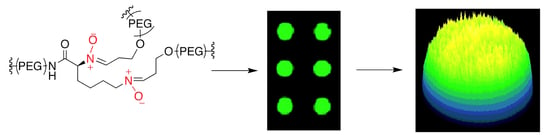
Fluorescent Dendritic Micro-Hydrogels: Synthesis, Analysis and Use in Single-Cell Detection
Abstract
:1. Introduction
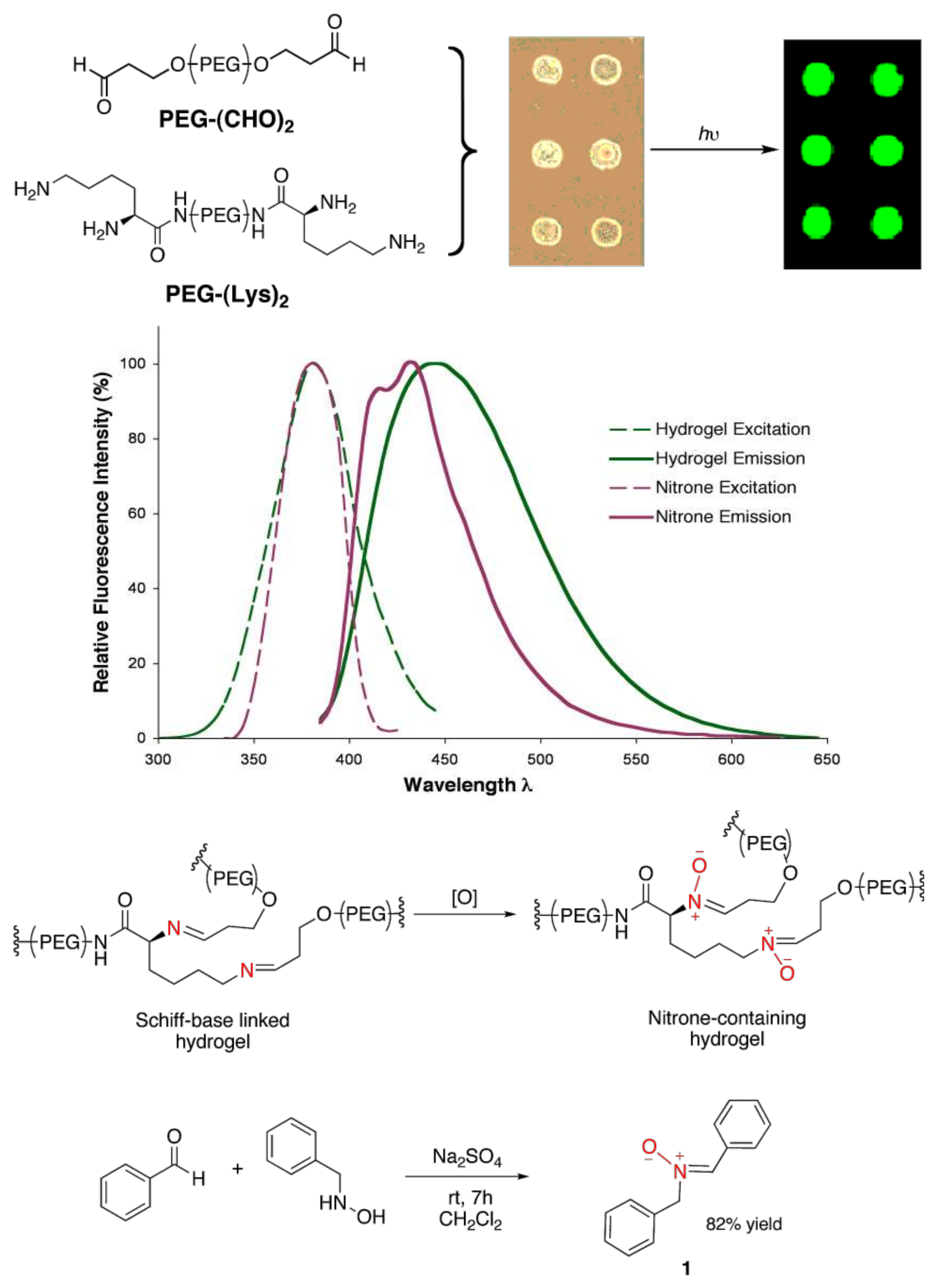
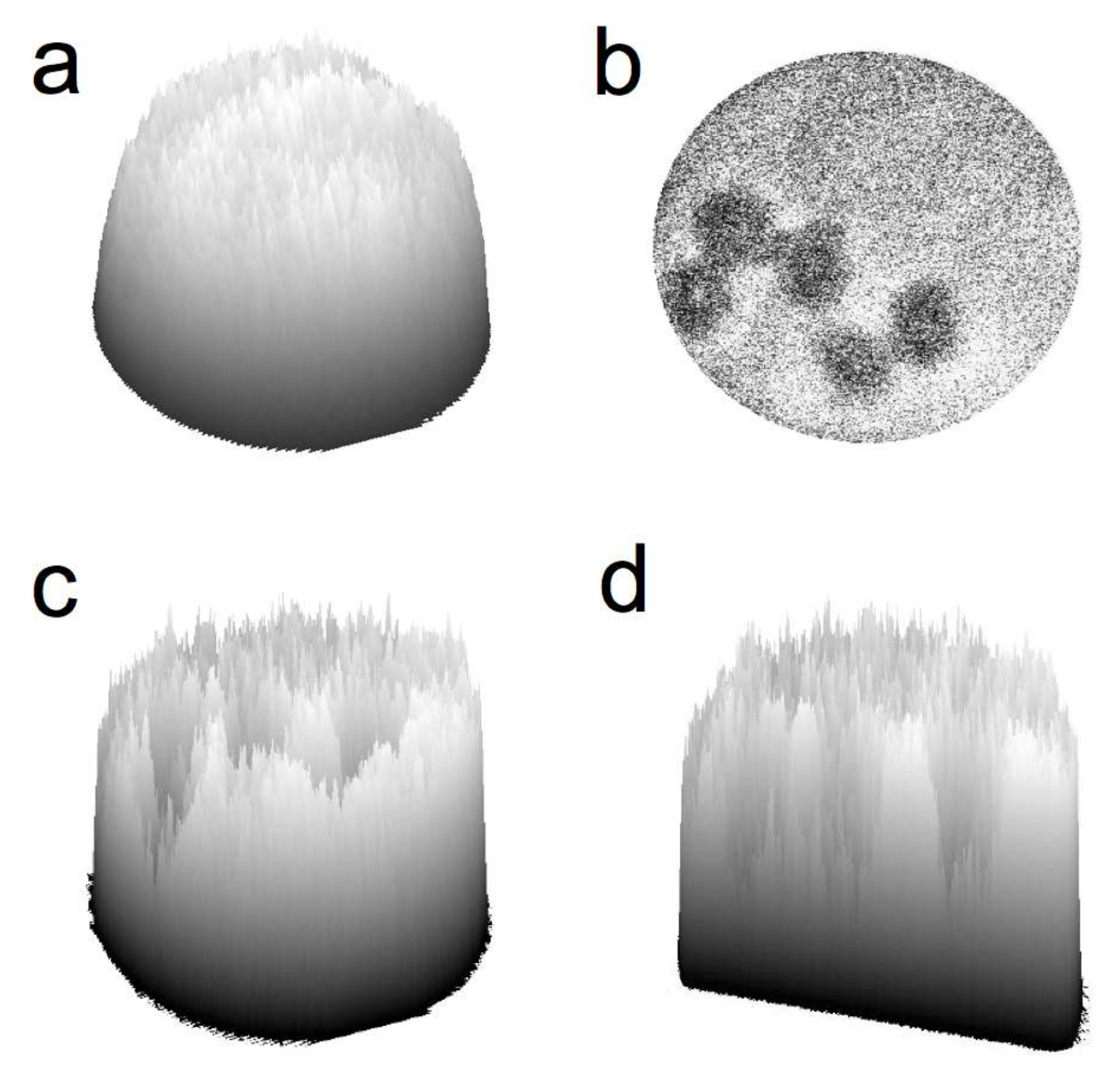
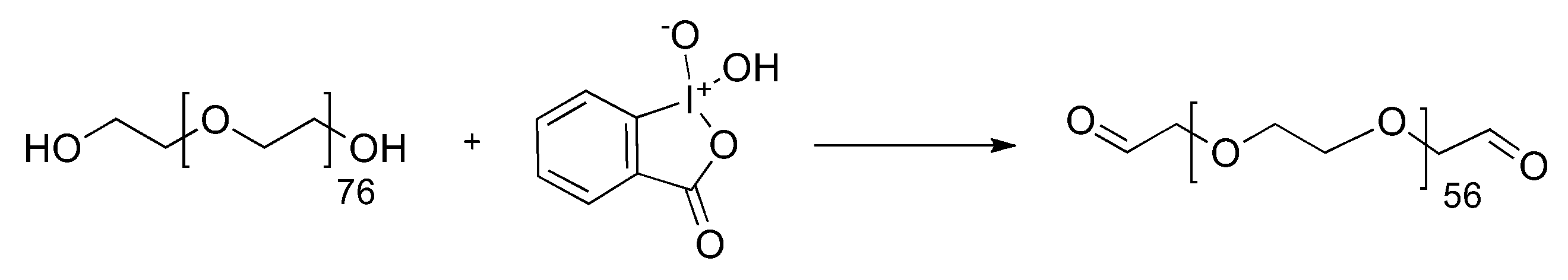
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Hydrogel Array Materials & Methods
3.3. Fluorescence Image Analysis
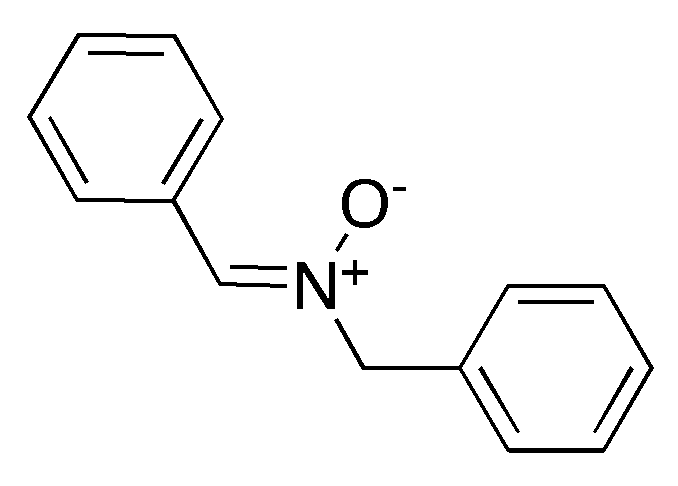
3.4. Nitrone Model
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Caminade, A.M.; Ouali, A.; Laurent, R.; Turrin, C.O.; Majoral, J.P. Coordination chemistry with phosphorus dendrimers. Applications as catalysts, for materials, and in biology. Coord. Chem. Rev. 2016, 308, 478–497. [Google Scholar] [CrossRef]
- Sun, H.J.; Zhang, S.; Percec, V. From structure to function via complex supramolecular dendrimer systems. Chem. Soc. Rev. 2015, 44, 3900–3923. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Astruc, D. Dendritic catalysis-Basic concepts and recent trends. Coord. Chem. Rev. 2013, 257, 2317–2334. [Google Scholar] [CrossRef]
- Newkome, G.R.; Shreiner, C.D. Poly(amidoamine), polypropylenimine, and related dendrimers and dendrons possessing different 1/2 branching motifs: An overview of the divergent procedures. Polymer 2008, 49, 1–173. [Google Scholar] [CrossRef]
- Molla, M.R.; Rangadurai, P.; Pavan, G.M.; Thayumanavan, S. Experimental and theoretical investigations in stimuli responsive dendrimer-based assemblies. Nanoscale 2015, 7, 3817–3837. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 6, 579–617. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, M.A.; Grinstaff, M.W. Biomedical Applications of Dendrimers: A Tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; MacKay, J.A.; Fréchet, J.M.J.; Szoka, F.C. Designing Dendrimers for Biological Applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S.; Tomalia, D.A. Dendrimers in Biomedical Application—Reflections on the Field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef] [PubMed]
