First Metal-Free Synthesis of Tetracyclic Pyrido and Pyrazino Thienopyrimidinone Molecules
Abstract
1. Introduction
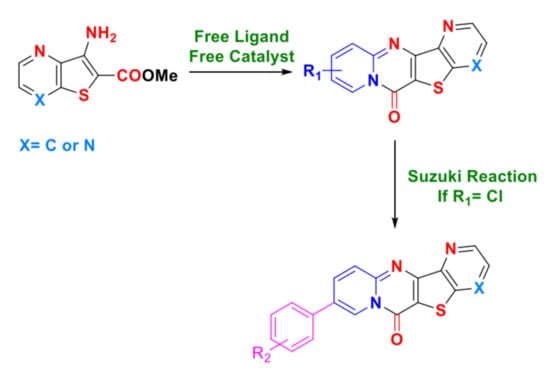
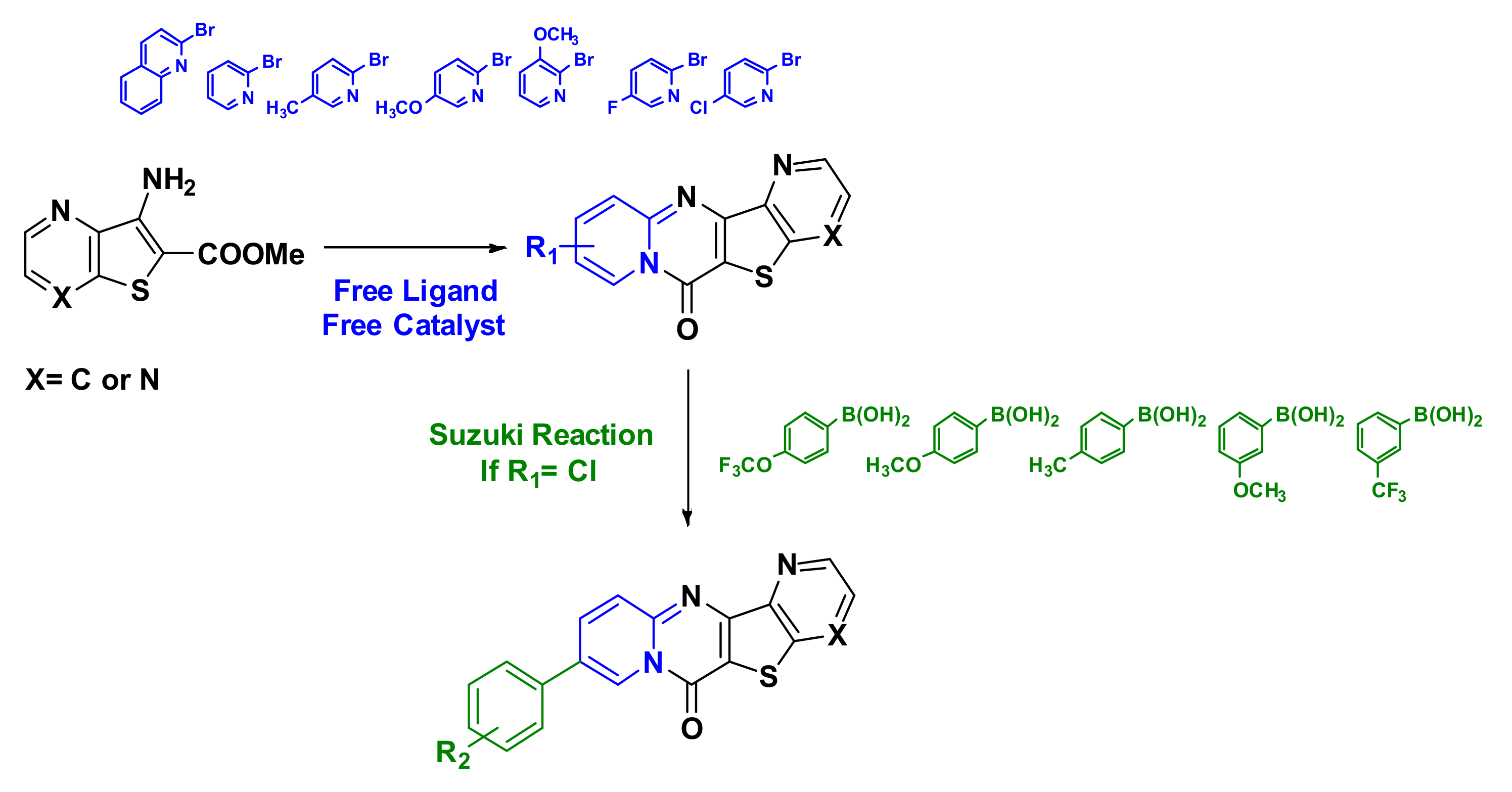
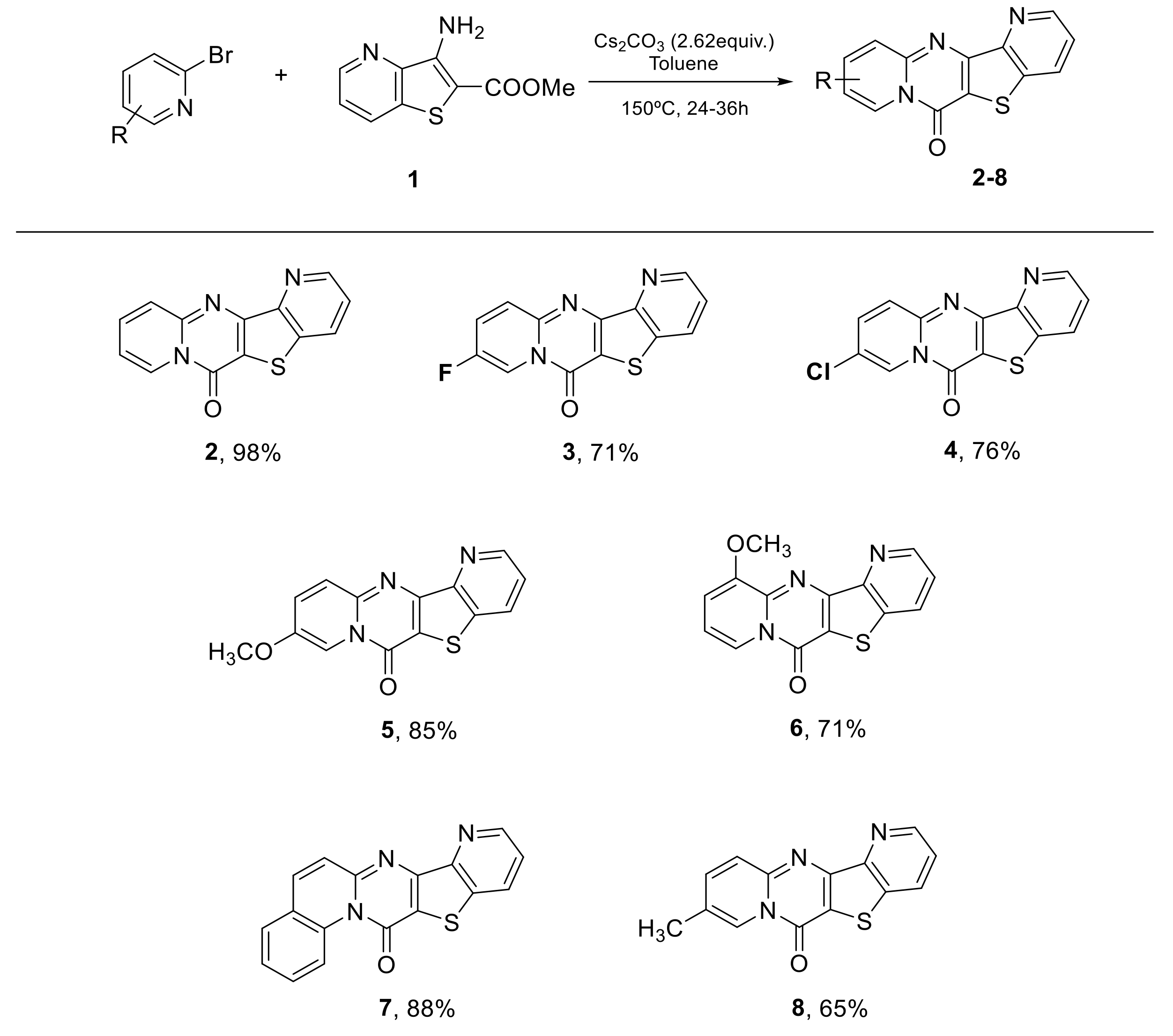
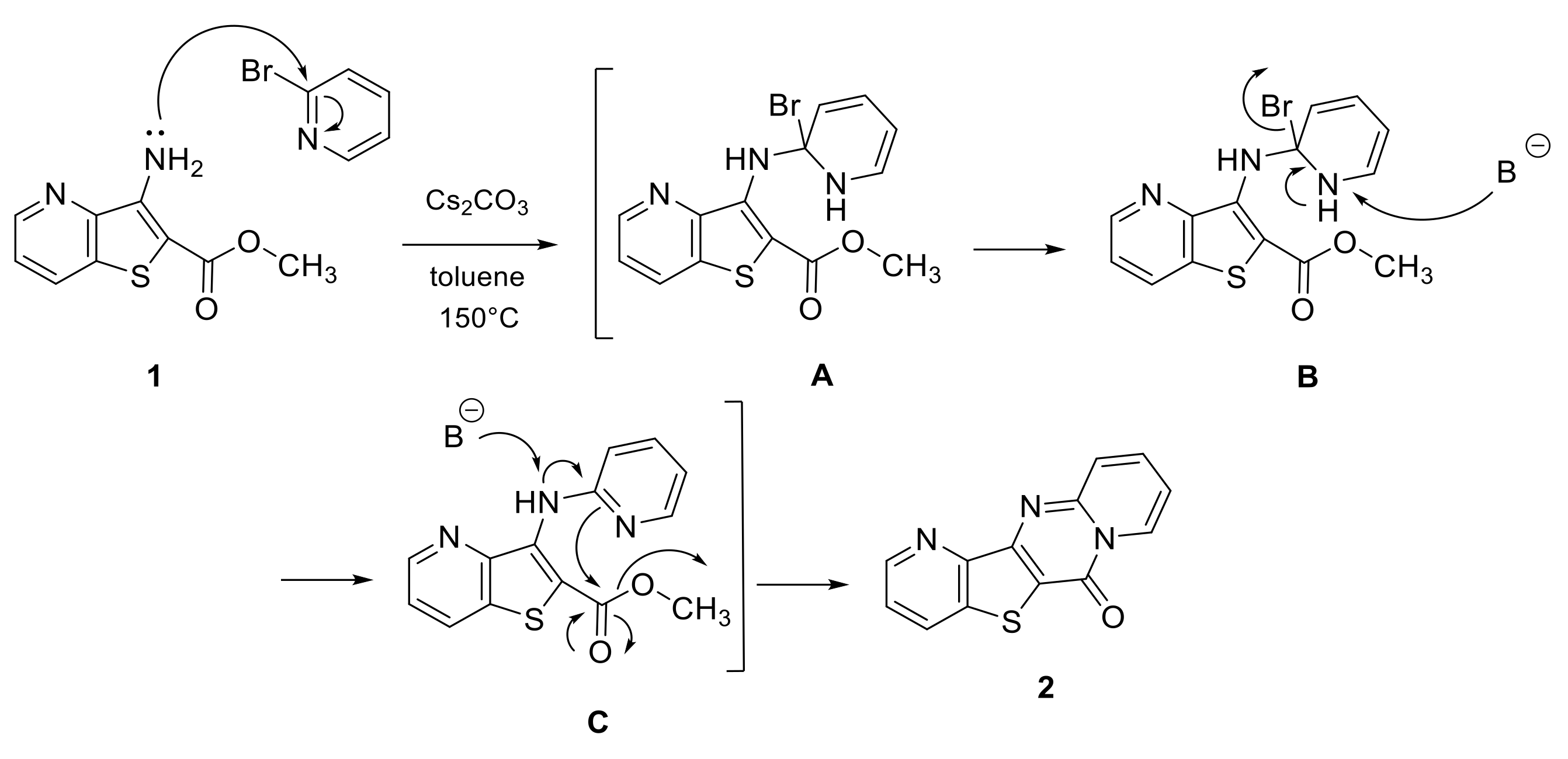
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. General Methods
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Davis, P.; Mousa, S. Anti-Angiogenesis Strategies in Cancer Therapeutics, 1st ed.; Academic Press: Amsterdam, The Netherlands, 2016; pp. 1–19. [Google Scholar]
- Loidreau, Y.; Deau, E.; Marchand, P.; Nourrisson, M.-R.; Loge, C.; Coadou, G.; Loaec, N.; Besson, T. Synthesis and molecular modelling studies of 8-arylpyrido[30,20:4,5] thieno[3,2-d]pyrimidin-4-amines as multitarget Ser/Thr kinases inhibitors. Eur. J. Med. Chem. 2015, 92, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Loidreau, Y.; Marchand, P.; Dubouilh-Benard, C.; Nourrisson, M.-R.; Duflos, M.; Loaec, N.; Meijer, L.; Besson, T. Synthesis and biological evaluation of N-aryl-7-methoxybenzo[b]furo[3,2-d]pyrimidin-4-amines and their