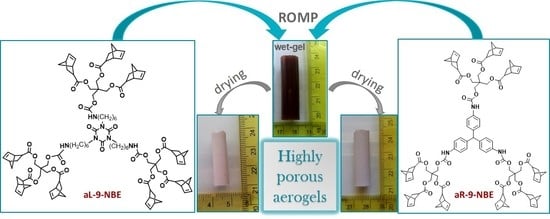
Poly(urethane-norbornene) Aerogels via Ring Opening Metathesis Polymerization of Dendritic Urethane-Norbornene Monomers: Structure-Property Relationships as a Function of an Aliphatic Versus an Aromatic Core and the Number of Peripheral Norbornene Moieties
Abstract
1. Introduction
2. Results and Discussion
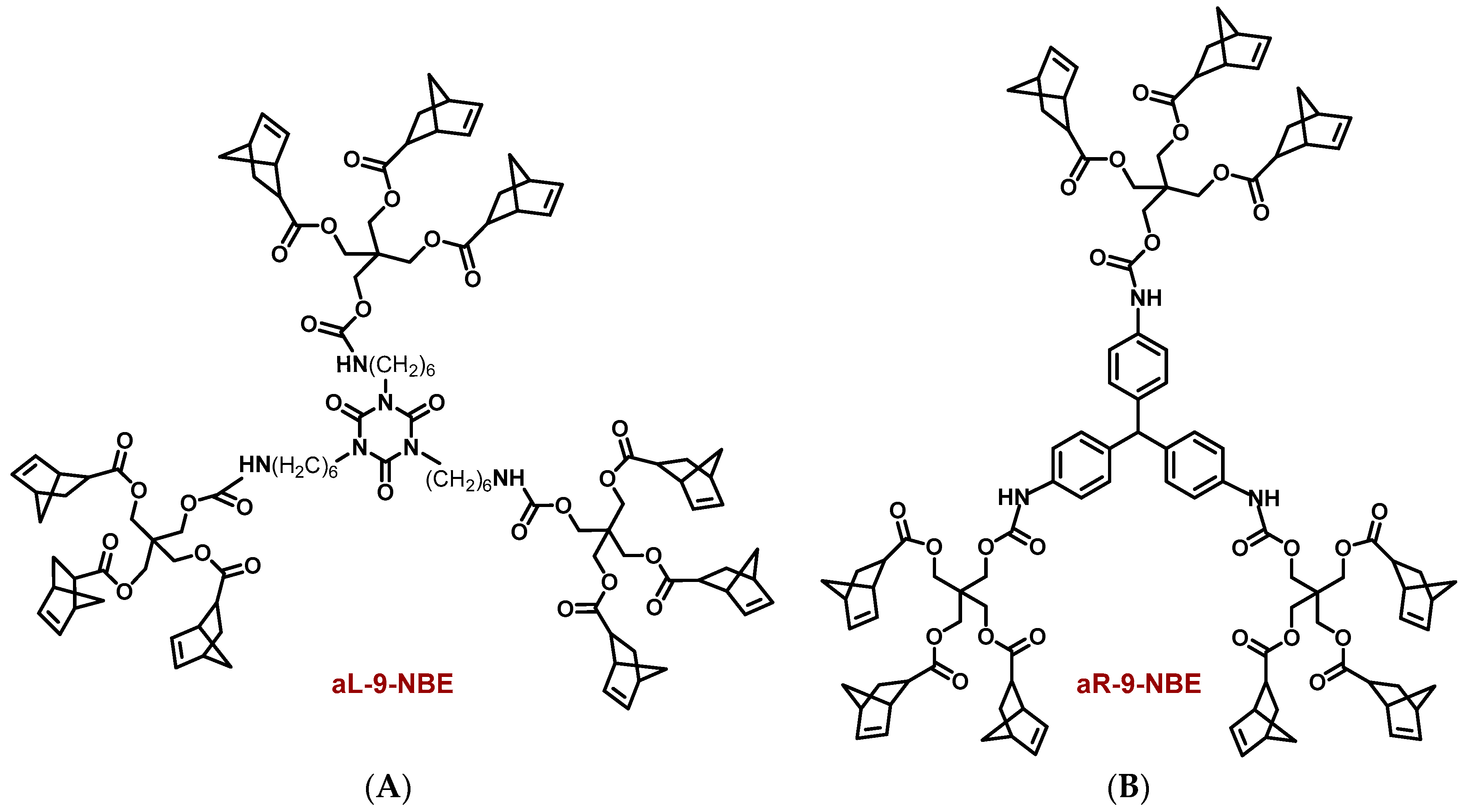
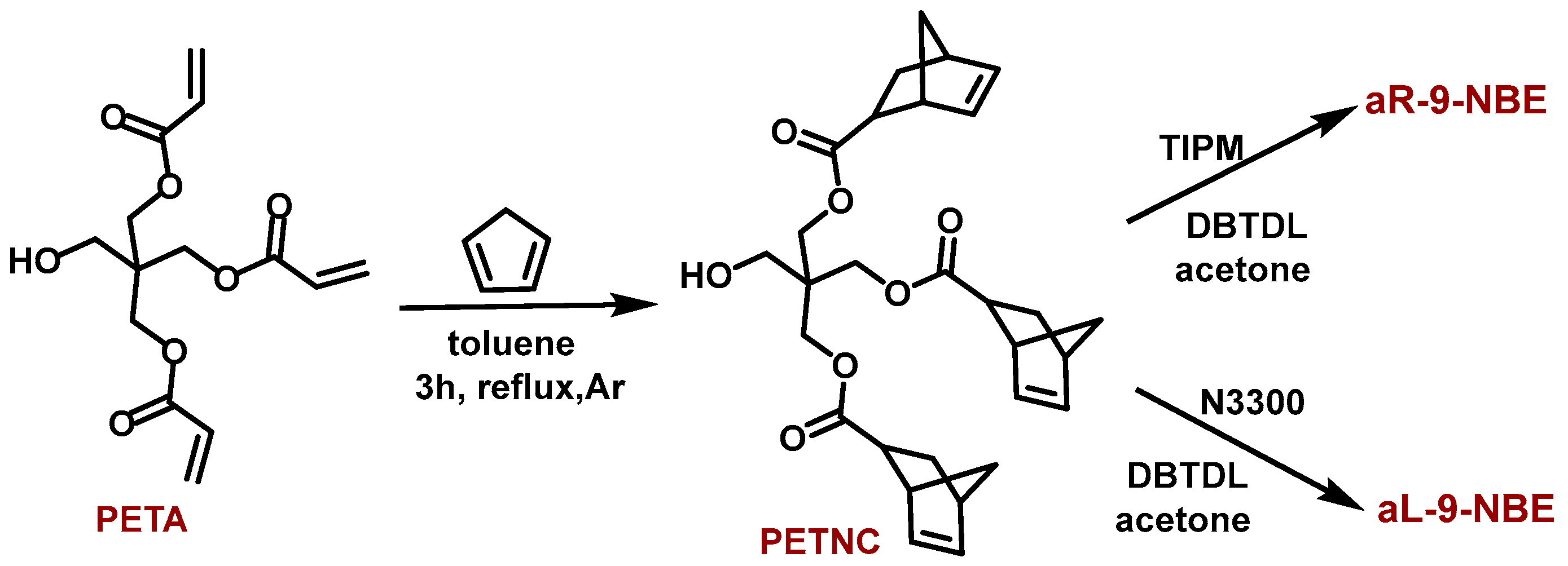
2.1. Synthesis and Characterization of Dendritic Monomers aL-9-NBE and aR-9-NBE
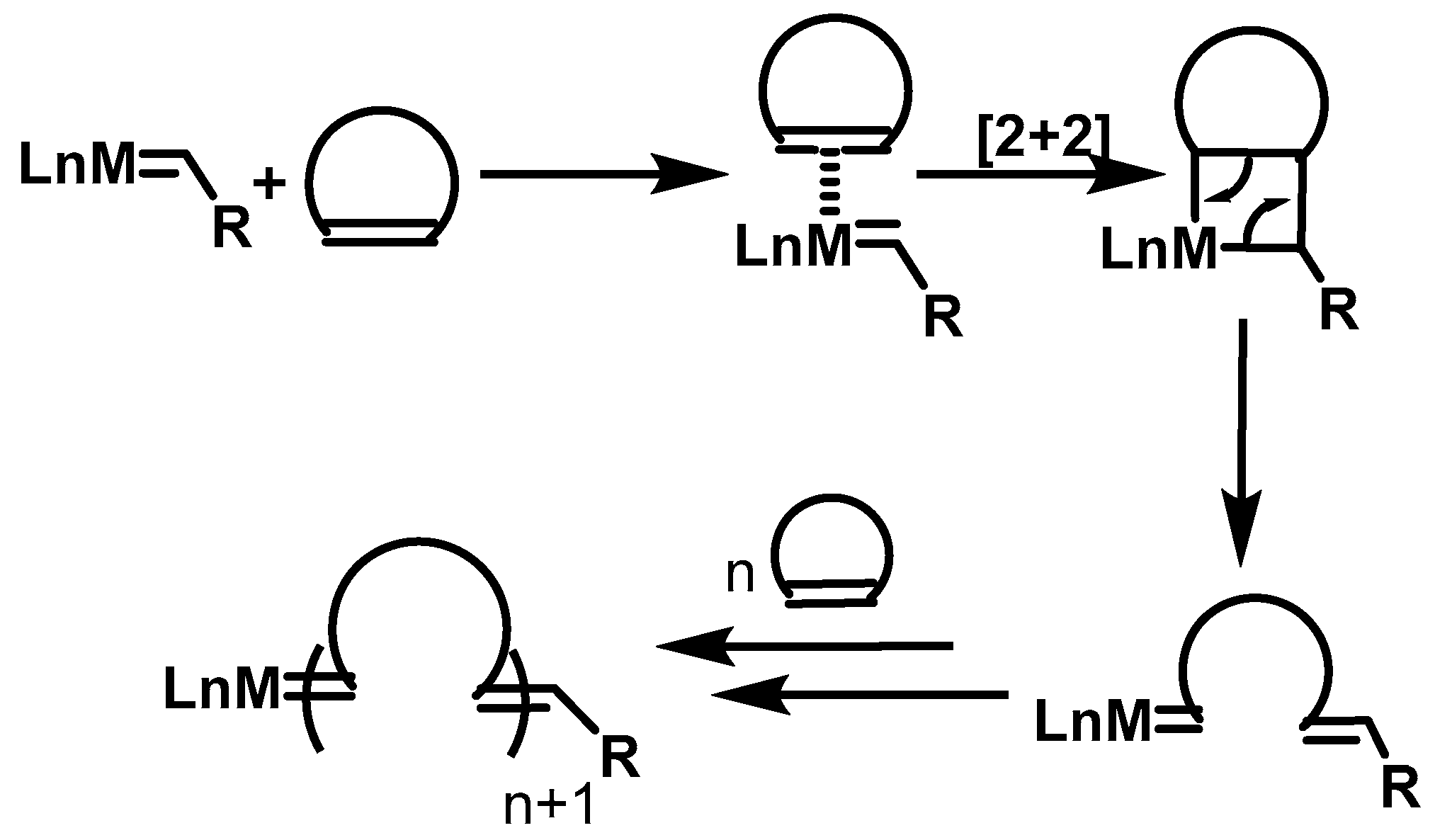
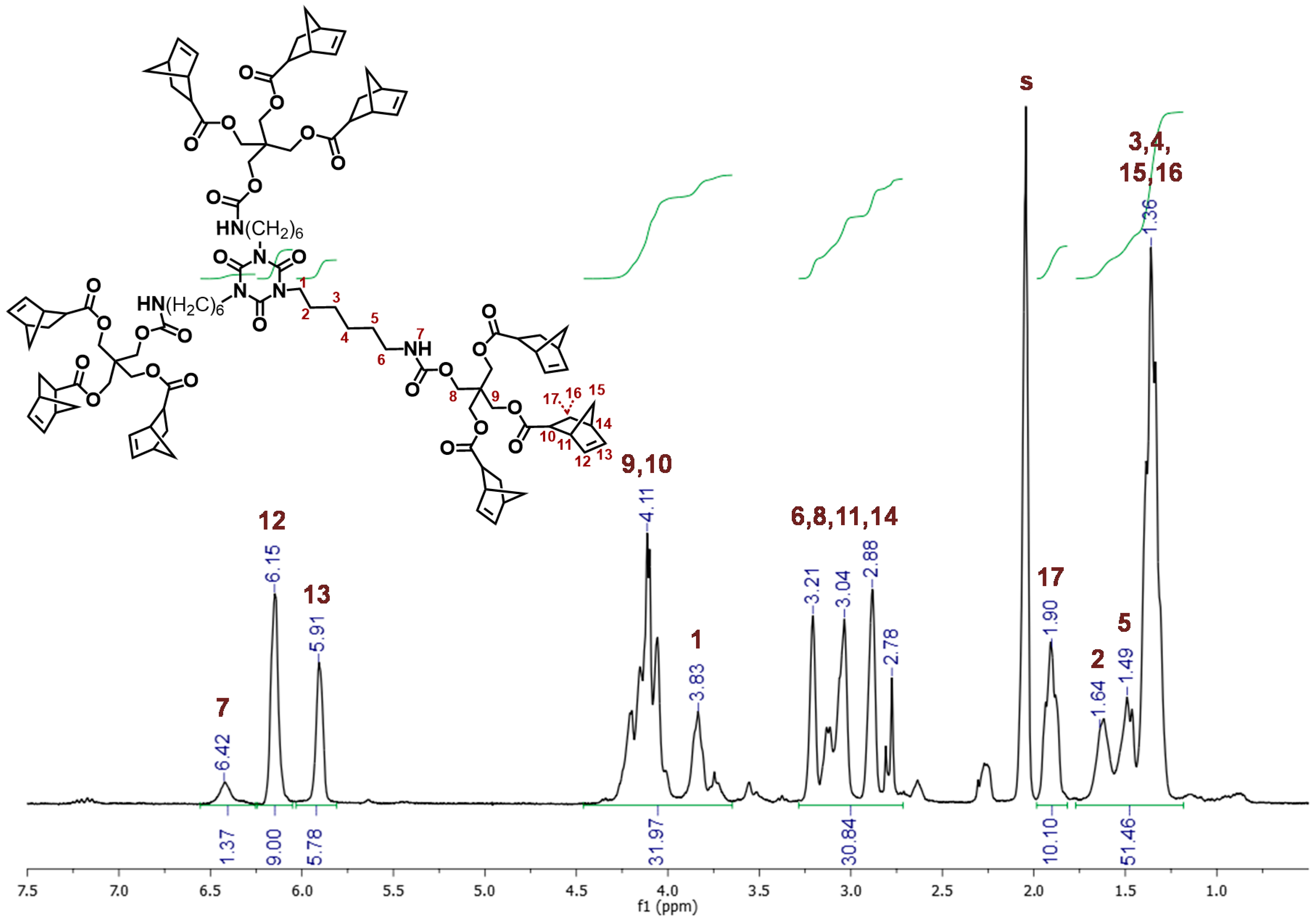
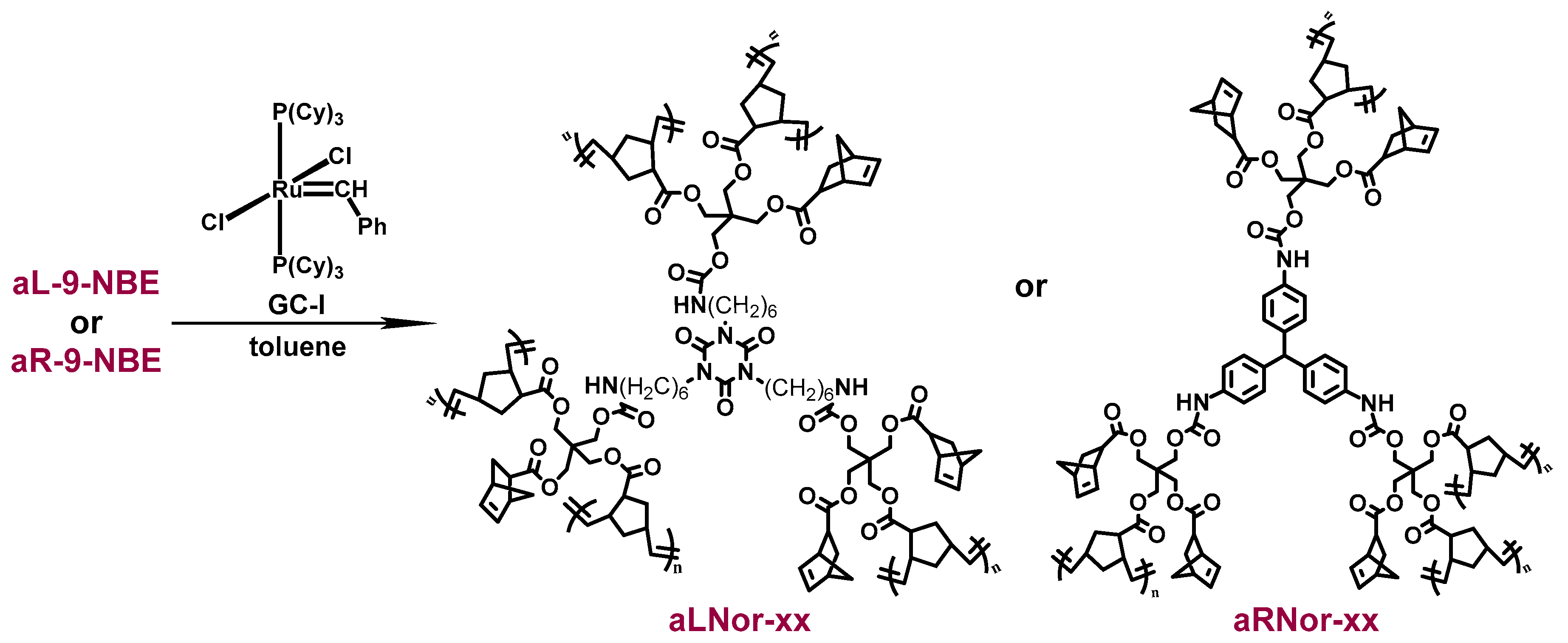
2.2. ROMP Synthesis of Aerogels
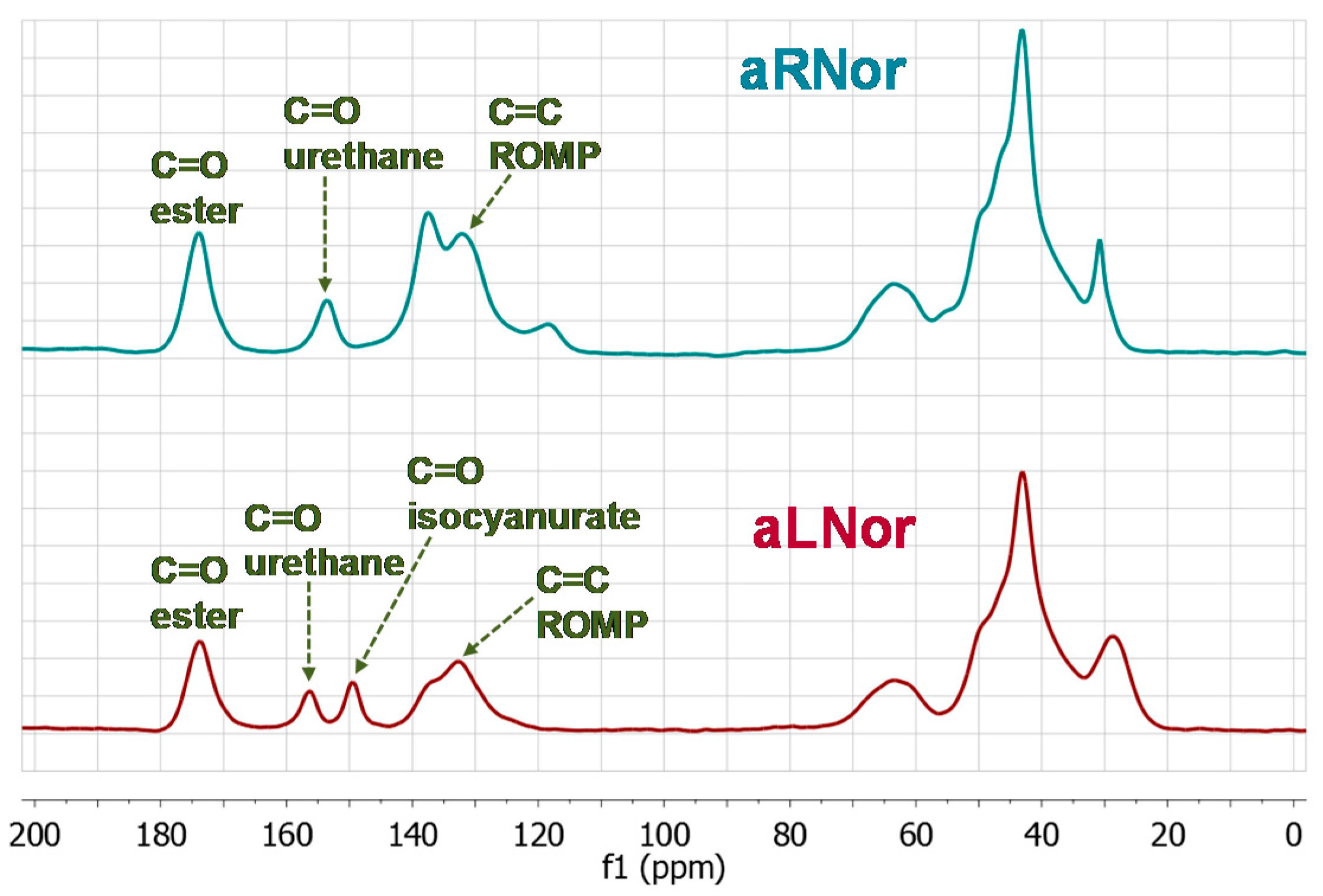
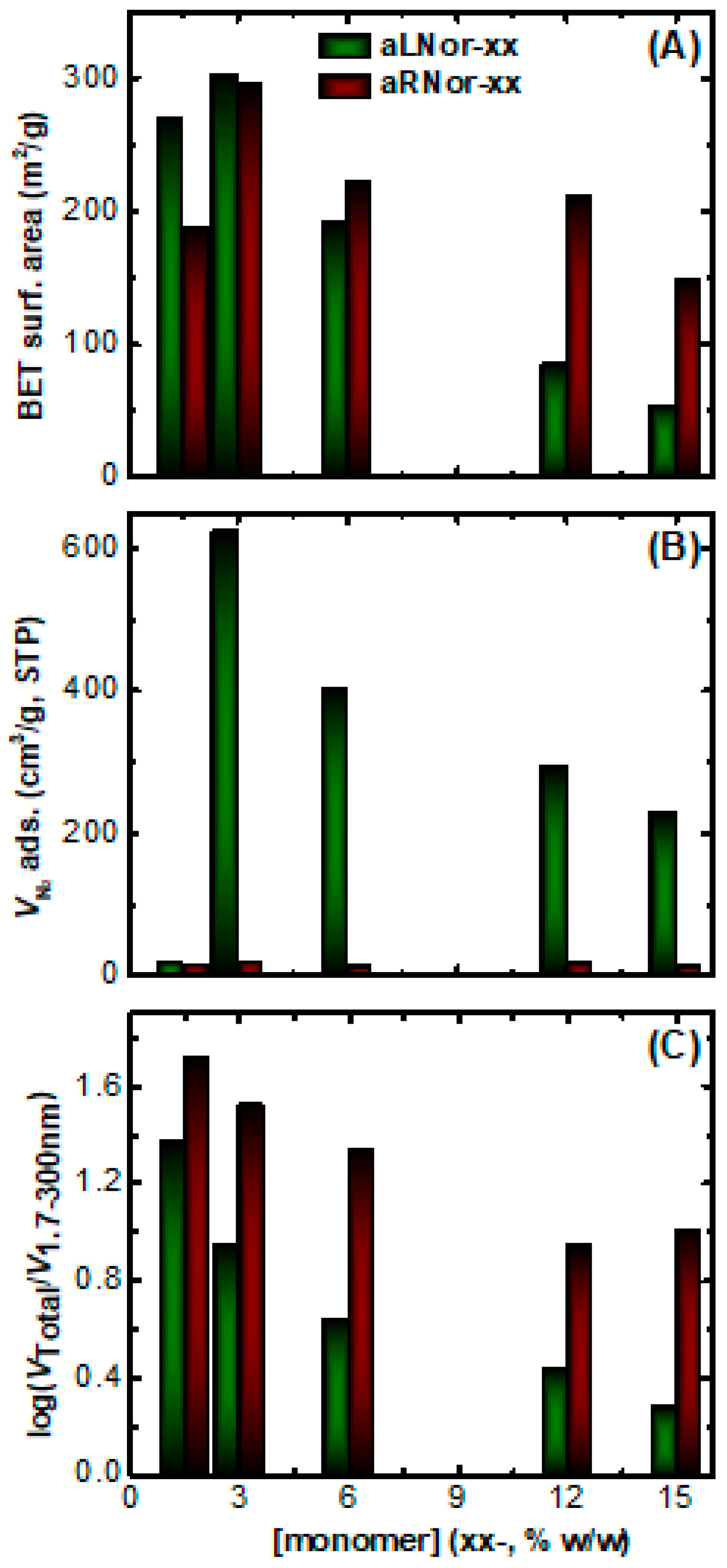
2.3. Characterization of Aliphatic and Aromatic Aerogels
3. Materials and Methods
3.1. General Information
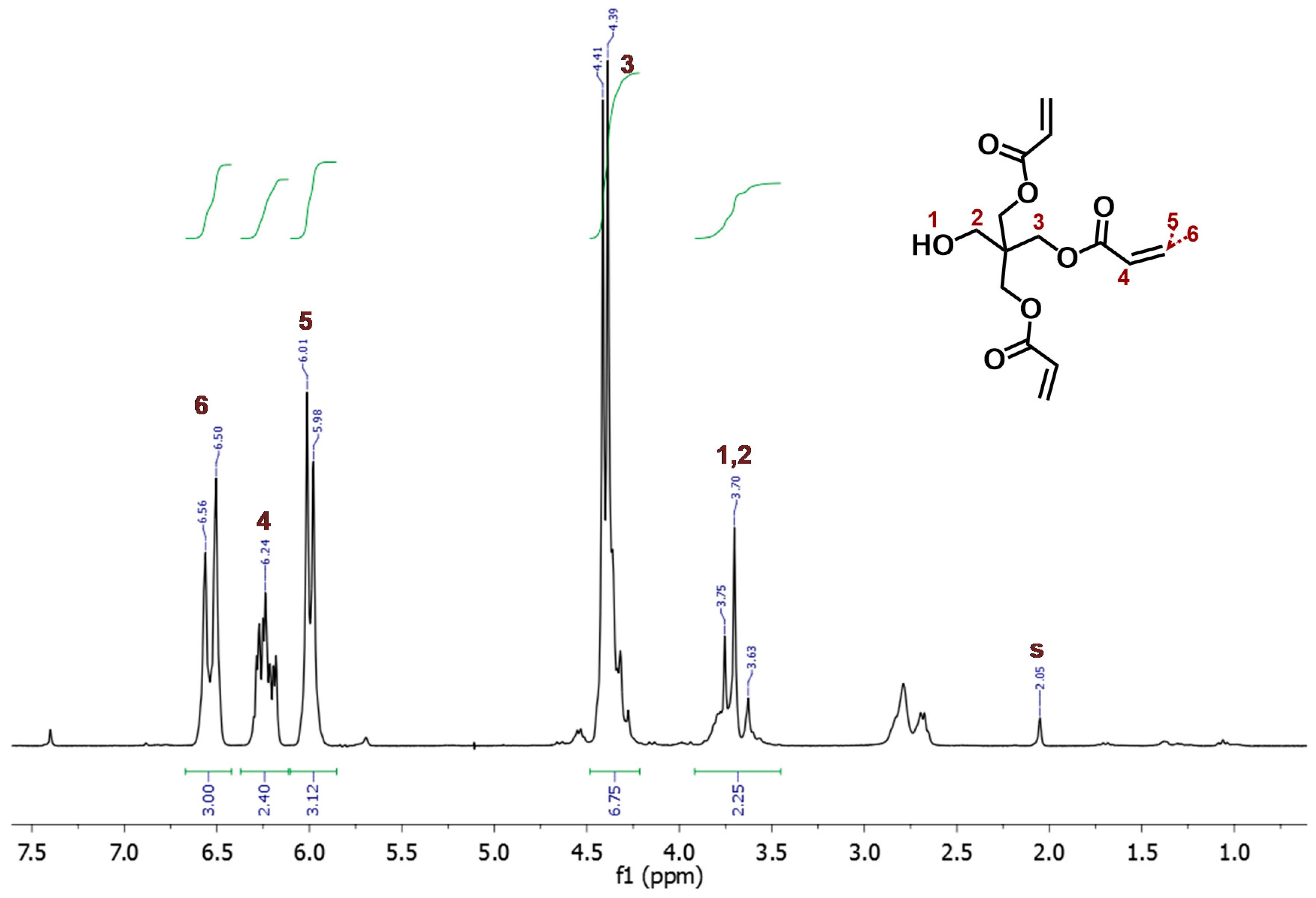
3.2. Synthesis of Pentaerythritol-Norbornene-Carboxylate (PETNC)
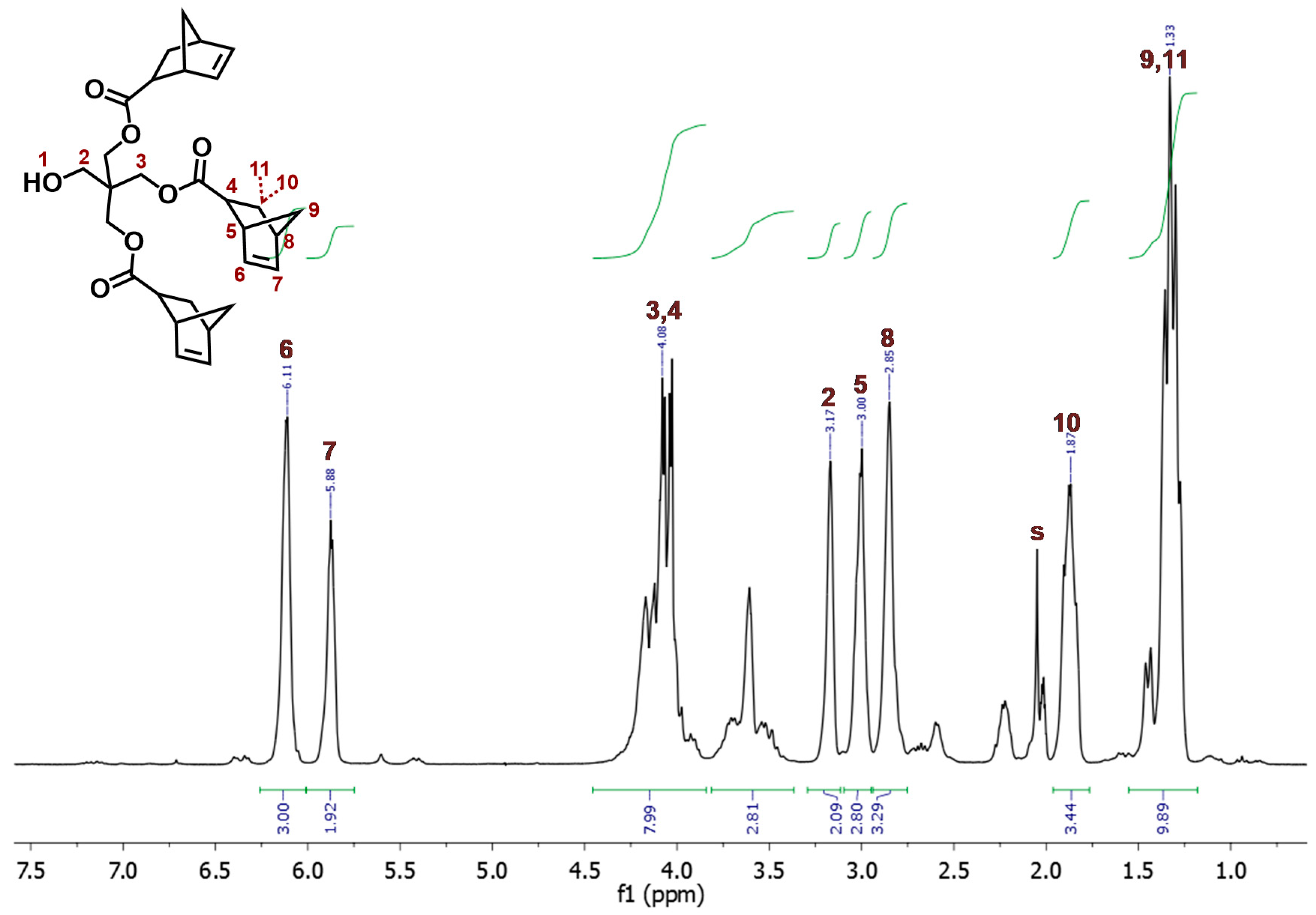
3.3. Synthesis of aL-9-NBE and aR-9-NBE Monomers
3.4. Polymerization Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Alemán, J.V.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, C.; Zhang, G.; Rawashdeh, A.-M.M. Nanoengineering Strong Silica Aerogels. Nano Lett. 2002, 2, 957–960. [Google Scholar] [CrossRef]
- Mulik, S.; Sotiriou-Leventis, C.; Leventis, N. Time-Efficient Acid-Catalyzed Synthesis of Resorcinol−Formaldehyde Aerogels. Chem. Mater. 2007, 19, 6138–6144. [Google Scholar] [CrossRef]
- Mulik, S.; Sotiriou-Leventis, C.; Leventis, N. Macroporous Electrically Conducting Carbon Networks by Pyrolysis of Isocyanate-Cross-Linked Resorcinol-Formaldehyde Aerogels. Chem. Mater. 2008, 20, 6985–6997. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, C.; Chandrasekaran, N.; Mulik, S.; Larimore, Z.J.; Lu, H.; Churu, G.; Mang, J.T. Multifunctional Polyurea Aerogels from Isocyanates and Water. A Structure−Property Case Study. Chem. Mater. 2010, 22, 6692–6710. [Google Scholar] [CrossRef]
