Trapping para-Quinone Methide Intermediates with Ferrocene: Synthesis and Preliminary Biological Evaluation of New Phenol-Ferrocene Conjugates
Abstract
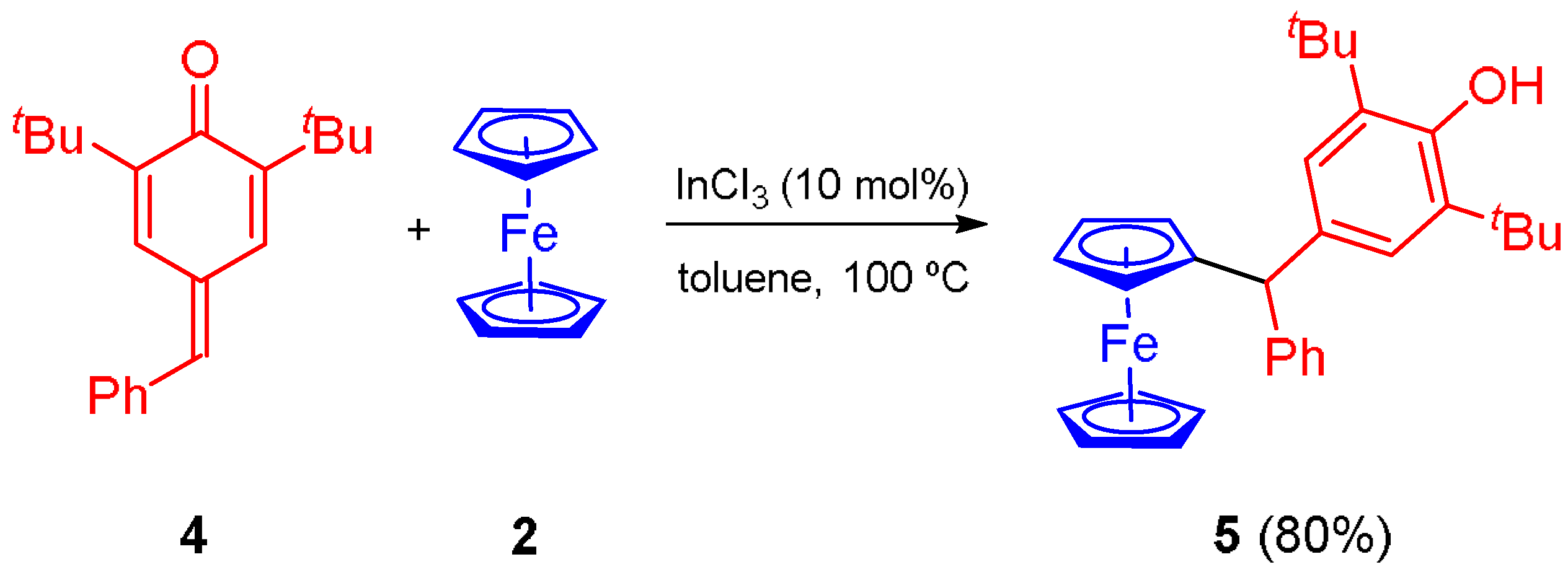
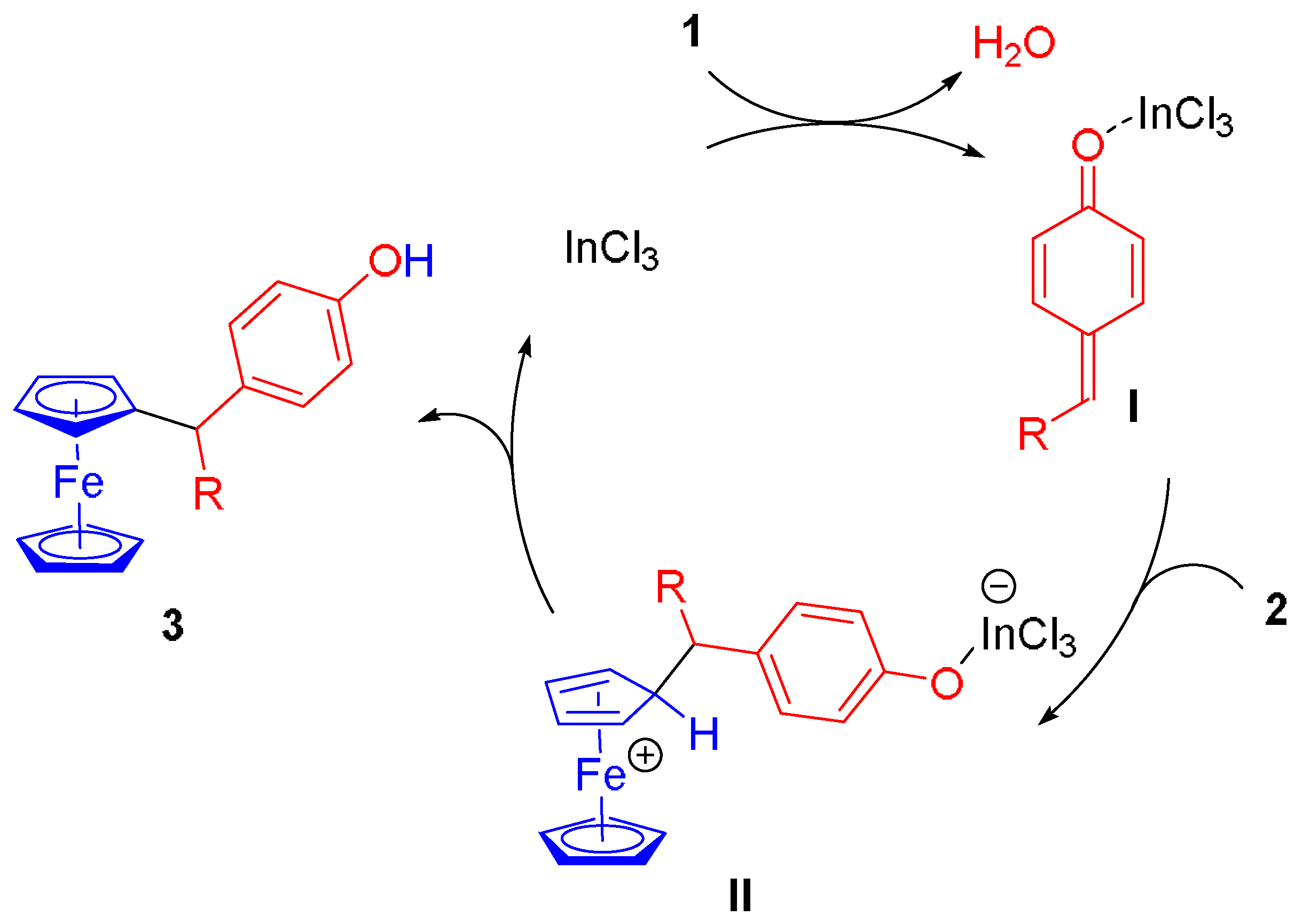
1. Introduction
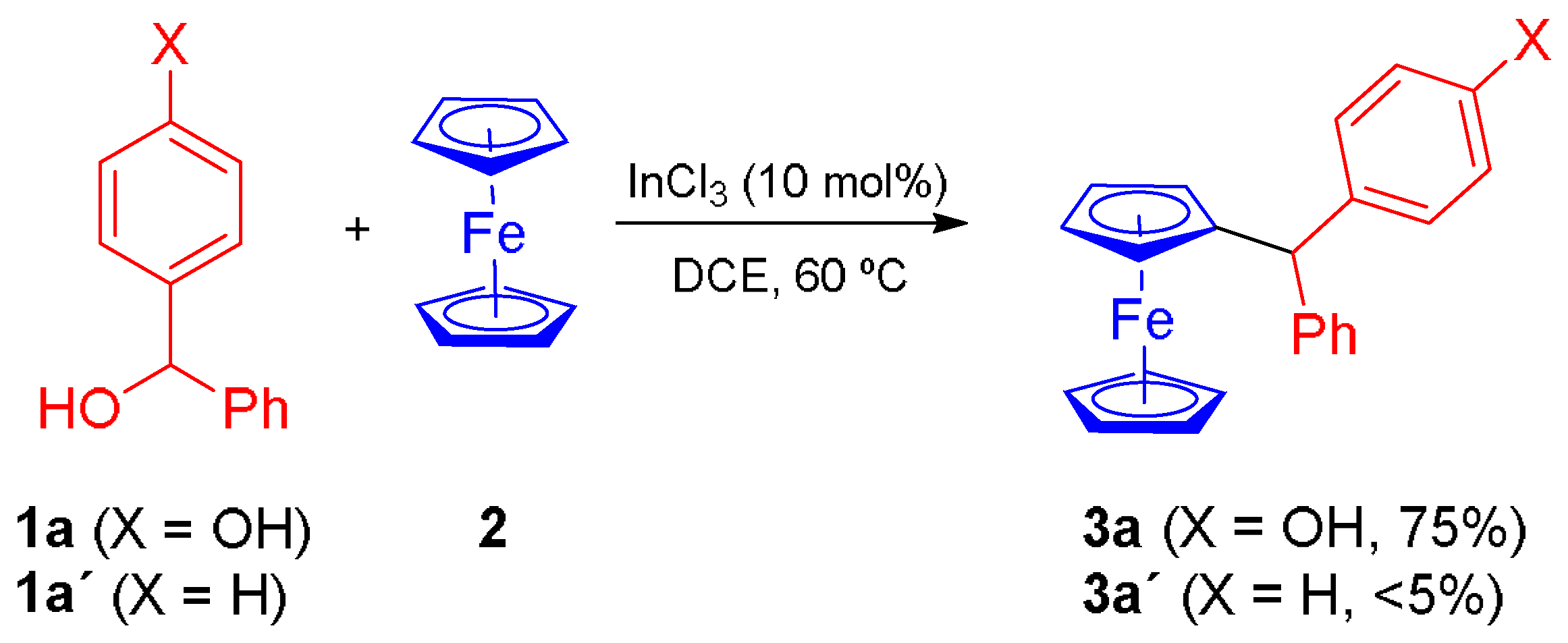
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. General Procedure for the Synthesis of Ferrocene Derivatives 3a–l
3.3. Synthesis of Ferrocene Derivative 5
3.4. Cytotoxic Assays
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
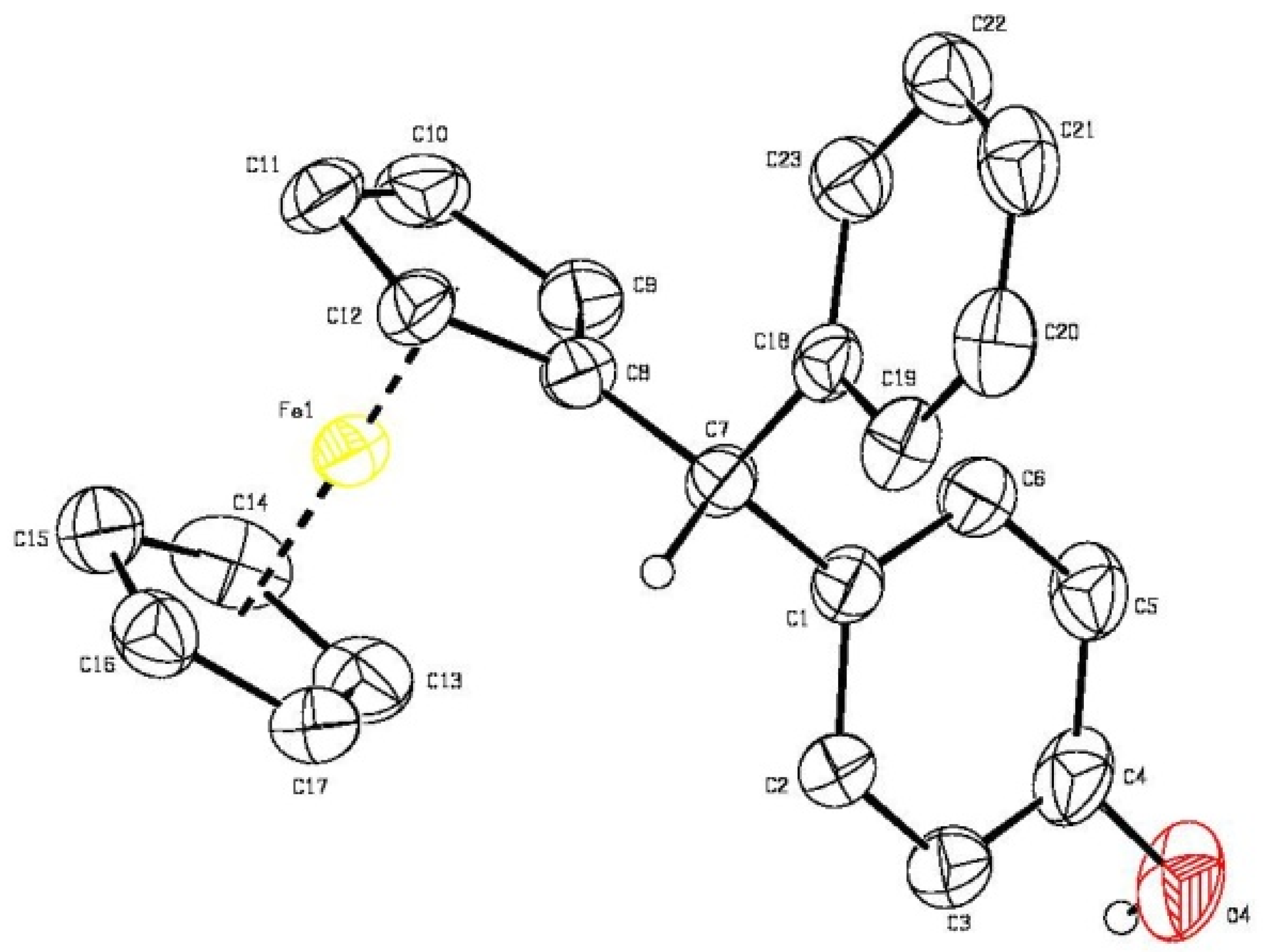
Appendix A. Crystal Data for Ferrocene Derivative 3a
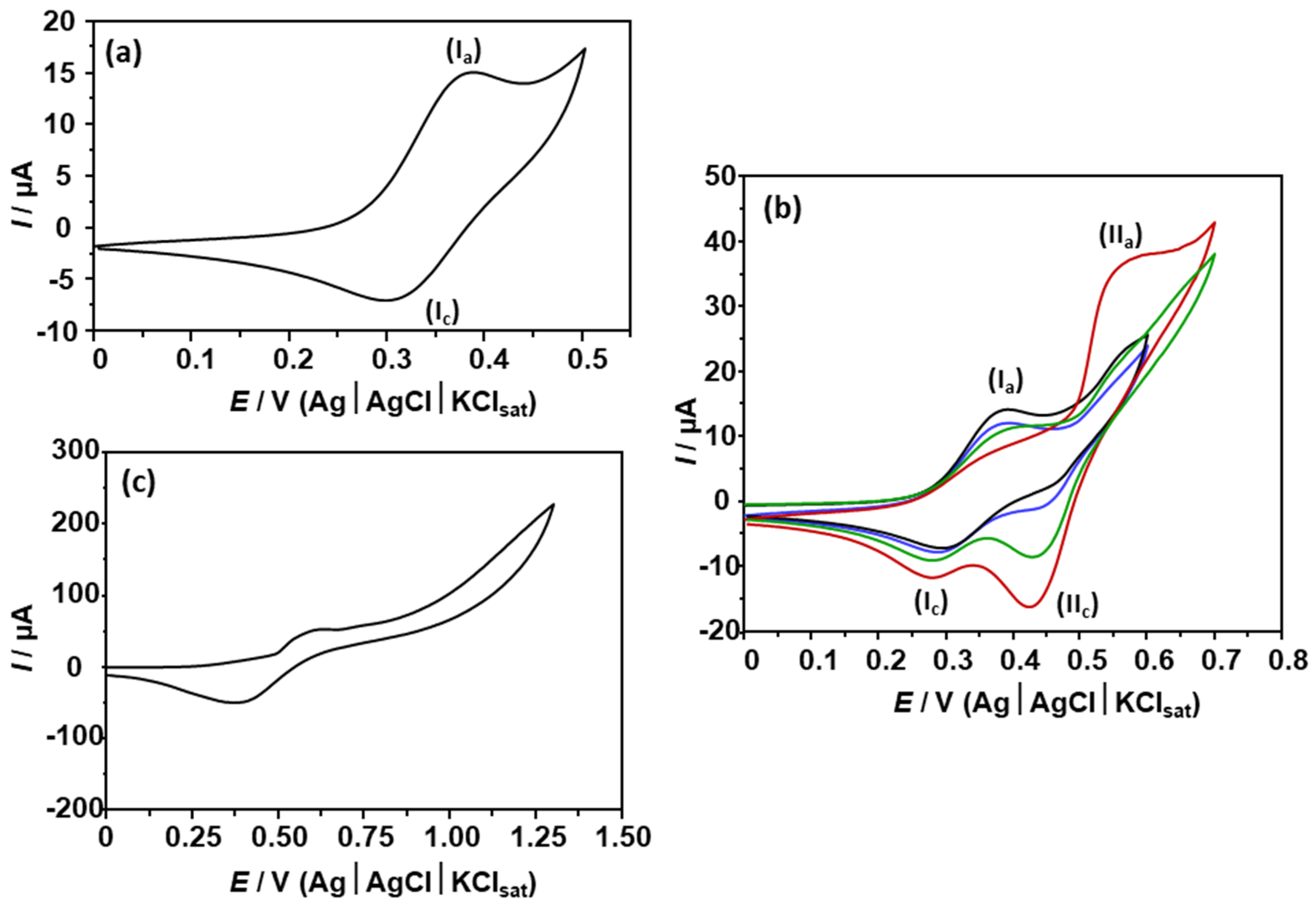
Appendix B. Electrochemical Study for Ferrocene Derivative 3a
References and Notes
- Kealy, T.J.; Pauson, P.L. A New Type of Organo-Iron Compound. Nature 1951, 168, 1039–1040. [Google Scholar] [CrossRef]
- Miller, S.A.; Tebboth, J.A.; Tremaine, J.F. Dicyclopentadienyliron. J. Chem. Soc. 1952, 632–635. [Google Scholar] [CrossRef]
- Štěpnička, P. Ferrocenes: Ligands, Materials and Biomolecules; Wiley: Chichester, UK, 2008. [Google Scholar]
- Phillips, E.S. Ferrocenes: Compounds, Properties and Applications; Nova Science Publishers: New York, NY, USA, 2011. [Google Scholar]