N-arylbenzo[b]thieno[3,2-d]pyrimidin-4-amine analogues as dual inhibitors of CLK1 and DYRK1A kinases. Eur. J. Med. Chem. 2013, 59, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Loidreau, Y.; Marchand, P.; Dubouilh-Benard, C.; Nourrisson, M.-R.; Duflos, M.; Lozach, O.; Loaec, N.; Meijer, L.; Besson, T. Synthesis and biological evaluation of N-arylbenzo[b]thieno[3,2-d]pyrimidin-4-amines and their pyrido and pyrazino analogues as Ser/Thr kinase inhibitors. Eur. J. Med. Chem. 2012, 58, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Bozorov, K.; Zhao, J.-Y.; Elmuradov, B.; Pataer, A.; Aisa, H.A. Recent developments regarding the use of thieno[2,3-d]pyrimidin-4-one derivatives in medicinal chemistry, with a focus on their synthesis and anticancer properties. Eur. J. Med. Chem. 2015, 102, 552–573. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Jangid, N.K.; Sharma, V. Metal- and nonmetal-catalyzed synthesis of five-membered S,N-heterocycles. J. Sulfur. Chem. 2018, 39, 193–236. [Google Scholar] [CrossRef]
- Kumari, S.; Kishore, D.; Paliwal, S.; Chauhan, R.; Dwivedi, J.; Mishra, A. Transition metal-free one-pot synthesis of nitrogen-containing heterocycles. Mol. Divers. 2016, 20, 185–232. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649. [Google Scholar] [CrossRef] [PubMed]
- Bentabed-Ababsa, G.; Sid Ely, S.C.; Hesse, S.; Nassar, E.; Chevallier, F.; Nguyen, T.T.; Derdour, A.; Mon-gin, F. Direct Metalation of Heteroaromatic Esters and Nitriles Using a Mixed Lithium−Cadmium Base. Subsequent Conversion to Dipyridopyrimidinones. J. Org. Chem. 2010, 75, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Ma, D. A One-Pot Coupling/Hydrolysis/Condensation Process to Pyrrolo[1,2-a]quinoxaline. J. Org. Chem. 2008, 73, 5159–5162. [Google Scholar] [CrossRef] [PubMed]
- Loubidi, M.; Moutardier, A.; Campos, J.F.; Berteina-Raboin, S. Pd-catalyzed Suzuki/Sonogashira cross-coupling reaction and the direct sp3 arylation of 7-chloro-5-methyl-[1,2,4]triazolo[1,5-a]pyrimidine. Tetrahedron Lett. 2018, 59, 1050–1054. [Google Scholar] [CrossRef]
- El Akkaoui, A.; Berteina-Raboin, S.; Mouaddib, A.; Guillaumet, G. Direct Arylation of Imidazo[1,2-b]pyridazines: Microwave-Assisted One-Pot Suzuki Coupling/Pd-Catalysed Arylation. Eur. J. Org. Chem. 2010, 5, 862–871. [Google Scholar] [CrossRef]
