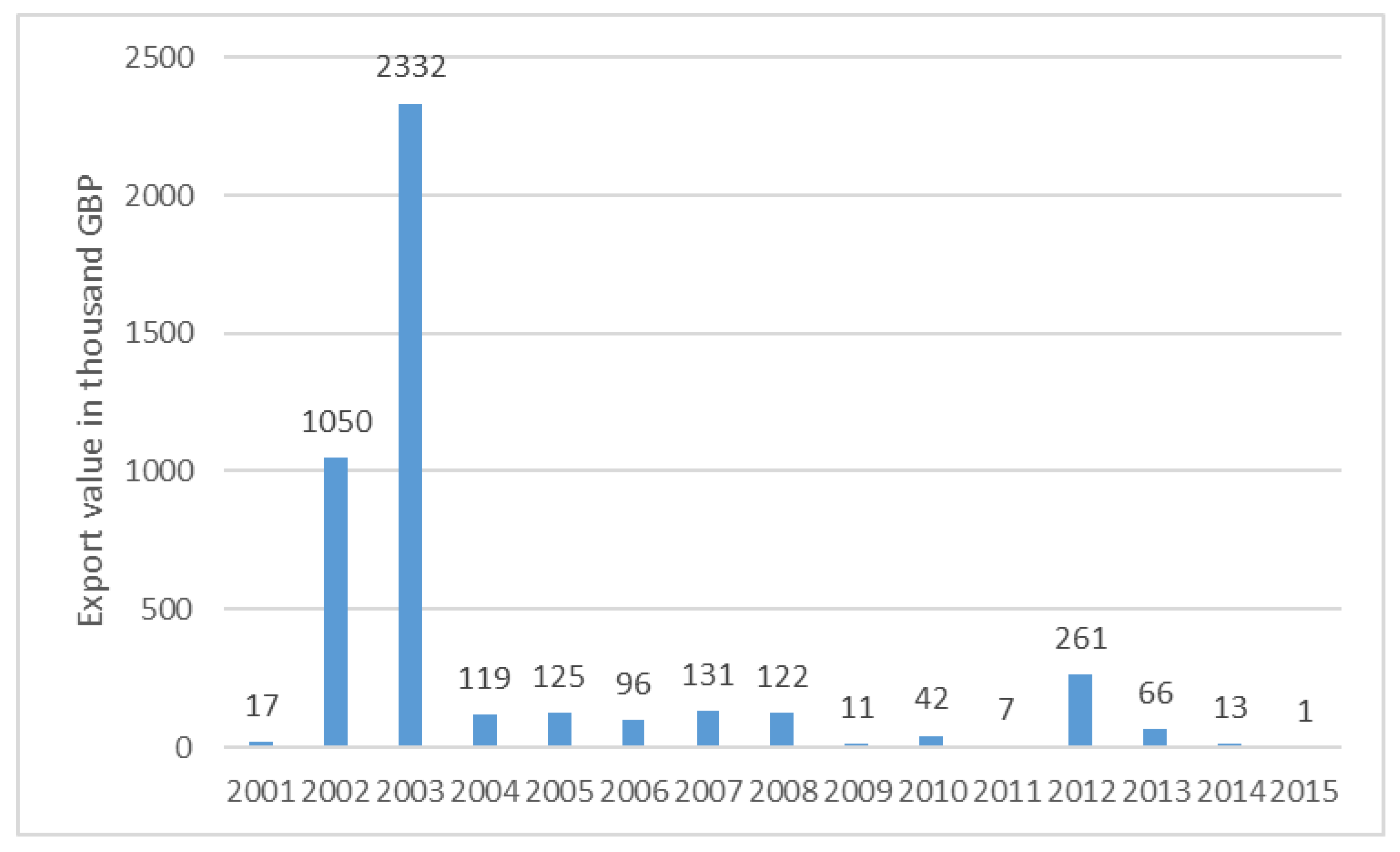
- Statista—The Statistics Portal. Value of Cocoa Shells, Husks, Skins and Other Cocoa Waste Exported from the United Kingdom (UK) from 2001 to 2015 (in 1000 GBP). 2017. Available online: https://www.statista.com/statistics/520014/cocoa-shells-husks-skins-and-other-cocoa-waste-export-value-united-kingdom-uk/ (accessed on 19 September 2017).