- Chidambareswarapattar, C.; Xu, L.; Sotiriou-Leventis, C.; Leventis, N. Robust monolithic multiscale nanoporous polyimides and conversion to isomorphic carbons. RSC Adv. 2013, 3, 26459–26469. [Google Scholar] [CrossRef]
- Leventis, N.; Chidambareswarapattar, C.; Mohite, D.P.; Larimore, Z.J.; Lu, H.; Sotiriou-Leventis, C. Multifunctional porous aramids (aerogels) by efficient reaction of carboxylic acids and isocyanates. J. Mater. Chem. 2011, 21, 11981–11986. [Google Scholar] [CrossRef]
- Mahadik-Khanolkar, S.; Donthula, S.; Sotiriou-Leventis, C.; Leventis, N. Polybenzoxazine Aerogels. 1. High-Yield Room-Temperature Acid-Catalyzed Synthesis of Robust Monoliths, Oxidative Aromatization, and Conversion to Microporous Carbons. Chem. Mater. 2014, 26, 1303–1317. [Google Scholar] [CrossRef]
- Chidambareswarapattar, C.; McCarver, P.M.; Luo, H.; Lu, H.; Sotiriou-Leventis, C.; Leventis, N. Fractal Multiscale Nanoporous Polyurethanes: Flexible to Extremely Rigid Aerogels from Multifunctional Small Molecules. Chem. Mater. 2013, 25, 3205–3224. [Google Scholar] [CrossRef]
- Mohite, D.P.; Mahadik-Khanolkar, S.; Luo, H.; Lu, H.; Sotiriou-Leventis, C.; Leventis, N. Polydicyclopentadiene aerogels grafted with PMMA: I. Molecular and interparticle crosslinking. Soft Matter 2013, 9, 1516–1530. [Google Scholar] [CrossRef]
- Kim, S.H.; Worsley, M.A.; Valdez, C.A.; Shin, S.J.; Dawedeit, C.; Braun, T.; Baumann, T.F.; Letts, S.A.; Kucheyev, S.O.; Wu, K.J.J.; et al. Exploration of the versatility of ring opening metathesis polymerization: An approach for gaining access to low density polymeric aerogels. RSC Adv. 2012, 2, 8672–8680. [Google Scholar] [CrossRef][Green Version]
