Comparison of Serum Metabolite Changes of Radiated Mice Administered with Panax quinquefolium from Different Cultivation Regions Using UPLC-Q/TOF-MS Based Metabolomic Approach
Abstract
:1. Introduction
2. Results
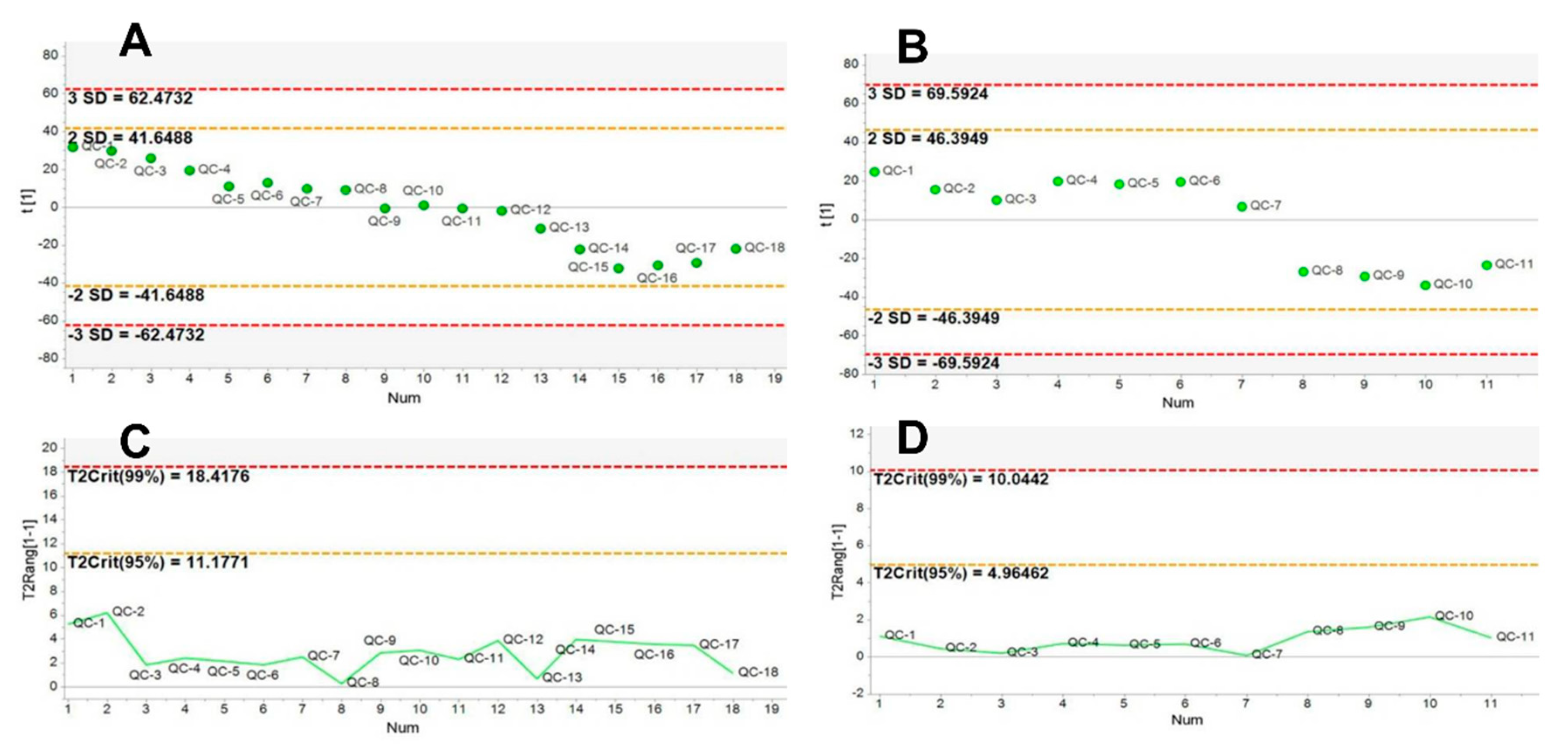
2.1. Method Validation
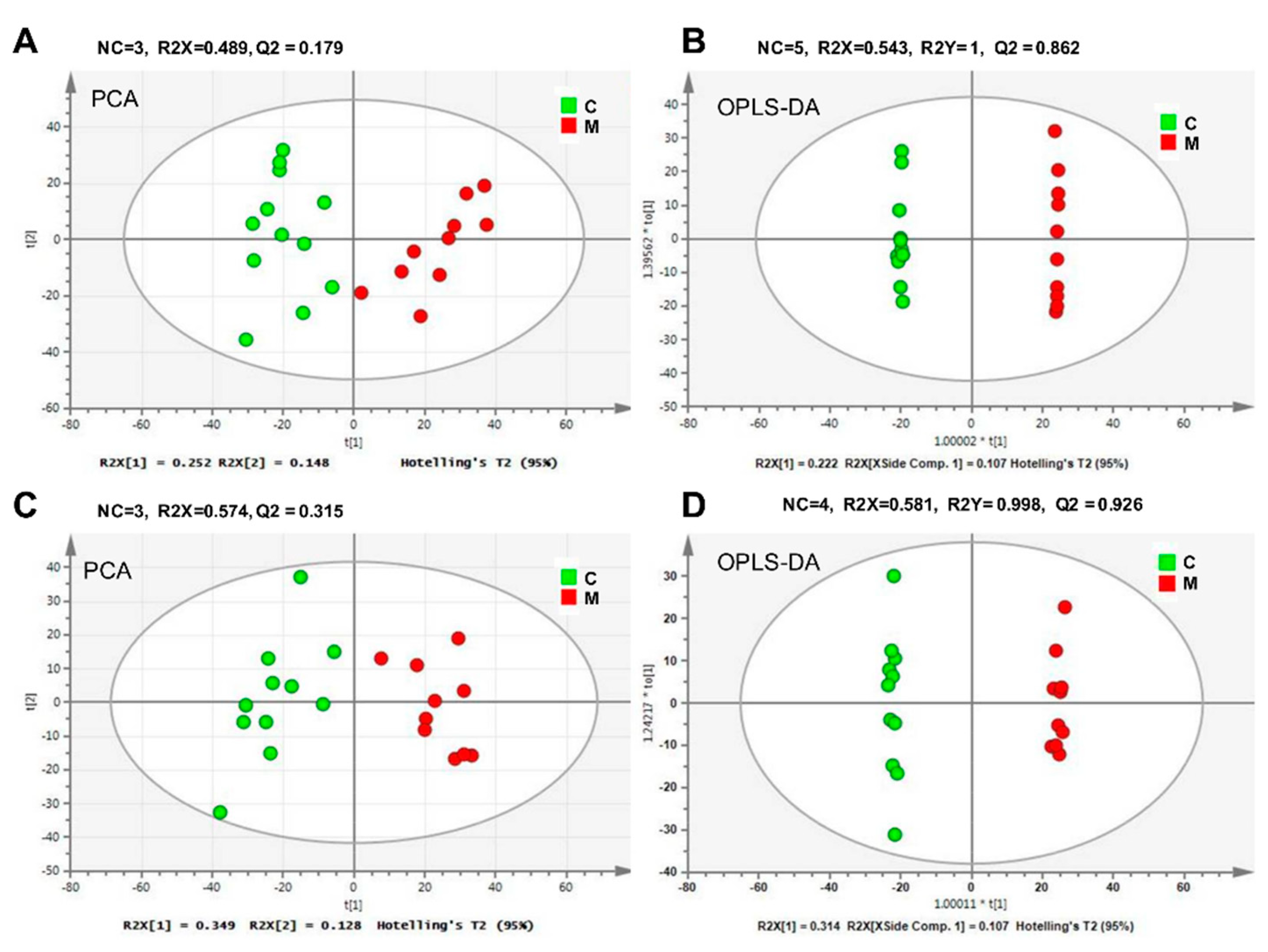
2.2. Cobalt-Radiation Induced Metabolite Changes in Mouse Serum
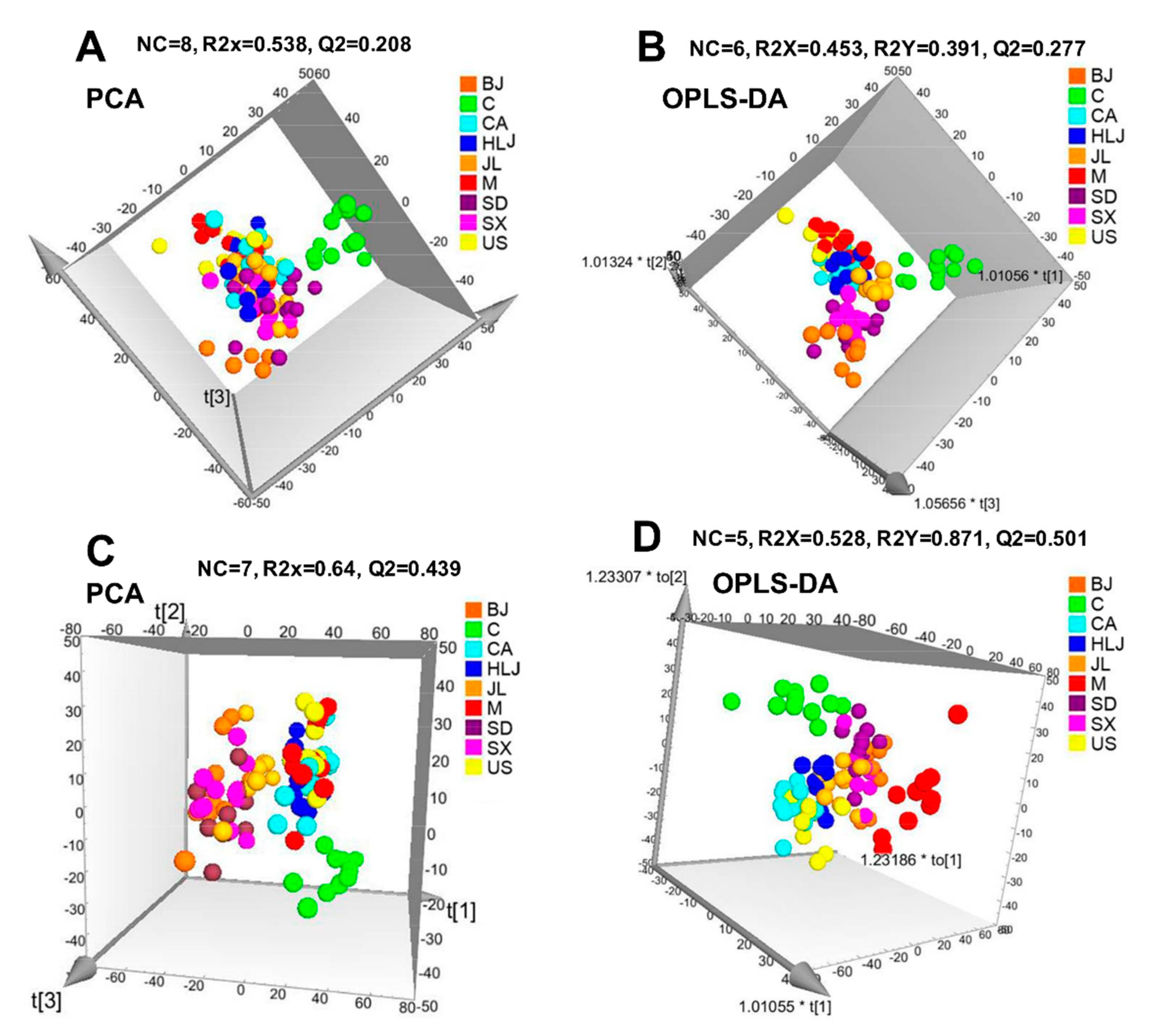
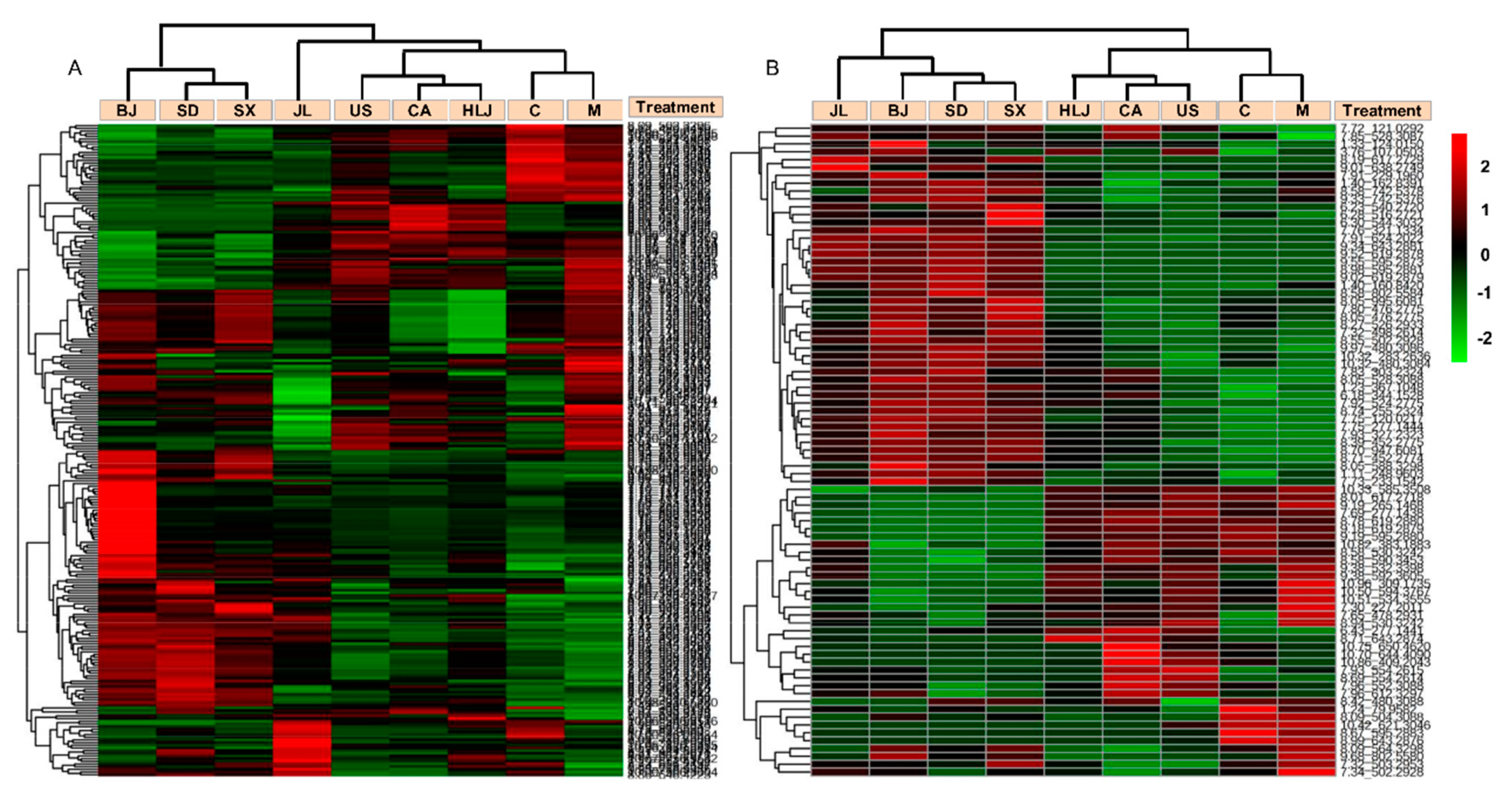
2.3. Serum Metabolite Changes in Radiated-Mice Administered with AG Decoction
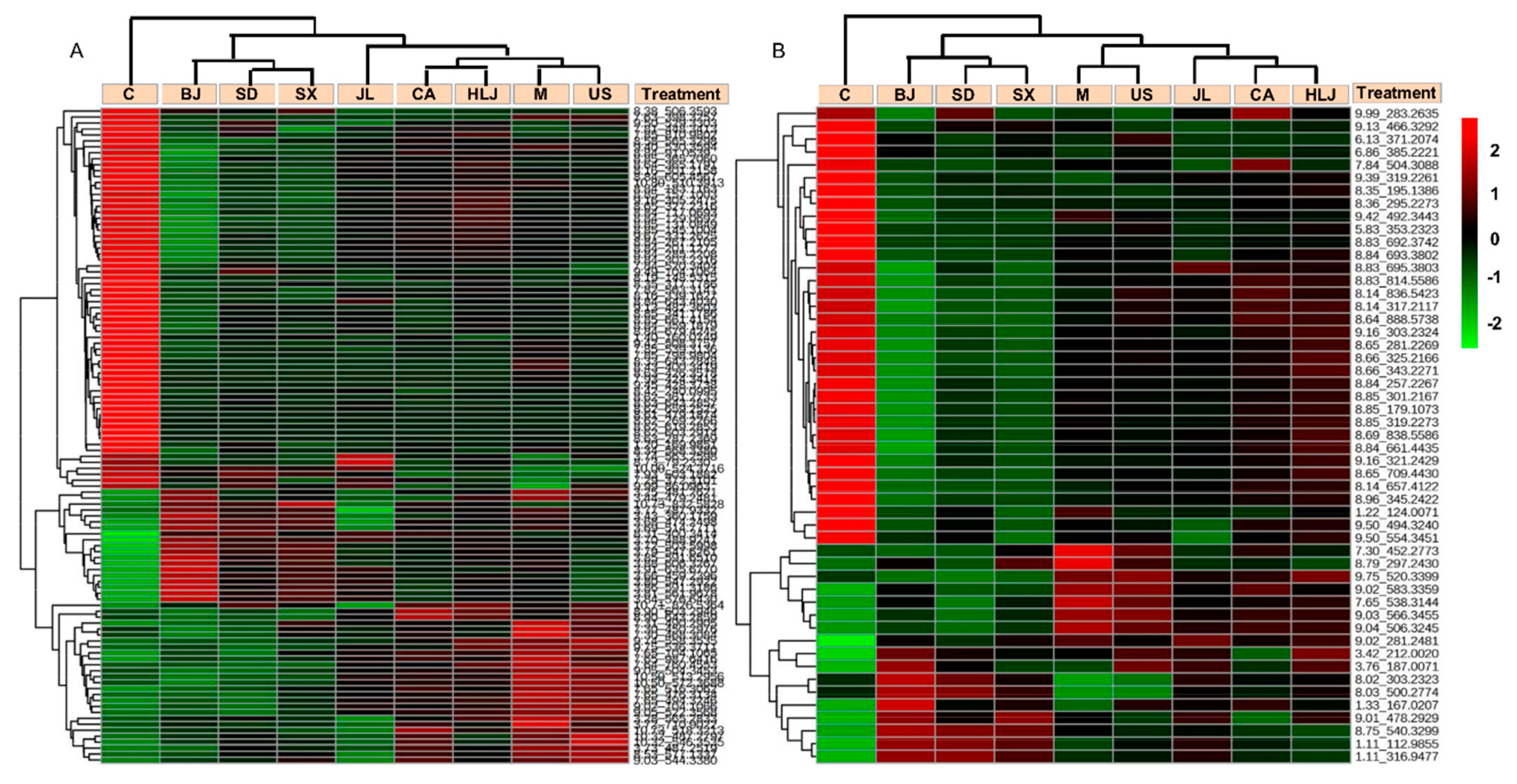
2.4. Biomarker Identification and Metabolic Pathway Analysis
3. Discussion
4. Materials and Methods
4.1. American Ginseng Root Samples and Decoction Preparation
4.2. Animals, Administration, and Co60 γ-Radiation Experiments
4.3. Serum Metabolomic Analysis
4.3.1. Serum Sample and QC Sample Preparation
4.3.2. UPLC/Q-TOF-MS Analysis
4.3.3. Multivariate Analysis of UPLC/Q-TOF-MS Data
4.4. Metabolite Concentration
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lemmon, H.R.; Sham, J.; Chau, L.A.; Madrenas, J. High molecular weight polysaccharides are key immunomodulators in North American ginseng extracts: Characterization of the ginseng genetic signature in primary human immune cells. J. Ethnopharmacol. 2012, 142, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.P.; Xu, D.D.; Lu, L.F.; Zheng, X.D.; Chen, W. Immunomodulatory effect of a formula developed from American ginseng and Chinese jujube extracts in mice. J. Zhejiang Univ. Sci. B 2016, 17, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Wang, S.; Wang, Q.; Zhang, H.; Bai, X.Y.; He, H.N.; Sun, W.J.; Liu, L.; Zhao, D.Q. Characterization and immunostimulating effects on murine peritoneal macrophages of a novel protein isolated from Panax quinquefolius L. J. Ethnopharmacol. 2016, 193, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D.; Wijewickreme, A.N.; Hu, C. Antioxidant properties of a North American ginseng extract. Mol. Cell. Biochem. 2000, 203, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Chen, S.; Feng, B.; Wu, Y.X.; Lui, E.; Chakrabarti, S. American ginseng (Panax quinquefolius) prevents glucose-induced oxidative stress and associated endothelial abnormalities. Phytomedicine 2011, 18, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Bowie, L.E.; Roscoe, W.A.; Lui, E.M.; Smith, R.; Karlik, S.J. Effects of an aqueous extract of North American ginseng on MOG(35–55)-induced EAE in mice. Can. J. Physiol. Pharmacol. 