- Queiroz, M.-J.R.P.; Begouin, A.; Ferreira, I.C.F.R.; Kirsch, G.; Calhelha, R.C.; Barbosa, S.; Estevinho, L.M. Palladium-Catalysed Amination of Electron-Deficient or Relatively Electron-Rich Benzo[b]thienyl Bromides-Preliminary Studies of Antimicrobial Activity and SARs. Eur. J. Org. Chem. 2004, 17, 3679–3685. [Google Scholar] [CrossRef]
- Queiroz, M.-J.R.P.; Calhelha, R.C.; Kirsch, G. Reactivity of several deactivated 3-aminobenzo[b]thiophenes in the Buchwald-Hartwig C-N coupling. Scope and limitations. Tetrahedron 2007, 63, 13000–13005. [Google Scholar] [CrossRef]
- Calhelha, R.C.; Queiroz, M.-J.R.P. Synthesis of new thieno[3,2-b]pyridine derivatives by palladium-catalyzed couplings and intramolecular cyclizations. Tetrahedron Lett. 2010, 51, 281–283. [Google Scholar] [CrossRef]
- Loubidi, M.; Pillard, C.; El Hakmaoui, A.; Bernard, P.; Akssira, M.; Guillaumet, G. A new synthetic approach to the imidazo[1,5-a]imidazole-2-one scaffold and effective functionalization through Suzuki-Miyaura cross coupling reactions. RSC Adv. 2016, 6, 7229–7238. [Google Scholar] [CrossRef]