- Lee, J.K.; Gould, G.L. Polydicyclopentadiene based aerogel: A new insulation material. J. Sol-Gel Sci. Technol. 2007, 44, 29–40. [Google Scholar] [CrossRef]
- Ivin, K.J.; Mol, J.C. Olefin Metathesis and Metathesis Polymerization; Academic Press: Cambridge, MA, USA, 1997; ISBN 978-0-08-053797-9. [Google Scholar]
- Dragutan, V.; Streck, R. Catalytic Polymerization of Cycloolefins: Ionic, Ziegler-Natta and Ring-Opening Metathesis Polymerization; Elsevier: New York, NY, USA, 2000; ISBN 978-0-08-052862-5. [Google Scholar]
- Cowie, J.M.G.; Arrighi, V. Polymers: Chemistry and Physics of Modern Materials, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-1-4200-0987-3. [Google Scholar]
- Schrock, R.R. Multiple Metal–Carbon Bonds for Catalytic Metathesis Reactions (Nobel Lecture). Angew. Chem. Int. Ed. 2006, 45, 3748–3759. [Google Scholar] [CrossRef] [PubMed]
- Grubbs, R.H. Olefin-Metathesis Catalysts for the Preparation of Molecules and Materials (Nobel Lecture). Angew. Chem. Int. Ed. 2006, 45, 3760–3765. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J. Organotransition Metal Chemistry: From Bonding to Catalysis, 1st ed.; University Science Books: Sausalito, CA, USA, 2009; ISBN 978-1-891389-53-5. [Google Scholar]
- Bielawski, C.W.; Grubbs, R.H. Living ring-opening metathesis polymerization. Prog. Polym. Sci. 2007, 32, 1–29. [Google Scholar] [CrossRef]
- Wei, J.; Trout, W.; Simon, Y.C.; Granados-Focil, S. Ring opening metathesis polymerization of triazole-bearing cyclobutenes: Diblock copolymer synthesis and evaluation of the effect of side group size on polymerization kinetics. J. Polym. Sci. Part Polym. Chem. 2017, 55, 1929–1939. [Google Scholar] [CrossRef]