- Grinstaff, M.W. Biodendrimers: New Polymeric Materials for Tissue Engineering. Chem. Eur. J. 2002, 8, 2838–2846. [Google Scholar] [CrossRef]
- Shaunak, S. Perspective: Dendrimer drugs for infection and inflammation. Biochem. Biophys. Res. Commun. 2015, 468, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.P.; Ficker, M.; Christensen, J.B.; Trohopoulos, P.N.; Moghimi, S.M. Dendrimers in Medicine: Therapeutic Concepts and Pharmaceutical Challenges. Bioconj. Chem. 2015, 26, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Bugno, J.; Hsu, H.; Hong, S. Recent advances in targeted drug delivery approaches using dendritic polymers. Biomater. Sci. 2015, 3, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, J.B.; Grinstaff, M.W. Therapeutic and Diagnostic Applications of Dendrimers for Cancer Treatment. Adv. Drug Deliv. Rev. 2008, 60, 1037–1055. [Google Scholar] [CrossRef] [PubMed]
- Gillies, E.R.; Frechet, J.M. Dendrimers and dendritic polymers in drug delivery. Drug Discov. Today 2005, 10, 35–43. [Google Scholar] [CrossRef]
- Lee, C.C.; Gillies, E.R.; Fox, M.E.; Guillaudeu, S.J.; Fréchet, J.M.J.; Dy, E.E.; Szoka, F.C. A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinoma. Proc. Natl. Acad. Sci. USA 2006, 103, 16649–16654. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Simanek, E.E. Synthesis of water-soluble dendrimers based on melamine bearing 16 paclitaxel groups. Org. Lett. 2008, 10, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Gurdag, S.; Khandare, J.S.S.; Matherly, L.H.; Kannan, R.M. Activity of dendrimer methotrexate conjugates on methotrexate-sensitive and resistant cell lines. Bioconj. Chem. 2006, 17, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.T.; Nakanishi, Y.; Kroll, D.J.; Griset, A.P.; Carnahan, M.A.; Wathier, M.; Oberlies, N.H.; Manikumar, G.; Wani, M.C.; Grinstaff, M.W. Dendrimer-encapsulated camptothecins: Increased solubility, cellular uptake, and cellular retention afford enhanced anticancer activity in vitro. Cancer Res. 2006, 66, 11913–11921. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.T.; Carnahan, M.A.; Immoos, C.E.; Ribeiro, A.A.; Finkelstein, S.; Lee, S.J.; Grinstaff, M.W. Dendritic molecular capsules for hydrophobic compounds. J. Am. Chem. Soc. 2003, 125, 15485–15489. [Google Scholar] [CrossRef] [PubMed]
- Hed, Y.; Oberg, K.; Berg, S.; Nordberg, A.; von Holst, H.; Malkoch, M. Multipurpose heterofunctional dendritic scaffolds as crosslinkers towards functional soft hydrogels and implant adhesives in bone fracture applications. J. Mater. Chem. B 2013, 1, 6015–6019. [Google Scholar] [CrossRef]
- Ghobril, C.; Grinstaff, M.W. The Chemistry and Engineering of Polymeric Hydrogel Adhesives for Wound Closure: A Tutorial. Chem. Soc. Rev. 2015, 44, 1820–1835. [Google Scholar] [CrossRef] [PubMed]
- Ghobril, C.; Charoen, K.; Rodriguez, E.K.; Nazarian, A.; Grinstaff, M.W. A Dendritic Thioester Hydrogel Based on Thiol-Thioester Exchange as a Dissolvable System for Wound Closure. Angew. Chem. Int. Ed. 2013, 52, 14070–14074. [Google Scholar] [CrossRef] [PubMed]
- Oelker, A.M.; Grinstaff, M.W. Ophthalmic Adhesives: A Materials Chemistry Perspective. J. Mater. Chem. 2008, 18, 2521–2536. [Google Scholar] [CrossRef]
- Wathier, M.; Jung, P.J.; Carnahan, M.A.; Kim, T.; Grinstaff, M.W. Dendritic Macromers as In Situ Polymerizing Biomaterials for Securing Cataract Incisions. J. Am. Chem. Soc. 2004, 126, 12744–12745. [Google Scholar] [CrossRef] [PubMed]
- Carnahan, M.A.; Middleton, C.; Kim, J.; Kim, T.; Grinstaff, M.W. Hybrid Dendritic-Linear Polyester-ethers for In Situ Photopolymerization. J. Am. Chem. Soc. 2002, 124, 5291–5293. [Google Scholar] [CrossRef] [PubMed]