- Adamafio, N.A. Theobromine Toxicity and Remediation of Cocoa By-products: An Overview. J. Biol. Sci. 2013, 37, 570–576. [Google Scholar] [CrossRef]
- Mylsamy, S. Studies on Synthesis And Characterization of Activated Carbon Prepared from Cocoa (Theobroma cacao) Shell And Its Adsorption Modeling Of Dissolved Organic Pollutants. PhD Thesis, Faculty of Science and Humanities, Anna University, Chennai, India, 14 September 2012. [Google Scholar]
- Okiyama, D.C.G.; Navarro, S.L.B.; Rodrigues, C.E.C. Cocoa shell and its compounds: Applications in the food industry. Trends Food Sci. Technol. 2017, 63, 103–112. [Google Scholar] [CrossRef]
- Chronopoulos, D.; Zuurbier, R.; Brandstetter, B.; Jung, C. Food Comprising Alkalized Cocoa Shells and Method Therefor. U.S. Patent 2011/0151098 A1, 23 June 2011. [Google Scholar]
- Nsor-Atindana, J.; Zhong, F.; Mothibe, K.J. In vitro hypoglycemic and cholesterol lowering effects of dietary fiber prepared from cocoa (Theobroma cacao L.) shells. Food Funct. 2012, 3, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Nsor-Atindana, J.; Zhong, F.; Mothibe, K.J.; Bangoura, M.L.; Lagnika, C. Quantification of total polyphenolic content and antimicrobial activity of cocoa (Theobroma cacao L.) bean shells. Pak. J. Nutr. 2012, 11, 672–677. [Google Scholar] [CrossRef]
- Vītola, V.; Ciproviča, I. The effect of cocoa beans heavy and trace elements on safety and stability of confectionery products. Rural Sustain. Res. 2016, 35, 19–23. [Google Scholar] [CrossRef]
- Silva, H.G.; Pires, A.J.V.; Silva, F.F.; Veloso, C.M.; Cravalho, G.G.P.; Cezário, A.S.; Santos, C.C. Farelo de Cacau (Theobroma cacao L.) e Torta de Dendê (Elaeis guineensis, Jacq) na Alimentação de Cabras em Lactação : Consumo e Produção de Leite. Rev. Bras. Zootecn. 2005, 34, 1786–1794. [Google Scholar] [CrossRef]
- Andrade, I.V.O.; Pires, A.J.V.; Carvalho, G.G.P.; de Veloso, C.M.; Bonomo, P. Perdas da características fermentativas e valor nutritivo da silagem de capim—elefante contendo subprodutos agrícolas—Losses, fermentation characteristics and nutritional. Rev. Bras. Zootecn. 2010, 39, 2578–2588. [Google Scholar] [CrossRef]
- Carvalho Junior, J.N.; Pires, A.J.V.; Veloso, C.M.; Silva, F.F.; Reis, R.A.; Carvalho, G.G.P. Digestibilidade aparente da dieta com capim-elefante ensilado com diferentes aditivos. Arq. Bras. Med. Vet. Zootec. 2010, 62, 889–897. [Google Scholar] [CrossRef]
- Beckett, S.T. Industrial Chocolate Manufacture and Use, 4th ed.; Wiley-Blackwell: Chichester, West Sussex, York, UK, 2009; ISBN 978-1-405-13949-6. [Google Scholar]
- Day, E.J.; Dilworth, B.C. Toxicity of jimson weed seed and cocoa shell meal to broiler. Poult. Sci. 1984, 63, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Emiola, I.A.; Ojebiyi, O.O.; Akande, T.O. Performance and Organ Weights of Laying Hens Fed Diets Containing Graded Levels of Sun-dried Cocoa Bean Shell (CBS). Int. J. Poult. Sci. 2011, 10, 987–990. [Google Scholar] [CrossRef]
- Olubamiwa, O.; Ikyo, S.M.; Adebowale, B.A.; Omojola, A.B.; Hamzat, R.A. Effect of Boiling Time on the Utilization of Cocoa Bean Shell in Laying Hen Feeds. Int. J. Poult. Sci. 2006, 5, 1137–1139. [Google Scholar] [CrossRef]
- Adeloye, A. Efficiencies of Conversion of Some Lignocellulosic Waste Materials by Goats. Bioresour. Technol. 1992, 40, 167–169. [Google Scholar] [CrossRef]