- Liu, J.; Burts, A.O.; Li, Y.; Zhukhovitskiy, A.V.; Ottaviani, M.F.; Turro, N.J.; Johnson, J.A. “Brush-First” Method for the Parallel Synthesis of Photocleavable, Nitroxide-Labeled Poly(ethylene glycol) Star Polymers. J. Am. Chem. Soc. 2012, 134, 16337–16344. [Google Scholar] [CrossRef] [PubMed]
- Raptopoulos, G.; Anyfantis, G.C.; Chriti, D.; Paraskevopoulou, P. Synthesis and structural characterization of poly(dicyclopentadiene) gels obtained with a novel ditungsten versus conventional W and Ru mononuclear catalysts. Inorg. Chim. Acta 2017, 460, 69–76. [Google Scholar] [CrossRef]
- Chriti, D.; Grigoropoulos, A.; Raptopoulos, G.; Charalambidis, G.; Nikolaou, V.; Coutsolelos, A.G.; Pitsikalis, M.; Mertis, K.; Paraskevopoulou, P. Metathesis Polymerization Reactions Induced by the Bimetallic Complex (Ph4P)2[W2(m-Br)3Br6]. Polymers 2015, 7, 2611–2624. [Google Scholar] [CrossRef]
- Floros, G.; Saragas, N.; Paraskevopoulou, P.; Psaroudakis, N.; Koinis, S.; Pitsikalis, M.; Hadjichristidis, N.; Mertis, K. Ring Opening Metathesis Polymerization of Norbornene and Derivatives by the Triply Bonded Ditungsten Complex Na[W2(m-Cl)3Cl4(THF)2]·(THF)3. Polymers 2012, 4, 1657–1673. [Google Scholar] [CrossRef]
- Saragas, N.; Floros, G.; Raptopoulos, G.; Pitsikalis, M.; Paraskevopoulou, P.; Mertis, K. Exploring the Reactivity of Na[W2(m-Cl)3Cl4(THF)2]∙(THF)3 towards the Polymerization of Selected Cycloolefins. Molecules 2015, 20, 21896–21908. [Google Scholar] [CrossRef] [PubMed]
- Schrock, R.R. Recent Advances in High Oxidation State Mo and W Imido Alkylidene Chemistry. Chem. Rev. 2009, 109, 3211–3226. [Google Scholar] [CrossRef] [PubMed]
- Basset, J.-M.; Leconte, M.; Lefebvre, F.; Hamilton, J.G.; Rooney, J.J. Stereoselectivity in cyclic and acyclic metathesis reactions. Macromol. Chem. Phys. 1997, 198, 3499–3506. [Google Scholar] [CrossRef]