- Fresneau, N.; Hiebel, M.-A.; Agrofoglio, L.A.; Berteina-Raboin, S. Efficient Synthesis of Unprotected C-5-Aryl/Heteroaryl-2′-deoxyuridine via a Suzuki-Miyaura Reaction in Aqueous Media. Molecules 2012, 17, 14409–14417. [Google Scholar] [CrossRef] [PubMed]
- El Akkaoui, A.; Bassoude, I.; Koubachi, J.; Berteina-Raboin, S.; Mouaddib, A.; Guillaumet, G. Pd-catalyzed regiocontrolled Sonogashira and Suzuki cross-coupling reaction of 3,6-dihalogenoimidazo[1,2-a]pyridines: One-pot double-coupling approach. Tetrahedron 2011, 67, 7128–7138. [Google Scholar] [CrossRef]
- Koubachi, J.; El Kazzouli, S.; Berteina-Raboin, S.; Mouaddib, A.; Guillaumet, G. Synthesis of Polysubstituted Imidazo[1,2-a]pyridines via Microwave-Assisted One-Pot Cyclization/Suzuki Coupling/Palladium-Catalyzed Heteroarylation. J. Org. Chem. 2007, 72, 7650–7655. [Google Scholar] [CrossRef] [PubMed]
- Wendeborn, S.; Berteina, S.; Brill, W.K.-D.; De Mesmaeker, A.D. Pd-mediated C-C and C-S bond formation on solid support: A scope and limitations study. Synlett. 1998, 6, 671–675. [Google Scholar] [CrossRef]