2012, 90, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wen, X.D.; Zhang, Z.; Zhang, C.F.; Wu, X.; He, X.; Liao, Y.; Wu, N.; Wang, C.Z.; Du, W.; et al. American ginseng significantly reduced the progression of high-fat-diet-enhanced colon carcinogenesis in Apc (Min/+) mice. J. Ginseng Res. 2015, 39, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Garbett, J.; Wilson, S.A.; Ralston, J.C.; De Boer, A.A.; Lui, E.M.; Wright, D.C.; Mutch, D.M. North American ginseng influences adipocyte-macrophage crosstalk regulation of inflammatory gene expression. J. Ginseng Res. 2016, 40, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Aung, H.H.; Zhang, B.; Sun, S.; Li, X.L.; He, H.; Xie, J.T.; He, T.C.; Du, W.; Yuan, C.S. Chemopreventive effects of heat-processed Pana xquinquefolius root on human breast cancer cells. Anticancer Res. 2008, 28, 2545–2551. [Google Scholar] [PubMed]
- Park, E.H.; Kim, Y.J.; Yamabe, N.; Park, S.H.; Kim, H.K.; Jang, H.J.; Kim, J.H.; Cheon, G.J.; Ham, J.; Kang, K.S. Stereospecific anticancer effects of ginsenoside Rg3 epimers isolated from heat-processed American ginseng on human gastric cancer cell. J. Ginseng Res. 2014, 38, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Querques, M.A.; Chakrabarti, S. North American ginseng (Panax quinquefolius) prevents hyperglycemia and associated pancreatic abnormalities in diabetes. J. Med. Food 2013, 16, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Mucalo, I.; Jovanovski, E.; Vuksan, V.; Božikov, V.; Romić, Z.; Rahelić, D. American ginseng extract (Panax quinquefolius L.) Is safe in long-term use in type 2 diabetic patients. Evid. Based Complement. Altern. Med. 2014, 969168. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.T. Comparative study on anti-aging action of Panax Ginseng (GQ) and quinquefolium (PQ). Chin. Pharmacol. Bull. 1997, 13, 229–232. [Google Scholar]
- Sun, J.H.; Chen, P. Differentiation of Panax quinquefolius grown in the USA and China using LC/MS-based chromatographic fingerprinting and chemometric approaches. Anal. Bioanal. Chem. 2011, 399, 1877–1889. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, P.; Cook, S.L.; Jackson, G.P.; Harnly, J.M.; Harrington, P.B. Classification of cultivation locations of Panax quinquefolius L samples using high performance liquid chromatography-electrospray ionization mass spectrometry and chemometric analysis. Anal. Chem. 2012, 84, 3628–3634. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Zhao, R.H.; Fu, J.G.; Li, Y. Investigation on production and commercial specification quality of Amercican ginseng cultivated in China. Mod. Chin. Med. 2013, 15, 324–328. [Google Scholar]