- Ferrocenes. Homogeneous Catalysis. Organic Synthesis. Materials Science; Togni, A., Hayashi, T., Eds.; VCH: Weinheim, Germany, 1995. [Google Scholar]
- Chiral Ferrocenes in Asymmetric Catalysis; Dai, L.-X., Hou, X.-L., Eds.; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Astruc, D. Why is Ferrocene so Exceptional? Eur. J. Inorg. Chem. 2017, 1, 6–29. [Google Scholar] [CrossRef]
- Arrayás, R.G.; Adrio, J.; Carretero, J.C. Recent Applications of Chiral Ferrocene Ligands in Asymmetric Catalysis. Angew. Chem. Int. Ed. 2006, 45, 7674–7715. [Google Scholar] [CrossRef] [PubMed]
- Van Staveren, D.R.; Metzler-Nolte, N. Bioorganometallic Chemistry of Ferrocene. Chem. Rev. 2004, 104, 5931–5986. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Gasser, G. The medicinal chemistry of ferrocene and its derivatives. Nat. Rev. Chem. 2017, 1, 066. [Google Scholar] [CrossRef]
- Dive, D.; Biot, C. Ferroquine as an Oxidative Shock Antimalarial. Curr. Top. Med. Chem. 2014, 14, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S.; Silva, A.M.S. A New Age for Iron: Antitumoral Ferrocenes. Organometallics 2013, 32, 5626–5639. [Google Scholar] [CrossRef]