- Adebowale, B.A.; Olubamiwa, O. Growth Response of Clarias gariepinus juvenile to Cocoa Husk Endocarp Based Diets. Agric. J. 2008, 3, 425–428. [Google Scholar]
- Falaye, A.E. Utilization of Cocoa Husk in the Nutrition of tilapia (Oreochromis niloticus). Ph.D. Thesis, University of Ibadan, Ibadan, Nigeria, 1988. [Google Scholar]
- Falay, A.E. Evaluation of the Chemical and Nutrient composition of Cocoa husk (Theobroma cacao) and its potential as a fish feed ingredient. Niger. J. Lasic Appl. Sci. 1990, 4, 157–164. [Google Scholar]
- Falaye, A.E. Utilization of agro-industrial waste as fish feedstuffs of Nigeria. In Proceedings of the 10th Annual Conference of the Fisheries Society of Nigeria (FISON), Abeokuta, Nigeria, 16–20 November 1992; pp. 47–57. [Google Scholar]
- Falaye, A.E.; Jauncey, K. Acceptability and digestibility by tilapia Oreochromis niloticus of feeds containing cocoa husk. Aquac. Nutr. 1999, 5, 157–161. [Google Scholar] [CrossRef]
- Pouomogne, V.; Gabriel, T.; Pouemegne, J.B. A preliminary evaluation of cacao husks diets for juvenile Nile tilapia (Oreochromis niloticus). Aquaculture 1997, 156, 211–219. [Google Scholar] [CrossRef]
- Ayinde, O.E.; Ojo, V.; Adeyina, A.A.; Adesoye, O. Economics of Using Cocoa Bean Shell as Feed Supplement for Rabbits. Pak. J. Nutr. 2010, 9, 195–197. [Google Scholar] [CrossRef]
- Magistrelli, D.; Zanchi, R.; Malagutti, L.; Galassi, G.; Canzi, E.; Rosi, F. Effects of Cocoa Husk Feeding on the Composition of Swine Intestinal Microbiota. J. Agric. Food Chem. 2016, 64, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Ogunsipe, M.H.; Ibidapo, I.; Oloruntola, O.D.; Agbede, J.O. Growth performance of pigs on dietary cocoa bean shell meal. Livest. Res. Rural Dev. 2017, 29, 1. [Google Scholar]
- Billeaud, L.A.; Zajicek, J.M. Influence of mulches on weed control, soil pH, soil nitrogen content, and growth of Ligustrum japonicum. J. Environ. Hortic. 1989, 7, 155–157. [Google Scholar]
- Bond, B.; Grundy, A.C. Non-chemical weed management in organic farming systems. Weed Res. 2001, 41, 383–405. [Google Scholar] [CrossRef]
- Arentoft, B.W.; Ali, A.; Streibig, J.C.; Andreasen, C. A new method to evaluate the weed-suppressing effect of mulches: A comparison between spruce bark and cocoa husk mulches. Weed Res. 2013, 53, 169–175. [Google Scholar] [CrossRef]
- Hale, S.; Alling, V.; Martinsen, V.; Mulder, J.; Breedveld, G.; Cornelissen, G. The sorption and desorption of phosphate-P, ammonium-N and nitrate-N in cacao shell and corn cob biochars. Chemosphere 2013, 91, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Awolu, O.O.; Oyeyemi, S.O. Optimization of bioethanol production from cocoa (Theobroma cacao) bean shell. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 506–514. [Google Scholar]
- Mancini, G.; Papirio, S.; Lens Piet, N.L.; Esposito, G. Solvent Pretreatments of Lignocellulosic Materials to Enhance Biogas Production: A Review. Environ. Eng. Sci. 2016, 33, 843–850. [Google Scholar] [CrossRef]
- Malaták, J.; Bradna, J. Use of waste material mixtures for energy purposes in small combustion devices. Res. Agric. Eng. 2014, 60, 50–59. [Google Scholar] [CrossRef]
- Directive of Ministry of the Environment of the Czech Republic to the Requirements for Awarding Marks—Hot Water Boilers for Central Heating Systems for Biomass; Directive No. 13; Ministry of the Environment of the Czech Republic: Prague, Czech Republic, 2006.