- Huang, L.F.; Suo, F.M.; Song, J.Y.; Wen, M.J.; Jia, G.L.; Xie, C.X.; Chen, S.L. Quality variation and ecotype division of Panax quinquefolium in China. Acta Pharm. Sin. 2013, 48, 580–589. [Google Scholar]
- Tang, Y.; Yan, S.M.; Wang, J.J.; Yuan, Y.; Yang, B. Quality evaluation of American ginseng using UPLC coupled with multivariate analysis. China J. Chin. Mater. Med. 2016, 41, 1678–1684. [Google Scholar]
- Xu, W.N.; Han, X.L.; Wang, K.P.; Wang, Z.; Dong, W.P.; Tian, Y.X. Comparison research of total saponins’s content of American ginseng from different regions. Ginseng Res. 2012, 4, 11–12. [Google Scholar]
- Wang, H.; Zeng, F.L.; Xie, C.X. Study on UPLC-UV-ELSD fingerprints for roots of Panax quinquefolium. Chin. Tradit. Herb. Drugs 2016, 47, 143–148. [Google Scholar]
- Zhang, D.; Zheng, K.Y.; Wang, Q.; Hou, F.J.; You, H.L.; Gao, Y.Z.; Wang, Q.Y.; Zheng, Y.G. Study on quality difference between domestic and imported Americanginseng. Mod. Chin. Med. 2017, 19, 833–838. [Google Scholar]
- Jia, C.; Chen, X.F.; WU, X.Y.; Li, X.E. Quality evaluation of American ginseng from different regions by infrared spectroscopy. Guid. J. Tradit. Chin. Med. Pharm. 2017, 14, 68–75. [Google Scholar]
- Wang, C.Z.; Yu, C.; Wen, X.D.; Chen, L.; Zhang, C.F.; Calway, T.; Qiu, Y.; Wang, Y.; Zhang, Z.; Anderson, S.; et al. American ginseng attenuates colitis-associated colon carcinogenesis in mice: Impact on gut microbiota and metabolomics. Cancer Prev. Res. 2016, 9, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Wang, C.Z.; Yu, C.; Qiu, Y.; Wen, X.D.; Zhang, C.F.; Yuan, C.S.; Jia, W. Metabonomic profiling reveals cancer chemopreventive effects of American ginseng on colon carcinogenesis in Apc(Min/+) mice. J. Proteome Res. 2015, 14, 3336–3347. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yu, Q.T.; Ge, Y.Z.; Zhang, W.S.; Fan, Y.; Ma, C.W.; Liu, Q.; Qi, L.W. Distinct urine metabolome after Asian ginseng and American ginseng intervention based on GC-MS metabolomics approach. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Michl, J.; Kite, G.C.; Wanke, S.; Zierau, O.; Vollmer, G.; Neinhuis, C.; Simmonds, M.S.; Heinrich, M. LC-MS- and (1)H NMR-based metabolomic analysis and in vitro toxicological assessment of 43 Aristolochia species. J. Nat. Prod. 2016, 79, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.H.; Wang, Y.X.; Sun, H.; Liao, N.; Li, X.E.; Qin, M.J.; Pang, X.C. Pharmacodynamic differences between hang maidong and chuan maidong based on metabonomics. Acta Pharm. Sin. 2014, 49, 1451–1456. [Google Scholar]