- Melendez, E. Metallocenes as target specific drugs for cancer treatment. Inorg. Chim. Acta 2012, 393, 36–52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ornelas, C. Application of ferrocene and its derivatives in cancer research. New J. Chem. 2011, 35, 1973–1985. [Google Scholar] [CrossRef]
- Schatzschneider, U.; Metzler-Nolte, N. New Principles in Medicinal Organometallic Chemistry. Angew. Chem. Int. Ed. 2006, 45, 1504–1507. [Google Scholar] [CrossRef] [PubMed]
- Gasser, G.; Ott, I.; Metzler-Nolte, N. Organometallic Anticancer Compounds. J. Med. Chem. 2011, 54, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Jaouen, G.; Vessières, A.; Top, S. Ferrocifen type anti cancer drugs. Chem. Soc. Rev. 2015, 44, 8802–8817. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Vessières, A.; Hillard, E.A.; Top, S.; Pigeon, P.; Jaouen, G. Ferrocifens and ferrocifenols as new potential weapons against breast cancer. Chimia 2007, 61, 716–724. [Google Scholar] [CrossRef]
- Vessières, A. Metal carbonyl tracers and the ferrocifen family: Two facets of biorganometallic chemistry. J. Organomet. Chem. 2013, 734, 3–16. [Google Scholar] [CrossRef]