- Wathier, M.; Johnson, S.M.; Kim, T.; Grinstaff, M.W. Hydrogels Formed by Multiple Peptide Ligation Reactions to Fasten Corneal Transplants. Bioconj. Chem. 2006, 17, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Berdahl, J.P.; Johnson, C.S.; Proia, A.D.; Grinstaff, M.W.; Kim, T. Comparison of Sutures and New Dendritic Polymer Adhesives for Corneal Laceration Repair in an In Vivo Chicken Model. Arch. Ophthalmol. 2009, 127, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Degoricija, L.; Söntjens, S.H.M.; Bansal, P.N.; Takahashi, M.; Joshi, N.; Snyder, B.; Grinstaff, M.W. Hydrogels for Osteochondral Regeneration Based on Photo-crosslinkable Carbamate Dendrimers. Biomacromolecules 2008, 9, 2863–2872. [Google Scholar] [CrossRef] [PubMed]
- Sontjens, S.H.M.; Nettles, D.L.; Carnahan, M.A.; Setton, L.A.; Grinstaff, M.W. Biodendrimer-based hydrogel scaffolds for cartilage tissue repair. Biomacromolecules 2006, 7, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Navath, R.S.; Menjoge, A.R.; Dai, H.; Romero, R.; Kannan, S.; Kannan, R.M. Injectable PAMAM dendrimer-PEG hydrogels for the treatment of genital infections: Formulation, in-vitro and in-vivo evaluation. Mol. Pharm. 2011, 8, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tyagi, P.; Kadam, R.S.; Holden, C.A.; Kompella, U.B. Hybrid dendrimer hydrogel/PLGA nanoparticle platform sustains drug delivery for one week and antiglaucoma effects for four days following one-time topical administration. ACS Nano 2012, 6, 7595–7606. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, M.M.; Wathier, M.; Grinstaff, M.W.; Schaus, S.E. Immobilized Hydrogels for High Throughput Screening of Molecular Interactions. Anal. Chem. 2007, 79, 1064–1066. [Google Scholar] [CrossRef] [PubMed]