- Abadie, M.J.; Dimonie, M.; Couve, C.; Dragutan, V. New catalysts for linear polydicyclopentadiene synthesis. Eur. Polym. J. 2000, 36, 1213–1219. [Google Scholar] [CrossRef]
- Bokaris, E.P.; Kosmas, M.M. All cis-poly(NBE) derived by the ROMP catalysts based on WCl6. J. Mol. Catal. Chem. 2003, 192, 263–273. [Google Scholar] [CrossRef]
- Schrock, R.R. Synthesis of Stereoregular Polymers through Ring-Opening Metathesis Polymerization. Acc. Chem. Res. 2014, 47, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Lenhardt, J.M.; Kim, S.H.; Worsley, M.A.; Leif, R.N.; Campbell, P.G.; Baumann, T.F.; Satcher, J.H. ROMP crosslinkers for the preparation of aliphatic aerogels. J. Non-Cryst. Solids 2015, 408, 98–101. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, C.; Mohite, D.P.; Larimore, Z.J.; Mang, J.T.; Churu, G.; Lu, H. Polyimide Aerogels by Ring-Opening Metathesis Polymerization (ROMP). Chem. Mater. 2011, 23, 2250–2261. [Google Scholar] [CrossRef]
- Bang, A.; Buback, C.; Sotiriou-Leventis, C.; Leventis, N. Flexible Aerogels from Hyperbranched Polyurethanes: Probing the Role of Molecular Rigidity with Poly(Urethane Acrylates) Versus Poly(Urethane Norbornenes). Chem. Mater. 2014, 26, 6979–6993. [Google Scholar] [CrossRef]
- Newmark, R.A.; Palazzotto, J. Carbon-13 NMR Analysis of Pentaerythritol Triacrylate. Appl. Spectrosc. 1990, 44, 804–807. [Google Scholar] [CrossRef]
- Craig, D. The Rearrangement of endo-3,6-Methylene-1,2,3,6-tetrahydro-cis-phthalic Anhydride. J. Am. Chem. Soc. 1951, 73, 4889–4892. [Google Scholar] [CrossRef]
- Vijayakumar, C.T.; Lederer, K.; Kramer, A. Thermogravimetric study of nadic-, methylnadic-, and allylnadic-bisimide monomers. J. Polym. Sci. Part Polym. Chem. 1991, 29, 929–931. [Google Scholar] [CrossRef]