- Hillard, E.A.; Vessières, A.; Jaouen, G. Ferrocene functionalized endocrine modulators as anticancer agents. Top. Organomet. Chem. 2010, 32, 81–117. [Google Scholar]
- Hillard, E.; Vessières, A.; Thouin, L.; Jaouen, G.; Amatore, C. Ferrocene-mediated proton-coupled electron transfer in a series of ferrocifen-type breast-cancer drug candidates. Angew. Chem. Int. Ed. 2006, 45, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Hamels, D.; Dansette, P.M.; Hillard, E.A.; Top, S.; Vessières, A.; Herson, P.; Jaouen, G.; Mansuy, D. Ferrocenyl quinone methides as strong antiproliferative agents: Formation by metabolic and chemical oxidation of ferrocenyl phenols. Angew. Chem. Int. Ed. 2009, 48, 9124–9126. [Google Scholar] [CrossRef] [PubMed]
- Messina, P.; Labbé, E.; Buriez, O.; Hillard, E.A.; Vessières, A.; Hamels, D.; Top, S.; Jaouen, G.; Frapart, Y.M.; Mansuy, D.; et al. Deciphering the activation sequence of ferrociphenol anticancer drug candidates. Chem. Eur. J. 2012, 18, 6581–6587. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pigeon, P.; Top, S.; McGlinchey, M.J.; Jaouen, G. Organometallic antitumor compounds: ferrocifens as precursors to quinone methides. Angew. Chem. Int. Ed. 2015, 54, 10230–10233. [Google Scholar] [CrossRef] [PubMed]