- Lemonakis, N.; Gikas, E.; Halabalaki, M.; Skaltsounis, A.L. Development and validation of a combined methodology for assessing the total quality control of herbal medicinal products—Application to oleuropein preparations. PLoS ONE 2013, 8, e78277. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Adhikari, J.S.; Rizvi, M.A.; Chaudhury, N.K. Melatonin attenuates 60Co γ-ray-induced hematopoietic; immunological and gastrointestinal injuries in C57BL/6 male mice. Environ. Toxicol. 2017, 32, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, Y.; Zhang, Z.; Ding, Y.; Dai, X.; Li, Y. Effect of polysaccharide from cultured Cordyceps sinensis on immune function and anti-oxidation activity of mice exposed to 60Co. Int. Immunopharmacol. 2011, 11, 2251–2257. [Google Scholar] [CrossRef] [PubMed]
- Shanthakumar, J.; Karthikeyan, A.; Bandugula, V.R.; Rajendra Prasad, N. Ferulic acid, a dietary phenolic acid, modulates radiation effects in Swiss albino mice. Eur. J. Pharmacol. 2012, 691, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Ossetrova, N.I.; Condliffe, D.P.; Ney, P.H.; Krasnopolsky, K.; Hieber, K.P.; Rahman, A.; Sandgren, D.J. Early-response biomarkers for assessment of radiation exposure in a mouse total-body irradiation model. Health Phys. 2014, 106, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yu, M.W.; Wang, X.M.; Zhang, Y.; Yang, G.W.; Luo, X.Q.; Peng, R.Y.; Gao, Y.B.; Zhao, L.; Wang, L.F. Heijiangdan ointment relieves oxidative stress from radiation dermatitis induced by (60)Co γ-ray in mice. Chin. J. Integr. Med. 2016, 22, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.F.; Weng, J.T.; Hsu, P.W.; Chi, Y.H.; Chen, C.K.; Liu, I.Y.; Chen, Y.C.; Wu, L.S. Gene expression profiling of biological pathway alterations by radiation exposure. Biomed. Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.T.; Li, X.; Fu, D.; Xiao, M. Circulating IL-18 binding protein (IL-18BP) and IL-18 as dual biomarkers of total-body irradiation in mice. Radiat. Res. 2016, 185, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Rana, P.; Devi, M.M.; Chaturvedi, S.; Javed, S.; Tripathi, R.P.; Khushu, S. Nuclear magnetic resonance spectroscopy-based metabonomic investigation of biochemical effects in serum of γ-irradiated mice. Int. J. Radiat. Biol. 2011, 87, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Laiakis, E.C.; Strassburg, K.; Bogumil, R.; Lai, S.; Vreeken, R.J.; Hankemeier, T.; Langridge, J.; Plumb, R.S.; Fornace, A.J., Jr; Astarita, G. Metabolic phenotyping reveals a lipid mediator response to ionizing radiation. J. Proteome Res. 2014, 13, 4143–4154. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, M.; Weber, W.M.; Mak, T.D.; Chung, J.; Doyle-Eisele, M.; Melo, D.R.; Brenner, D.J.; Guilmette, R.A.; Fornace, A.J., Jr. Metabolomic and lipidomic analysis of serum from mice exposed to an internal emitter, cesium-137, using a shotgun LC-MS(E) approach. J. Proteome Res. 2015, 14, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Pannkuk, E.L.; Laiakis, E.C.; Mak, T.D.; Astarita, G.; Authier, S.; Wong, K.; Fornace, A.J., Jr. A lipidomic and metabolomic serum signature frommonhuman primates exposed to ionizing radiation. Metabolomics 2016, 12. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; O’Brien, K.F.; Wang, W.; Sheng, C.; Wang, T.; Johnke, R.M.; Allison, R.R. American Ginseng modifies Cs-induced DNA damage and oxidative stress in human lymphocytes. Open Nucl. Med. J. 2009, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; O’Brien, K.F.; Wang, W.D.; Johnke, R.M.; Sheng, C.; Benhabib, S.M.; Wang, T.; Allison, R.R. Radioprotective effect of American ginseng on human lymphocytes at 90 minutes postirradiation: A study of 40 cases. Altern. Complement. Med. 2010, 16, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.D.; Smith, J.; Portman, D.; Kim, R.; Oberoi-Jassal, R.; Rajasekhara, S.; Davis, M. Single institute experience with methylphenidate and American ginseng in cancer-related fatigue. Am. J. Hosp. Palliat. Care 2018, 35, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.X. Experience of Chinese medicine utilization in postradiation lung cancer. Chin. J. Integr. Tradit. West. Med. 1994, S1, 287–288. [Google Scholar]
- Li, X.F. Chinese medicine treatment of radiotherapy-induced oral ulcer. J. Emerg. Tradit. Chin. Med. 2008, 17, 255–256. [Google Scholar]
- Wang, C.G.; Jia, X.J.; Dong, L.H.; Wu, Z.F.; Gong, S.L.; Ma, X.Y.; Chen, Y.P. Ef fects of PQL on levels of immunal proteins, C3, C4 and expressions of CD4, CD8, and CD25 on lymphocytes of peripherial blood in patients with breast carcinoma undergoing radiotherapy. J. Jilin Univ. Med. Ed. 2005, 31, 287–289. [Google Scholar]
- Barton, D.L.; Soori, G.S.; Bauer, B.A.; Sloan, J.A.; Johnson, P.A.; Figueras, C.; Duane, S.; Mattar, B.; Liu, H.; Atherton, P.J.; et al. Pilot study of Panax quinquefolius (American ginseng) to improve cancer-related fatigue: A randomized, double-blind, dose-finding evaluation: NCCTG trial N03CA. Support. Care Cancer 2010, 18, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.L.; Liu, H.; Dakhil, S.R.; Linquist, B.; Sloan, J.A.; Nichols, C.R.; McGinn, T.W.; Stella, P.J.; Seeger, G.R.; Sood, A.; et al. Wisconsin ginseng (Panax quinquefolius) to improve cancer-related fatigue: A randomized, double-blind trial, N07C2. J. Natl. Cancer Inst. 2013, 105, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Worley, B.; Powers, R. Multivariate analysis in metabolomics. Curr. Metab. 2013, 1, 92–107. [Google Scholar]