- Wan, C.; Lu, Y.; Jiao, Y.; Jin, C.; Sun, Q.; Li, J. Ultralight and hydrophobic nanofibrillated cellulose aerogels from coconut shell with ultrastrong adsorption properties. J. Appl. Polym. Sci. 2015, 132, 42037. [Google Scholar] [CrossRef]
- Nemoto, J.; Saito, T.; Isogai, A. Simple Freeze-Drying Procedure for Producing Nanocellulose Aerogel-Containing, High-Performance Air Filters. ACS Appl. Mater. Interfaces 2015, 7, 19809–19815. [Google Scholar] [CrossRef] [PubMed]
- Leventis, N.; Palczer, A.; McCorkle, L.; Zhang, G.; Sotiriou-Leventis, C. Nanoengineered Silica-Polymer Composite Aerogels with No Need for Supercritical Fluid Drying. J. Sol-Gel Sci. Technol. 2005, 35, 99–105. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available. |
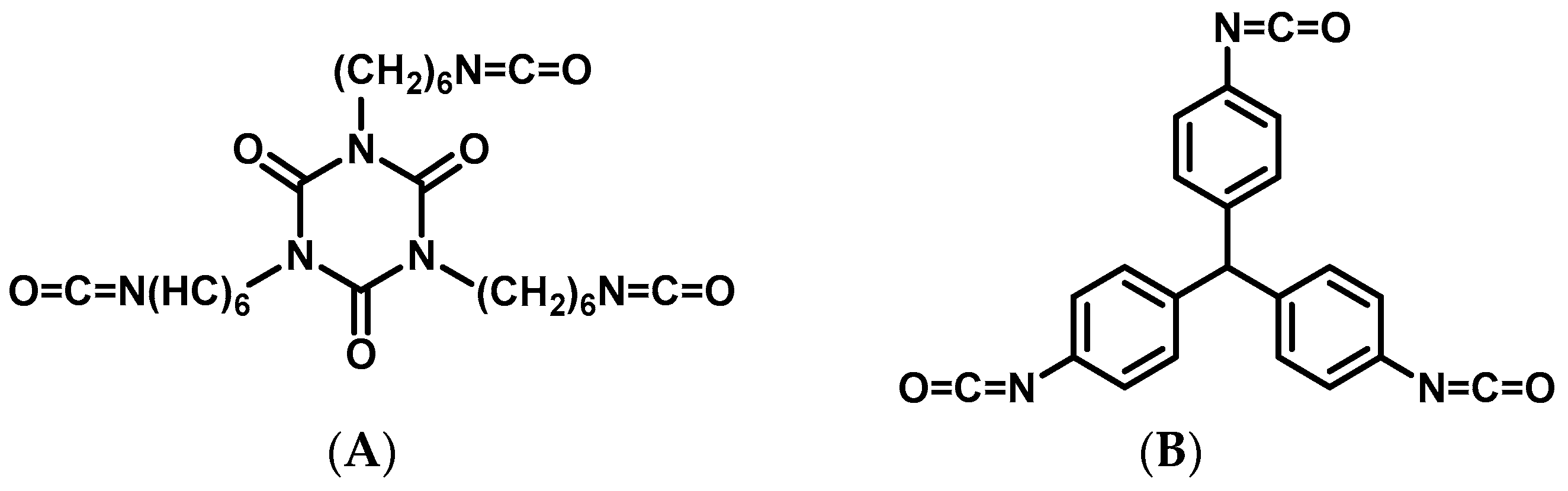



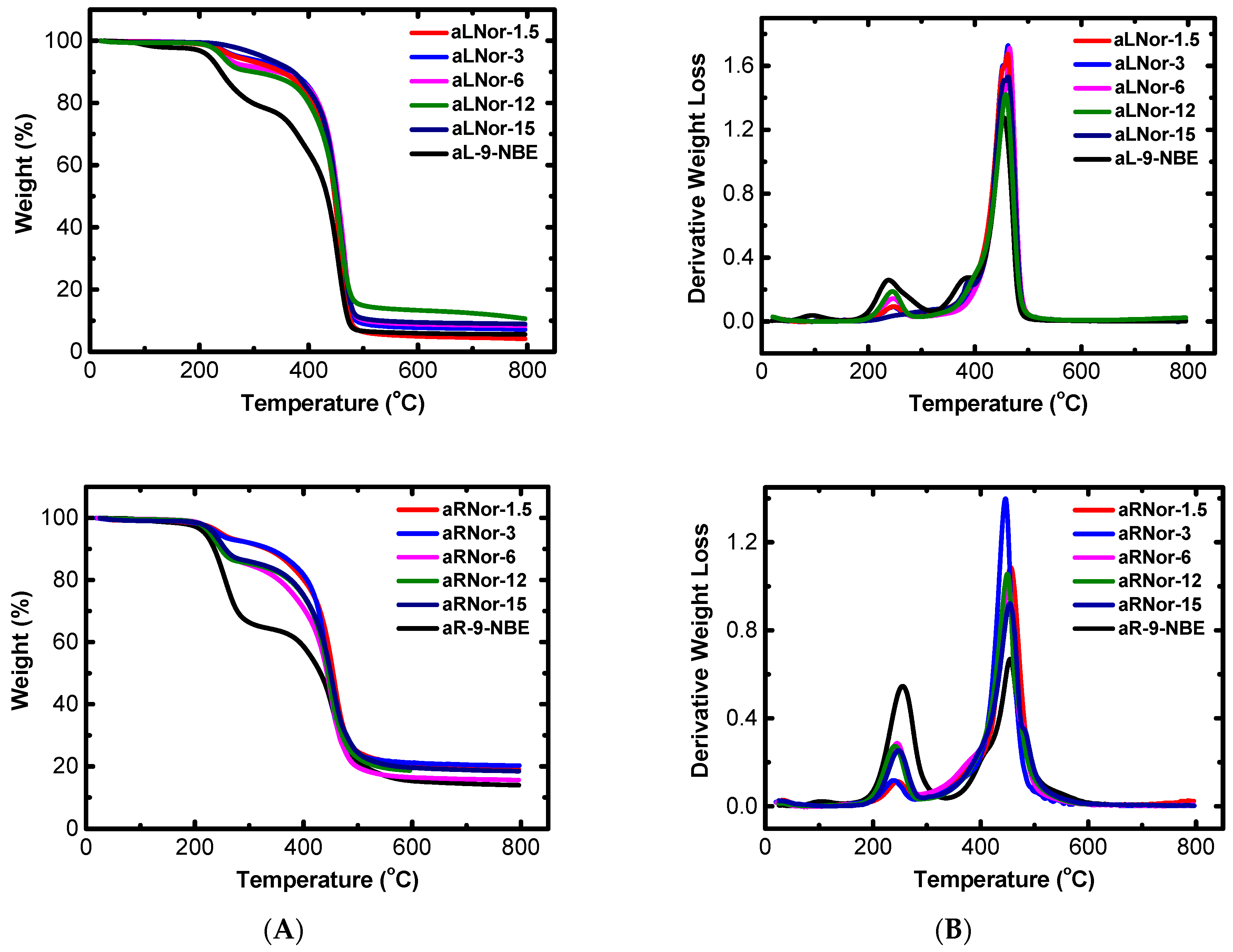


| Sample | Start (°C) | End (°C) | 1st Peak (°C) | 2nd Peak (°C) |
|---|---|---|---|---|
| aL-9-NBE | 166.6 | 512.7 | 238.8 | 454.2 |
| aR-9-NBE | 156.3 | 628.9 | 254.9 | 456.1 |
| Entry | GC-I (mg, [mmol]) | [GC-I] (% w/w, [mM]) | GC-I/Monomer (mol/mol) | Apparent Gelation Time 2 (min) |
|---|---|---|---|---|
| 1 | 20 [0.024] | 0.20 [2.4] | 1/25 | 15 |
| 2 | 10 [0.012] | 0.10 [1.2] | 1/50 | 60 |
| 3 | 5 [0.006] | 0.05 [0.6] | 1/100 | 140 |
| 4 | 1 [0.0012] | 0.01 [0.12] | 1/500 | - |
| Sample | Linear Shrinkage 5 (%) | Bulk Density ρb (g cm−3) |
|---|---|---|
| aLNor-12-SCF 2 | 33 ± 5 | 0.5 ± 0.1 |
| aLNor-12-FD 3 | 40 ± 1 | 0.8 ± 0.1 |
| aLNor-12-PENT 4 | 53.45 ± 0.04 | 1.10 ± 0.02 |
| Sample | Monomer | Monomer | GC-I/Monomer | Apparent Gelation Time 2 | |
|---|---|---|---|---|---|
| (g) | (mmol) | (% w/w) | (mol/mol) | (min) | |
| aLNor-1.5 | 0.13 | 0.065 | 1.5 | 1/5 | 110 |
| aLNor-3 | 0.30 | 0.150 | 3.4 | 1/12 | 65 |
| aLNor-6 | 0.57 | 0.288 | 6.1 | 1/24 | 25 |
| aLNor-12 | 1.15 | 0.576 | 11.2 | 1/48 | 20 |
| aLNor-15 | 1.50 | 0.752 | 15 | 1/63 | 23 |