- Rangabhashiyam, S.; Anu, N.; Selvaraju, N. Sequestration of dye from textile industry wastewater using agricultural waste products as adsorbents. J. Environ. Chem. Eng. 2013, 1, 629–641. [Google Scholar] [CrossRef]
- Fioresi, F.; Vieillard, J.; Bargougui, R.; Bouazizi, N.; Nkuigue Fotsing, P.; Djoufac Woumfo, E.; Brun, N.; Mofaddel, N.; Le Derf, F. Chemical modification of the cocoa shell surface using diazonium salts. J. Colloid Interface Sci. 2017, 494, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Bernaet, H.; Ruysscher, I. Methods for Extraction Cocoa. U.S. Patent 2013/0302473 A1, 14 November 2013. [Google Scholar]
- Turcotte, V.; Blais, J.-F.; Mercier, G.; Drogui, P. Use of cocoa shells as biofiltration support for the treatment of effluents from the food industry. Can. J. Civ. Eng. 2009, 36, 1059–1070. [Google Scholar] [CrossRef]
- Ahmad, F.; Daud, W.M.A.W.; Ahmad, M.A.; Radzi, R. Using cocoa (Theobroma cacao) shell-based activated carbon to remove 4-nitrophenol from aqueous solution: Kinetics and equilibrium studies. Chem. Eng. J. 2011, 178, 461–467. [Google Scholar] [CrossRef]
- Ahmad, F.; Daud, W.M.A.W.; Ahmad, M.A.; Radzi, R. Cocoa (Theobroma cacao) shell-based activated carbon by CO2 activation in removing of Cationic dye from aqueous solution: Kinetics and equilibrium studies. Chem. Eng. Res. Des. 2012, 90, 1480–1490. [Google Scholar] [CrossRef]
- Ahmad, F.; Daud, W.M.A.W.; Ahmad, M.A.; Radzi, R. The effects of acid leaching on porosity and surface functional groups of cocoa (Theobroma cacao)-shell based activated carbon. Chem. Eng. Res. Des. 2013, 91, 1028–1038. [Google Scholar] [CrossRef]
- Kalaivani, S.S.; Vidhyadevi, T.; Murugesan, A.; Baskaralingam, P.; Anuradha, C.D.; Ravikumar, L.; Sivanesan, S. Equilibrium and kinetic studies on the adsorption of Ni(II) ion from an aqueous solution using activated carbon prepared from Theobroma cacao (cocoa) shell. Desalin. Water Treat. 2014, 54, 1629–1641. [Google Scholar] [CrossRef]
- Ribas, M.C.; Adebayo, M.A.; Prola, L.D.T.; Lima, E.C.; Cataluña, R.; Feris, L.A.; Calvete, T. Comparison of a homemade cocoa shell activated carbon with commercial activated carbon for the removal of reactive violet 5 dye from aqueous solutions. Chem. Eng. J. 2014, 248, 315–326. [Google Scholar] [CrossRef]
- Saucier, C.; Adebayo, M.A.; Lima, E.C.; Cataluña, R.; Thue, P.S.; Prola, L.D.; Puchana-Rosero, M.J.; Machado, F.M.; Pavan, F.A.; Dotto, G.L. Microwave-assisted activated carbon from cocoa shell as adsorbent for removal of sodium diclofenac and nimesulide from aqueous effluents. J. Hazard. Mater. 2015, 289, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Recobert, M.; Trautwein, G.; Perez-Cadenas, M.; Alca~niz-Monge, J. Preparation of binderless activated carbon monoliths from cocoa bean husk. Microporous Mesoporous Mater. 2017, 243, 28–38. [Google Scholar] [CrossRef]
- Tran, T.N.; Bayer, I.S.; Heredia-Guerrero, J.A.; Frugone, M.; Lagomarsino, M.; Maggio, F.; Athanassiou, A. Cocoa Shell Waste Biofilaments for 3D Printing Applications. Macromol. Mater. Eng. 2017, 302, 1700219. [Google Scholar] [CrossRef]
- Tu, C. Study about Stability of Cacao Husk Pigment and Its Dyeing Properties on Cotton. Key Eng. Mater. 2016, 671, 133–138. [Google Scholar] [CrossRef]