- Worley, B.; Powers, R. PCA as a practical indicator of OPLS-DA model reliability. Curr. Metab. 2016, 4, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, S.; Johansson, E.; Sjöström, L.; Mellerowicz, E.J.; Edlund, U.; Shockcor, J.P.; Gottfries, J.; Moritz, T.; Trygg, J. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal. Chem. 2008, 80, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zheng, K.Y.; Wang, L.F.; Zhao, R.H.; Hou, F.J.; Wang, Q.; You, H.L.; Du, L.H.; Huang, L.Q.; Zheng, Y.G. Correlation analysis between quality characteristics and commercial specifications of Panacis Quinquefolii Radix. China J. Chin. Mater. Med. 2016, 41, 3329–3335. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
| ESI Mode | C | US | CA | HLJ | JL | SD | SX | BJ |
|---|---|---|---|---|---|---|---|---|
| + | 46/64 | 6/11 | 19/28 | 21/48 | 39/59 | 37/43 | 31/54 | 71/75 |
| − | 15/36 | 7/6 | 13/10 | 13/8 | 18/11 | 20/19 | 26/22 | 27/30 |
| RT (min) | Quasi-Molecular Ion (m/z) | Cal. m/z | Mass Error (ppm) | HMDB ID | Metabolites | Formula | Metabolic Pathway | Fold Change | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-M | M-US | M-CA | M-HLJ | M-JL | M-SD | M-SX | M-BJ | ||||||||
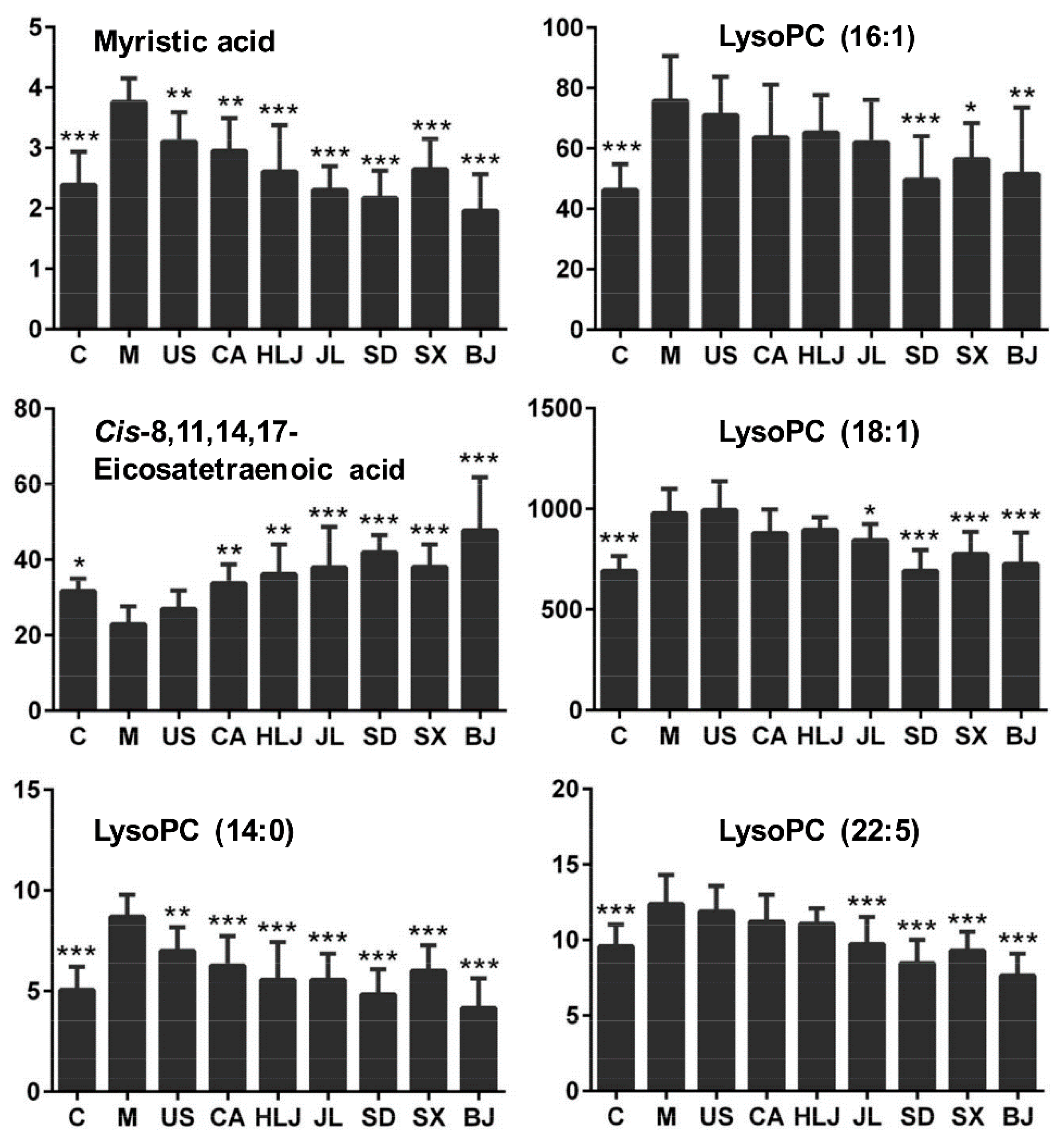
| * 7.30 | 468.3084 [M + H]+ | 468.3085 | −0.140 | HMDB10379 | LysoPC (14:0) | C22H46NO7P | Lipid metabolism | 1.72 c | 0.80 b | 0.72 c | 0.64 c | 0.64 c | 0.56 c | 0.69 c | 0.48 c |
| * 8.65 | 476.2746 [M + Na]+ | 476.2748 | −0.337 | HMDB11503 | LysoPE (16:0) | C21H44NO7P | Lipid metabolism | 0.97 c | 1.34 | 1.43 | 1.69 | 1.98 c | 1.64 | 1.73 | 1.61 |
| 7.65 | 494.3246 [M + H]+ | 494.3241 | 0.980 | HMDB10383 | LysoPC (16:1) | C24H48NO7P | Lipid metabolism | 1.64 c | 0.94 | 0.84 a | 0.86 | 0.82 | 0.66 b | 0.75 a | 0.68 b |
| * 10.80 | 510.3913 [M + H]+ | 510.3918 | −0.982 | HMDB11149 | LysoPC (O-18:0) | C26H56NO6P | Lipid metabolism | 0.72 c | 0.92 | 0.94 | 0.97 | 0.87 | 0.84 | 0.78 a | 0.67 c |
| * 7.84 | 520.3404 [M + H]+ | 520.3398 | 1.219 | HMDB10386 | LysoPC (18:2) | C26H50NO7P | Lipid metabolism | 0.67 c | 0.87 | 1.01 | 1.00 | 0.98 | 1.09 | 0.99 | 0.89 |
| * 9.05 | 522.3568 [M + H]+ | 522.3554 | 2.650 | HMDB02815 | LysoPC (18:1) | C26H52NO7P | Lipid metabolism | 1.42 c | 1.02 | 0.90 | 0.92 | 0.86 | 0.71 b | 0.79 b | 0.74 b |