| aRNor-1.5 | 0.12 | 0.065 | 1.5 | 1/5 | 10 |
| aRNor-3 | 0.28 | 0.151 | 3.1 | 1/12 | 12 |
| aRNor-6 | 0.54 | 0.291 | 5.4 | 1/24 | 35 |
| aRNor-12 | 1.10 | 0.591 | 11.3 | 1/48 | 30 |
| aRNor-15 | 1.42 | 0.765 | 14.3 | 1/63 | 15 |
| -xx | aLNor-xx | aRNor-xx | ||
|---|---|---|---|---|
| CPD (mol) | CPD/aL-9-NBE (mol/mol) | CPD (mol) | CPD/aR-9-NBE (mol/mol) | |
| monomer | 0.454 | 9.1 | 0.526 | 9.8 |
| 1.5 | 0.100 | 2.0 | 0.109 | 2.0 |
| 3 | 0.085 | 1.7 | 0.118 | 2.1 |
| 6 | 0.123 | 2.4 | 0.222 | 4.1 |
| 12 | 0.148 | 3.0 | 0.216 | 4.0 |
| 15 | 0.318 | 6.4 | 0.211 | 3.9 |
| Sample | Linear Shrinkage 1 (%) | Bulk Density ρb (g cm−3) | Skeletal Density ρs (g cm−3) | Porosity 2 Π (% v/v) | BET Surf. Area σ (m2g−1) | VTotal3 (V1.7-300nm) 4 (cm3 g-1) | Av. Pore Diameter 5 (nm) |
|---|---|---|---|---|---|---|---|
| aLNor-1.5 | 27 ± 3 | 0.070 ± 0.001 | 1.61 ± 0.08 | 96 | 270 | 13.66 (0.58) | 88 |
| aLNor-3 | 30 ± 1 | 0.125 ± 0.003 | 1.38 ± 0.03 | 91 | 302 | 7.28 (0.82) | 13 |
| aLNor-6 | 34 ± 8 | 0.24 ± 0.05 | 1.274 ± 0.009 | 81 | 191 | 3.38 (0.77) | 13 |
| aLNor-12 | 33 ± 5 | 0.5 ± 0.1 | 1.242 ± 0.005 | 60 | 85 | 1.19 (0.43) | 24 |
| aLNor-15 | 35 ± 1 | 0.676 ± 0.002 | 1.229 ± 0.005 | 45 | 54 | 0.66 (0.35) | 24 |
| aRNor-1.5 | 14 ± 1 | 0.032 ± 0.004 | 1.8 ± 0.1 | 98 | 188 | 30.69 (0.58) | 123 |
| aRNor-3 | 15 ± 2 | 0.045 ± 0.001 | 1.7 ± 0.1 | 97 | 294 | 21.63 (0.65) | 88 |
| aRNor-6 | 18 ± 2 | 0.10 ± 0.02 | 1.42 ± 0.02 | 93 | 221 | 9.30 (0.42) | 78 |
| aRNor-12 | 18 ± 1 | 0.17 ± 0.01 | 1.43 ± 0.04 | 88 | 211 | 5.18 (0.59) | 11 |
| aRNor-15 | 12 ± 1 | 0.195 ± 0.008 | 1.31 ± 0.01 | 85 | 148 | 4.36 (0.43) | 116 |
| Sample-xx | Particle Radius r (nm) | |
|---|---|---|
| aLNor-xx | aRNor-xx | |
| 1.5 | 6.9 | 8.9 |
| 3 | 7.2 | 6.0 |
| 6 | 12.3 | 9.6 |
| 12 | 28.4 | 9.9 |