- Hillard, E.; Vessières, A.; Le Bideau, F.; Plazuk, D.; Spera, D.; Huché, M.; Jaouen, G. A Series of Unconjugated Ferrocenyl Phenols: Prospects as Anticancer Agents. Chem. Med. Chem. 2006, 1, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Plazuk, D.; Vessières, A.; Le Bideau, F.; Jaouen, G.; Zakrzewski, J. Synthesis of Benzyl- and Benzhydrylferrocenes via Friedel-Crafts Alkylation of Ferrocene. Access to Ferrocenyl Bisphenols with High Affinities for Estrogen Receptors. Tetrahedron Lett. 2004, 45, 5425–5427. [Google Scholar] [CrossRef]
- López, E.; Lonzi, G.; López, L.A. Gold-Catalyzed C-H Bond Functionalization of Metallocenes: Synthesis of Densely Functionalized Ferrocene Derivatives. Organometallics 2014, 33, 5924–5927. [Google Scholar] [CrossRef]
- López, E.; Borge, J.; López, L.A. Gold-Catalyzed Intermolecular Formal Insertion of Aryldiazo Esters into Cp-H Bonds of Iron and Ruthenium Metallocenes. Chem. Eur. J. 2017, 23, 3091–3097. [Google Scholar] [CrossRef] [PubMed]