Sample Availability: Samples of all compounds are available from the authors. |
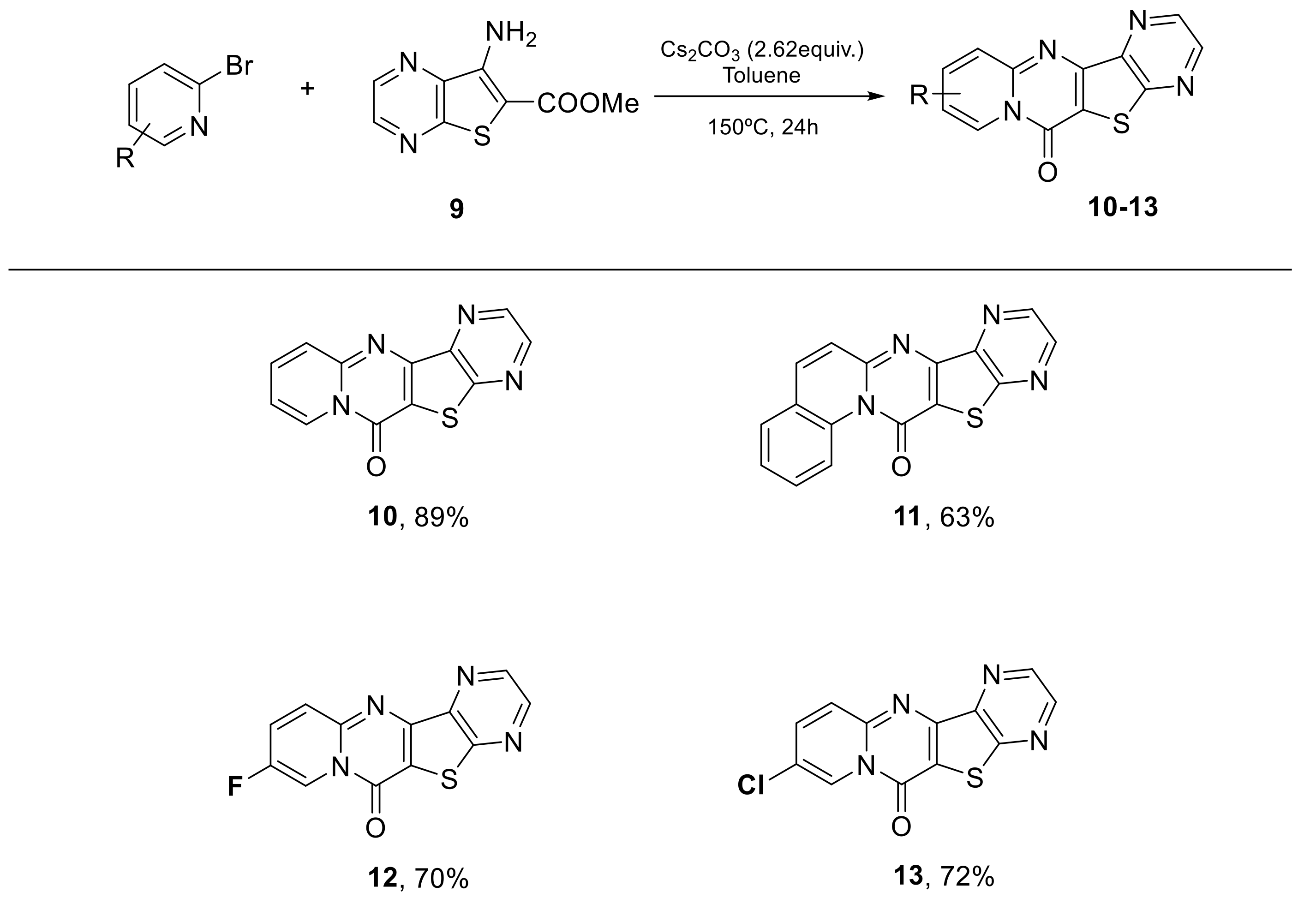


| Entry | [Cat] (mol %) | Solvent | Ligand (mol %) | T (°C) | Base 2.62 equiv | Yield (%) [a] |
|---|---|---|---|---|---|---|
| 1 | Pd(OAc)2 (3) | Dioxane | Xantphos (4) | 120 | Cs2CO3 | 67 |
| 2 | Pd(OAc)2 (3) | Dioxane | PPh3 (6) | 120 | Cs2CO3 | 33 |
| 3 | Pd(OAc)2 (5) | Dioxane | 10-phenanthroline (10) | 120 | Cs2CO3 | 18 |
| 4 | Pd(OAc)2 (3) | Dioxane | - | 120 | Cs2CO3 | 14 |
| 5 | Pd(OAc)2 (3) | Toluene | - | 120 | Cs2CO3 | [b] |
| 6 | Pd(OAc)2 (3) | Toluene | PPh3 (6) | 120 | Cs2CO3 | 8 |
| 7 | Pd(OAc)2 (3) | Toluene | PPh3 (6) | 150 | Cs2CO3 | 10 |
| 8 | Pd(OAc)2 (3) | Toluene/EtOH (2:1) | - | 120 | Cs2CO3 | - |
| 9 | Pd(OAc)2 (3) | Toluene | - | 150 | Cs2CO3 | 41 |
| 10 | Pd(OAc)2 (3) | Toluene | - | 150 | K3PO4 | - |
| 11 | Pd(OAc)2 (3) | DMA | - | 150 | Cs2CO3 | [c] |
| 12 | Pd(TFA)2 (5) | Toluene | - | 120 | Cs2CO3 | 61 |
| 13 | Pd(dppf)Cl2·DCM (3) | Toluene | - | 120 | Cs2CO3 | - |
| 14 | - | Toluene | - | 150 | Cs2CO3 | 98 |
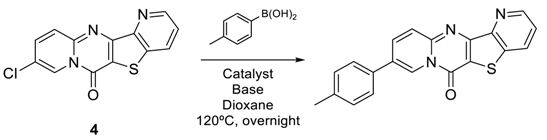
| Entry | [Cat] | Base | % [a] |