| 15 | 45.2 | 15.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanellou, A.; Anyfantis, G.C.; Chriti, D.; Raptopoulos, G.; Pitsikalis, M.; Paraskevopoulou, P. Poly(urethane-norbornene) Aerogels via Ring Opening Metathesis Polymerization of Dendritic Urethane-Norbornene Monomers: Structure-Property Relationships as a Function of an Aliphatic Versus an Aromatic Core and the Number of Peripheral Norbornene Moieties. Molecules 2018, 23, 1007. https://doi.org/10.3390/molecules23051007
Kanellou A, Anyfantis GC, Chriti D, Raptopoulos G, Pitsikalis M, Paraskevopoulou P. Poly(urethane-norbornene) Aerogels via Ring Opening Metathesis Polymerization of Dendritic Urethane-Norbornene Monomers: Structure-Property Relationships as a Function of an Aliphatic Versus an Aromatic Core and the Number of Peripheral Norbornene Moieties. Molecules. 2018; 23(5):1007. https://doi.org/10.3390/molecules23051007
Chicago/Turabian StyleKanellou, Aspasia, George C. Anyfantis, Despoina Chriti, Grigorios Raptopoulos, Marinos Pitsikalis, and Patrina Paraskevopoulou. 2018. "Poly(urethane-norbornene) Aerogels via Ring Opening Metathesis Polymerization of Dendritic Urethane-Norbornene Monomers: Structure-Property Relationships as a Function of an Aliphatic Versus an Aromatic Core and the Number of Peripheral Norbornene Moieties" Molecules 23, no. 5: 1007. https://doi.org/10.3390/molecules23051007
APA StyleKanellou, A., Anyfantis, G. C., Chriti, D., Raptopoulos, G., Pitsikalis, M., & Paraskevopoulou, P. (2018). Poly(urethane-norbornene) Aerogels via Ring Opening Metathesis Polymerization of Dendritic Urethane-Norbornene Monomers: Structure-Property Relationships as a Function of an Aliphatic Versus an Aromatic Core and the Number of Peripheral Norbornene Moieties. Molecules, 23(5), 1007. https://doi.org/10.3390/molecules23051007