- López, E.; Suárez, T.; Ballesteros, A.; López, L.A. Gold(I)-Catalyzed Reaction of Ferrocene and Propargylic Esters: Synthesis of Functionalized Ferrocene Derivatives. Eur. J. Inorg. Chem. 2017, 225–228. [Google Scholar] [CrossRef]
- González-Pelayo, S.; López, E.; Borge, J.; de-los-Santos-Álvarez, N.; López, L.A. Ferrocene-Decorated Phenol Derivatives by Trapping of ortho-Quinone Methide Intermediates with Ferrocene. Eur. J. Org. Chem. 2018. [Google Scholar] [CrossRef]
- The Lewis acid-catalyzed monoalkylation of ferrocene with some benzyl alcohols has been reported. This process takes place in molten ferrocene as solvent at high temperature (176 °C): Abdullah, R.; Chung, K.; Kim, Y.-W.; Hong, I.S. An Efficient Synthesis of Mono-Substituted Benzyl Ferrocene Derivatives. Bull. Korean Chem. Soc. 2015, 36, 32–35. [Google Scholar] [CrossRef]
- For a similar methodology for the alkylation of ferrocene, see: Neuse, E.W.; Trifan, D.S. Alkylation of Ferrocene with α-Aryl Alcohols. J. Am. Chem. Soc. 1962, 84, 1850–1856. [Google Scholar]
- COD 3000199 Contains the Supplementary Crystallographic Data for This Paper. These Data Can Be Obtained Free of Charge via. See also Supplementary Materials. Available online: http://www.crystallography.net/search.html (accessed on 30 May 2018).