|---|---|---|---|
| 1 | Pd(dppf)Cl2·DCM | K2CO3 | 85 |
| 2 | Pd(dppf)Cl2·DCM | Na2CO3 | 57 |
| 3 | Pd(PPh3)4 | Na2CO3 | [b] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aounzou, M.; Campos, J.F.; Loubidi, M.; Berteina-Raboin, S. First Metal-Free Synthesis of Tetracyclic Pyrido and Pyrazino Thienopyrimidinone Molecules. Molecules 2018, 23, 1159. https://doi.org/10.3390/molecules23051159
Aounzou M, Campos JF, Loubidi M, Berteina-Raboin S. First Metal-Free Synthesis of Tetracyclic Pyrido and Pyrazino Thienopyrimidinone Molecules. Molecules. 2018; 23(5):1159. https://doi.org/10.3390/molecules23051159
Chicago/Turabian StyleAounzou, Mohammed, Joana F. Campos, Mohammed Loubidi, and Sabine Berteina-Raboin. 2018. "First Metal-Free Synthesis of Tetracyclic Pyrido and Pyrazino Thienopyrimidinone Molecules" Molecules 23, no. 5: 1159. https://doi.org/10.3390/molecules23051159
APA StyleAounzou, M., Campos, J. F., Loubidi, M., & Berteina-Raboin, S. (2018). First Metal-Free Synthesis of Tetracyclic Pyrido and Pyrazino Thienopyrimidinone Molecules. Molecules, 23(5), 1159. https://doi.org/10.3390/molecules23051159