- Jozinović, A.; Panak Balentić, J.; Ačkar, Đ.; Babić, J.; Pajin, B.; Miličević, B.; Guberac, S.; Šubarić, D. Cocoa husk application in enrichment of extruded snack products. In Proceedings of the Fourth International Congress on Cocoa Coffee and Tea, Torino, Italy, 25–28 June 2017; p. 68. [Google Scholar]
- Sanchez, D.; Moulay, L.; Muguerza, B.; Quiñones, M.; Miguel, M.; Aleixandre, A. Effect of a Soluble Cocoa Fiber-Enriched Diet in Zucker Fatty Rats. J. Med. Food 2010, 13, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Mundo, M.L.; Jaramillo Flores, M.E.; Espinosa Solis, V.; Chávez-Reyes, Y.; Díaz Ramírez, M.; Salgado Cruz, M.P.; Calderón Domínguez, G. Muffins enriched with cocoa shell fiber. In Proceedings of the Fourth International Congress on Cocoa Coffee and Tea, Torino, Italy, 25–28 June 2017; p. 124. [Google Scholar]
- Martínez-Cervera, S.; Salvador, A.; Muguerza, B.; Moulay, L.; Fiszman, S.M. Cocoa fibre and its application as a fat replacer in chocolate muffins. LWT Food Sci. Technol. 2011, 44, 729–736. [Google Scholar] [CrossRef]
- Collar, C.; Rosell, C.M.; Muguerza, B.; Moulay, L. Breadmaking performance and keeping behavior of cocoa-soluble fiber-enriched wheat breads. Food Sci. Technol. Int. 2009, 15, 79–87. [Google Scholar] [CrossRef]
- Bradbury, A.; Kopp, G. Polyphenol-Enriched Composition from Cocoa Shell Extraction. U.S. Patent 0879946A2, 30 November 2006. [Google Scholar]
- Hernández-Hernández, C.; Viera-Alcaide, I.; Sillero, A.M.M.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Bioactive compounds in Mexican genotypes of cocoa cotyledon and husk. Food Chem. 2018, 240, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Pereira, L.; Guglielmetti, A.; Zeppa, G. Pulsed Electric Field Assisted Extraction of Bioactive Compounds from Cocoa Bean Shell and Coffee Silverskin. Food Bioproc. Technol. 2018, 11, 818–835. [Google Scholar] [CrossRef]
- Arlorio, M.; Coisson, J.D.; Restani, P.; Martelli, A. Characterization of Pectins and Some Secondary Compounds from Theobroma cacao Hulls. J. Food Sci. 2001, 66, 653–656. [Google Scholar] [CrossRef]
- Mazzutti, S.; Goncalves Rodrigues, L.G.; Mezzomo, N.; Venturi, V.; Ferreira, S.R.S. Integrated green-based processes using supercritical CO2 and pressurized ethanol applied to recover antioxidant compouds from cocoa (Theobroma cacao) bean hulls. J. Supercrit. Fluids 2018, 135, 52–59. [Google Scholar] [CrossRef]
- Ooshima, T.; Osaka, Y.; Sasaki, H.; Osawa, K.; Yasuda, H.; Matsumura, M.; Sobue, S.; Matsumoto, M. Caries inhibitory activity of cacao bean husk extract in in-vitro and animal experiments. Arch. Oral Biol. 2000, 45, 639–645. [Google Scholar] [CrossRef]
- Osawa, K.; Miyazaki, K.; Shimura, S.; Okuda, J.; Matsumoto, M.; Ooshima, T. Identification of Cariostatic Substances in the Cacao Bean Husk: Their Anti-glucosyltransferase and Antibacterial Activities. J. Dent. Res. 2001, 80, 2000–2004. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Park, H.J.; Park, H.H.; Kim, H.S.; Kwon, I.B. Manufacturing process of glucosyltransferase inhibitors from cacao bean husk. U.S. Patent US006159451A, 12 December 2000. [Google Scholar]