| * 8.01 | 524.2754 [M + Na]+ | 524.2748 | 1.220 | HMDB11517 | LysoPE (20:4/0:0) | C25H44NO7P | Lipid metabolism | 0.64 a | 1.38 | 1.67 a | 2.03 c | 1.91 c | 2.82 c | 2.26 c | 2.74 c |
| * 7.49 | 540.3062 [M + Na]+ | 540.3061 | 0.258 | HMDB10387 | LysoPC (18:3) | C26H48NO7P | Lipid metabolism | 0.97 | 1.37 | 1.43 | 1.41 | 1.40 | 1.79 b | 1.72 a | 1.59 |
| * 7.76 | 548.2756 [M + Na]+ | 548.2748 | 1.532 | HMDB11526 | LysoPE (22:6/0:0) | C27H44NO7P | Lipid metabolism | 1.03 | 1.23 | 1.43 | 1.48 | 1.05 | 2.22 c | 1.94 b | 1.90 |
| * 8.34 | 568.3380 [M + Na]+ | 568.3374 | 1.125 | HMDB10394 | LysoPC (20:3) | C28H52NO7P | Lipid metabolism | 0.67 c | 0.91 | 0.90 | 0.81 | 0.87 | 0.93 | 0.84 | 0.69 |
| * 9.39 | 570.3533 [M + H]+ | 570.3554 | −3.710 | HMDB10402 | LysoPC (22:5) | C30H52NO7P | Lipid metabolism | 1.29 b | 0.96 | 0.91 | 0.90 | 0.79 a | 0.68 c | 0.75 b | 0.62 c |
| * 7.22 | 282.2789 [M + H]+ | 282.2791 | −0.855 | HMDB02117 | Oleamide | C18H35NO | Amino acid metabolism | 0.65 c | 0.40 c | 0 c | 0 c | 0 c | 0 c | 0 c | 0 c |
| 2.91 | 132.0643 [M + H]+ | 132.0655 | −9.236 | HMDB02113 | 3-Hydroxy-l-proline | C5H9NO3 | Amino acid metabolism | 1.12 | 0.88 | 0.65 | 0.57 b | 0.82 | 0.91 | 1.03 | 0.94 |
| * 8.16 | 301.2158 [M + Na]+ | 301.2138 | 6.635 | HMDB01388 | Alpha-Linolenic acid | C18H30O2 | Lipid metabolism | 0.42 c | 1.00 | 1.00 | 1.25 | 1.10 | 0.76 | 0.60 | 0.38 a |
| * 8.84 | 303.2316 [M + H]+ | 303.2319 | −0.847 | HMDB01999 | Eicosapentaenoic acid | C20H30O2 | Lipid metabolism | 0.51 c | 0.94 | 1.05 | 1.14 | 1.03 | 0.89 | 0.77 | 0.51 |
| 9.23 | 319.1939 [M + H]+ | 319.1904 | 11.010 | HMDB06709 | Ubiquinone-2 | C19H26O4 | Energy metabolism | 0.36 | 0.45 | 1.20 | 1.19 | 9.15 b | 3.04 | 1.57 | 1.60 |
| 7.81 | 448.3413 [M + Na]+ | 448.3397 | 3.502 | HMDB06464 | Elaidic carnitine | C25H47NO4 | Lipid metabolism | 0.48 c | 1.15 | 1.28 | 1.28 | 1.20 | 1.34 | 0.71 | 1.09 |
| 7.29 | 372.3101 [M + H]+ | 372.3108 | −1.975 | HMDB05066 | Tetradecanoyl carnitine | C21H41NO4 | Lipid metabolism | 0.49 c | 1.29 | 1.47 | 1.27 | 1.55 | 1.56 | 1.15 | 1.32 |
| * 8.84 | 285.2208 [M + H]+ | 285.2213 | −1.725 | HMDB01358 | Retinal | C20H28O | Lipid metabolism | 0.52 c | 0.97 | 1.08 | 1.16 | 1.02 | 0.89 | 0.79 | 0.51 b |
| * 10.32 | 283.2636 [M − H]− | 283.2631568 | 1.565 | HMDB00827 | Stearic acid | C18H36O2 | Lipid metabolism | 0.92 | 0.93 | 1.02 | 1.11 | 1.15 | 1.30 b | 1.22 a | 1.23 a |
| * 8.85 | 301.2167 [M − H]− | 301.2162067 | 1.638 | HMDB01999 | Eicosapentaenoic acid | C20H30O2 | Lipid metabolism | 0.60 c | 0.92 | 1.03 | 1.10 | 0.93 | 0.82 | 0.72 | 0.49 c |