- A detailed analysis revealed a non-classical intermolecular interaction of the phenolic OH group with the π cloud of the aromatic ring. For selected examples of aromatic rings as weak hydrogen bond acceptors in crystals, see: Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Seminal contribution on Friedel-Crafts acylation of ferrocene: Woodward, R.B.; Rosenblum, M.; Whiting, M.C. A new aromatic system. J. Am. Chem. Soc. 1952, 74, 3458–3459. [Google Scholar] [CrossRef]
- For a recent related arylation process involving electron-rich arenes, see: Gao, S.; Xu, X.; Yuan, Z.; Zhou, H.; Yao, H.; Lin, A. 1,6-Addition arylation of para-quinone methides: An approach to unsymmetrical triarylmethanes. Eur. J. Org. Chem. 2016, 2016, 3006–3012. [Google Scholar] [CrossRef]
- In this study, NIH 3T3 cells were used as normal control cell line
- Wong, Y.F.; Wang, Z.; Sun, J. Chiral phosphoric acid catalyzed asymmetric addition of naphthols to para-quinone methides. Org. Biomol. Chem. 2016, 14, 5751–5754. [Google Scholar] [CrossRef] [PubMed]
- McNulty, J.; McLeod, D. A scalable process for the synthesis of (E)-Pterostilbene involving aqueous Wittig olefination chemistry. Tetrahedron Lett. 2013, 54, 6303–6306. [Google Scholar] [CrossRef]
- Chu, W.-D.; Zhang, L.-F.; Bao, X.; Zhao, X.-H.; Zeng, C.; Du, J.-Y.; Zhang, G.-B.; Wang, F.-X.; Ma, X.-Y.; Fan, C.-A. Asymmetric catalytic 1,6-conjugate addition/aromatization of para-quinone methides: Enantioselective introduction of functionalized diarylmethine sterogenic centers. Angew. Chem. Int. Ed. 2013, 52, 9229–9233. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

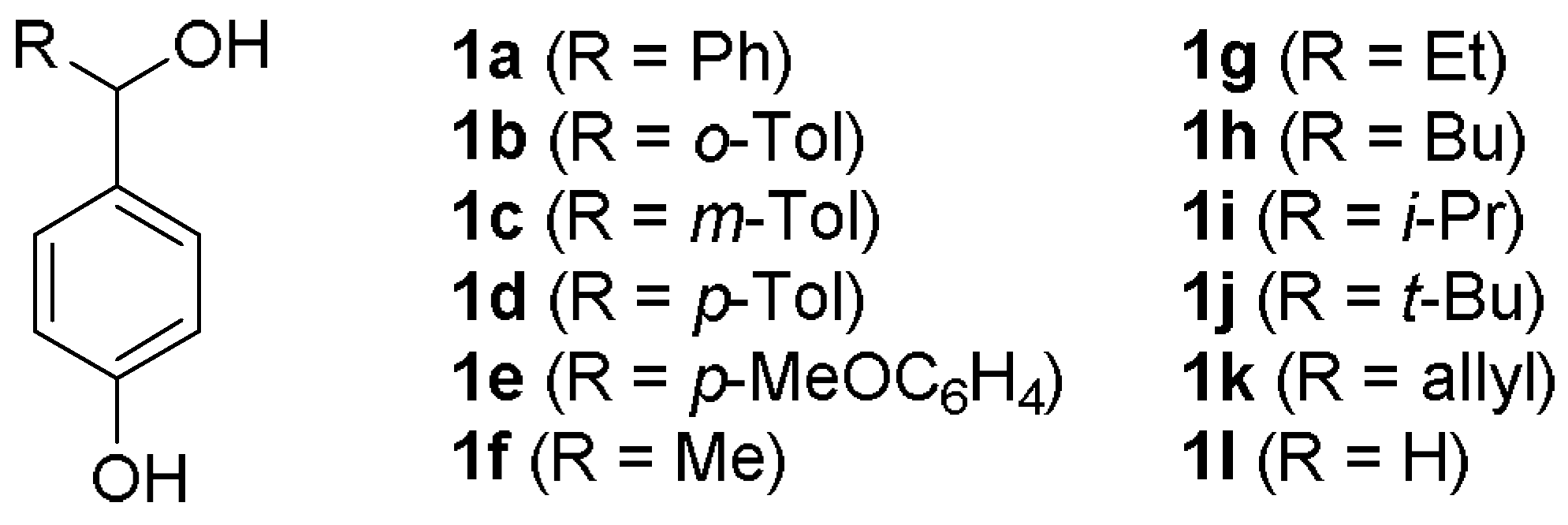
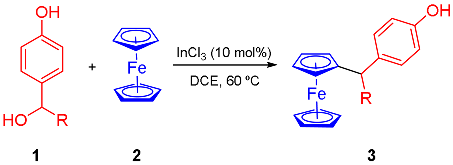
| Entry | Substrate | R | 3 | Yield (%) a |