- Kim, K.H.; Lee, K.W.; Kim, D.Y.; Park, H.H.; Kwon, I.B.; Lee, H.J. Extraction and fractionation of glucosyltransferase inhibitors from cacao bean husk. Process Biochem. 2004, 39, 2043–2046. [Google Scholar] [CrossRef]
- Matsumoto, M.; Tsuji, M.; Okuda, J.; Sasaki, H.; Nakano, K.; Osawa, K.; Shimura, S.; Ooshima, T. Inhibitory effects of cacao bean husk extract on plaque formation in vitro and in vivo. Eur. J. Oral Sci. 2004, 112, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Percival, R.S.; Devine, D.A.; Duggal, M.S.; Chartron, S.; Marsh, P.D. The effect of cocoa polyphenols on the growth, metabolism and biofilm formation by Streptococcus mutans and Streptococcus sanguinis. Eur. J. Oral Sci. 2006, 114, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh Babu, N.S.; Vivek, D.K.; Ambika, G. Comparative evaluation of chlorhexidine mouthrinse versus cacao bean husk extract mouthrinse as antimicrobial agents in children. Eur. Arch. Paediatr. Dent. 2011, 12, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Unten, S.; Ushijima, H.; Shimizu, H.; Tsuchie, H.; Kitamura, T.; Moritome, N.; Sakacamh, I. Effect of cacao husk extract on human immunodeficiency virus infection. Lett. Appl. Microbiol. 1991, 14, 251–254. [Google Scholar] [CrossRef]
- Hartati, I. Hydrotropic Extraction of Theobromine from Cocoa Bean Shell. Momentum 2010, 6, 17–20. [Google Scholar]
- Bentil, J.A.; Dzogbefia, V.B.; Alemawor, F. Enhancement of the nutritive value of cocoa (Theobroma cacao) bean shells for use as feed for animals through a two-stage solid state fermentation with Pleurotus ostreatus and Aspergillus niger. Int. J. Appl. Microbiol. Biotechnol. Res. 2015, 3, 20–30. [Google Scholar]
- Timbie, D.J.; Sechrist, L.; Keeney, P.G. Application of high-pressure liquid chromatography to the study of variables affecting theobromine and caffeine concentrations in cocoa beans. J. Food Sci. 1978, 43, 560–565. [Google Scholar] [CrossRef]
- Azam, S.; Hadi, N.; Khan, N.U.; Hadi, S.M. Antioxidant and prooxidant properties of caffeine, theobromine and xanthine. Med. Sci. Monit. 2003, 9, 325–330. [Google Scholar]
- Fredholm, B.B. Methylxanthines. In Handbook of Experimental Pharmacology; Fredholm, B.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 200, ISBN 978 3 642 13442 5. [Google Scholar]
- Arlorio, M.; Coïsson, J.D.; Travaglia, F.; Varsaldi, F.; Miglio, G.; Lombardi, G.; Martelli, A. Antioxidant and biological activity of phenolic pigments from Theobroma cacao hulls extracted with supercritical CO2. Food Res. Int. 2005, 38, 1009–1014. [Google Scholar] [CrossRef]
- Redgwell, R.; Trovato, V.; Merinat, S.; Curti, D.; Hediger, S.; Manez, A. Dietary fibre in cocoa shell: Characterisation of component polysaccharides. Food Chem. 2003, 81, 103–112. [Google Scholar] [CrossRef]
- Mollea, C.; Chiampo, F.; Conti, R. Extraction and characterization of pectins from cocoa husks: A preliminary study. Food Chem. 2008, 107, 1353–1356. [Google Scholar] [CrossRef]
- Chan, S.-Y.; Choo, W.-S. Effect of extraction conditions on the yield and chemical properties of pectin from cocoa husks. Food Chem. 2013, 141, 3752–3758. [Google Scholar] [CrossRef] [PubMed]
- Martin-Cabrejas, M.A.; Valiente, C.; Esteban, R.M.; Molla, E.; Waldron, K. Cocoa hull: A potential source of dietary fibre. J. Sci. Food Agric. 1994, 66, 307–311. [Google Scholar] [CrossRef]