| * 8.02 | 303.2323 [M − H]− | 303.2318567 | 1.462 | HMDB02177 | Cis-8,11,14,17-Eicosatetraenoic acid | C20H32O2 | Lipid metabolism | 0.72 a | 1.14 | 1.47 b | 1.58 b | 1.66 c | 1.84 c | 1.61 c | 2.08 c |
| 9.39 | 319.2261 [M − H]− | 319.2267713 | −2.103 | HMDB06245 | 18-Hydroxyarachidonic acid | C20H32O3 | Lipid metabolism | 0.07 c | 3.77 | 4.16 | 5.27 | 4.94 | 3.09 | 2.23 | 1.29 |
| 8.14 | 836.5423 [M − H]− | 836.5436105 | −1.567 | HMDB10166 | PS (18:0/22:5) | C46H80NO10P | Amino acid metabolism | 0.41 c | 1.51 | 1.71 a | 1.49 | 1.37 | 0.73 | 0.72 | 0.35 a |
| * 1.22 | 124.0071 [M − H]− | 124.0062903 | 6.530 | HMDB00251 | Taurine | C2H7NO3S | Amino acid metabolism | 0.48 c | 0.39 | 0.33 | 0.59 | 0.46 | 0.60 | 0.05 b | 0.19 a |
| 9.02 | 281.2481 [M − H]− | 281.2475068 | 2.109 | HMDB00207 | Oleic acid | C18H34O2 | Lipid metabolism | 1.31 c | 0.96 | 0.96 | 0.99 | 1.02 | 0.91 | 0.96 | 0.95 |
| * 8.74 | 255.2324 [M − H]− | 255.2318567 | 2.129 | HMDB00220 | Palmitic acid | C16H32O2 | Lipid metabolism | 1.04 | 1.07 | 1.10 | 1.09 | 1.17 | 1.29 b | 1.23 a | 1.27 b |
| * 7.30 | 227.2011 [M − H]− | 227.2005566 | 2.392 | HMDB00806 | Myristic acid | C14H28O2 | Lipid metabolism | 1.57 c | 0.82 b | 0.78 b | 0.69 c | 0.61 c | 0.56 c | 0.73 c | 0.52 c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Z.; Jia, C.; Liao, D.; Chen, X.; Li, X. Comparison of Serum Metabolite Changes of Radiated Mice Administered with Panax quinquefolium from Different Cultivation Regions Using UPLC-Q/TOF-MS Based Metabolomic Approach. Molecules 2018, 23, 1014. https://doi.org/10.3390/molecules23051014
Qin Z, Jia C, Liao D, Chen X, Li X. Comparison of Serum Metabolite Changes of Radiated Mice Administered with Panax quinquefolium from Different Cultivation Regions Using UPLC-Q/TOF-MS Based Metabolomic Approach. Molecules. 2018; 23(5):1014. https://doi.org/10.3390/molecules23051014
Chicago/Turabian StyleQin, Zhenxian, Chan Jia, Dengqun Liao, Xiaofang Chen, and Xian’en Li. 2018. "Comparison of Serum Metabolite Changes of Radiated Mice Administered with Panax quinquefolium from Different Cultivation Regions Using UPLC-Q/TOF-MS Based Metabolomic Approach" Molecules 23, no. 5: 1014. https://doi.org/10.3390/molecules23051014
APA StyleQin, Z., Jia, C., Liao, D., Chen, X., & Li, X. (2018). Comparison of Serum Metabolite Changes of Radiated Mice Administered with Panax quinquefolium from Different Cultivation Regions Using UPLC-Q/TOF-MS Based Metabolomic Approach. Molecules, 23(5), 1014. https://doi.org/10.3390/molecules23051014