|---|---|---|---|---|
| 1 | 1a | C6H5 | 3a | 75 |
| 2 | 1b | o-MeC6H4 | 3b | 76 |
| 3 | 1c | m-MeC6H4 | 3c | 51 |
| 4 | 1d | p-MeC6H4 | 3d | 82 |
| 5 | 1e | p-MeOC6H4 | 3e | 48 |
| 6 | 1f | Me | 3f | 62 |
| 7 | 1g | Et | 3g | 60 |
| 8 | 1h | n-Bu | 3h | 42 |
| 9 | 1i | i-Pr | 3i | 62 |
| 10 | 1j | t-Bu | 3j | 43 |
| 11 | 1k | Allyl | 3k | 44 |
| 12 | 1l | H | 3l | 64 |
| 3a | 3g | |
|---|---|---|
| A2780 | 1.07 | 2.23 |
| A549 | 3.55 | 4.89 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Pelayo, S.; López, E.; Borge, J.; De-los-Santos-Álvarez, N.; López, L.A. Trapping para-Quinone Methide Intermediates with Ferrocene: Synthesis and Preliminary Biological Evaluation of New Phenol-Ferrocene Conjugates. Molecules 2018, 23, 1335. https://doi.org/10.3390/molecules23061335
González-Pelayo S, López E, Borge J, De-los-Santos-Álvarez N, López LA. Trapping para-Quinone Methide Intermediates with Ferrocene: Synthesis and Preliminary Biological Evaluation of New Phenol-Ferrocene Conjugates. Molecules. 2018; 23(6):1335. https://doi.org/10.3390/molecules23061335
Chicago/Turabian StyleGonzález-Pelayo, Silvia, Enol López, Javier Borge, Noemí De-los-Santos-Álvarez, and Luis A. López. 2018. "Trapping para-Quinone Methide Intermediates with Ferrocene: Synthesis and Preliminary Biological Evaluation of New Phenol-Ferrocene Conjugates" Molecules 23, no. 6: 1335. https://doi.org/10.3390/molecules23061335
APA StyleGonzález-Pelayo, S., López, E., Borge, J., De-los-Santos-Álvarez, N., & López, L. A. (2018). Trapping para-Quinone Methide Intermediates with Ferrocene: Synthesis and Preliminary Biological Evaluation of New Phenol-Ferrocene Conjugates. Molecules, 23(6), 1335. https://doi.org/10.3390/molecules23061335