- Bonvehi, J.S.; Beneria, M.A. Composition of dietary fibre in cocoa husk. Zeitschrift für Lebensmitteluntersuchung und -Forschung A 1998, 207, 105–109. [Google Scholar]
- Chung, B.Y.; Iiyama, K.; Han, K.-W. Compositional characterization of cacao (Theobroma cacao L.) hull. Agric. Chem. Biotechnol. 2003, 46, 12–16. [Google Scholar]
- Castillejo, G.; Bulló, M.; Anguera, A.; Escribano, J.; Salas-Salvadó, J. A controlled, randomized, double-blind trial to evaluate the effect of a supplement of cocoa husk that is rich in dietary fiber on colonic transit in constipated pediatric patients. Pediatrics 2006, 118, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Moulay, L.; Granado-Serrano, A.B.; Vilanova, O.; Muguerza, B.; Goya, L.; Bravo, L. Hypolipidemic Effect in Cholesterol-Fed Rats of a Soluble Fiber-Rich Product Obtained from Cocoa Husks. J. Agric. Food Chem. 2008, 56, 6985–6993. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Mundo, M.L.; Martínez Mendez, D.; Chávez-Reyes, Y.; Espinosa-Solis, V.; Jaramillo-Flores, M.-E.; Torruco-Uco, J.G. Chemical and nutritional characteristics of biscuits added with cocoa shell powder. In Proceedings of the Fourth International Congress on Cocoa Coffee and Tea, Torino, Italy, 25–28 June 2017; p. 147. [Google Scholar]


| Hernández-Hernández et al. [58] | Barbosa-Pereira et al. [59] | Arlorio et al. (2001) [60] | |||||
|---|---|---|---|---|---|---|---|
| Extraction method | Methanol-water extraction | Ethanol-acidified water extraction | Water extraction | Methanol-acidified water extraction | Acidified water extraction | Pulsed Electric Field Extraction | Supercritical CO2 extraction |
| Total phenols (mg/g) | 14.64 | 49.46 | 5.77 | 20.39 | 9.40 | 24.93 - 32.30 | 18.2 |
| Theobromine (mg/g) | 10.20 | 11.00 | 8.47 | 11.62 | 6.6 | 4.64 – 10.92 | 12.9 |
| Caffeine (mg/g) | n.d. | n.d. | n.d. | n.d. | n.d. | 1.59 - 4.21 | n.d. |
| Catechin (mg/g) | 1.02 | 1.97 | 1.65 | 4.00 | 6.16 | n.d. | n.d. |
| Epicatechin (mg/g) | 15.84 | 9.00 | 6.93 | 17.70 | 7.04 | 0.21 - 2.12 | n.d. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panak Balentić, J.; Ačkar, Đ.; Jokić, S.; Jozinović, A.; Babić, J.; Miličević, B.; Šubarić, D.; Pavlović, N. Cocoa Shell: A By-Product with Great Potential for Wide Application. Molecules 2018, 23, 1404. https://doi.org/10.3390/molecules23061404
Panak Balentić J, Ačkar Đ, Jokić S, Jozinović A, Babić J, Miličević B, Šubarić D, Pavlović N. Cocoa Shell: A By-Product with Great Potential for Wide Application. Molecules. 2018; 23(6):1404. https://doi.org/10.3390/molecules23061404
Chicago/Turabian StylePanak Balentić, Jelena, Đurđica Ačkar, Stela Jokić, Antun Jozinović, Jurislav Babić, Borislav Miličević, Drago Šubarić, and Nika Pavlović. 2018. "Cocoa Shell: A By-Product with Great Potential for Wide Application" Molecules 23, no. 6: 1404. https://doi.org/10.3390/molecules23061404
APA StylePanak Balentić, J., Ačkar, Đ., Jokić, S., Jozinović, A., Babić, J., Miličević, B., Šubarić, D., & Pavlović, N. (2018). Cocoa Shell: A By-Product with Great Potential for Wide Application. Molecules, 23(6), 1404. https://doi.org/10.3390/molecules23061404