- Larson, C.L.; Tucker, S.A. Intrinsic fluorescence of carboxylate-terminated polyamido amine dendrimers. Appl. Spectrosc. 2001, 55, 679–683. [Google Scholar] [CrossRef]
- Lee, W.I.; Bae, Y.B.; Bard, A.J. Strong blue photoluminescence and ECL from OH-terminated PAMAM dendrimers in the absence of gold nanoparticles. J. Am. Chem. Soc. 2004, 126, 8358–8359. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Imae, T. Fluorescence emission from dendrimers and its pH dependence. J. Am. Chem. Soc. 2004, 126, 13204–13205. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Imae, T.; Miki, M. Fluorescence emission from PAMAM and PPI dendrimers. J. Colloid Interface Sci. 2007, 306, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.J.; Hu, C.C.; Chu, C.C.; Imae, T. Intrinsically fluorescent PAMAM dendrimer as gene carrier and nanoprobe for nucleic acids delivery: Bioimaging and transfection study. Biomacromolecules 2011, 12, 4283–4290. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Qian, Y. A study using quantum chemical theory methods on the intrinsic fluorescence emission and the possible emission mechanisms of PAMAM. RSC Adv. 2014, 4, 58788–58794. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, X.-L.; Qian, Y. Poly-amidoamine structure characterization: Amide resonance structure of imidic acid (HO–C[double bond, length as m-dash]N) and tertiary ammonium. RSC Adv. 2014, 4, 49535–49540. [Google Scholar] [CrossRef]
- Ballistreri, F.P.; Barbuzzi, E.; Tomaselli, G.A.; Toscano, R.M.; Mol, J.C. Oxidation of N, N-benzylalkylamines to nitrones by Mo(VI) and W(VI) polyperoxo complexes. Catal. A Chem. 1997, 114, 229. [Google Scholar] [CrossRef]
- Cardona, F.; Bonanni, M.; Soldaini, G.; Goti, A. One-Pot Synthesis of Nitrones from Primary Amines and Aldehydes Catalyzed by Methyltrioxorhenium. ChemSusChem 2008, 1, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Feuer, H. Nitrile Oxides, Nitrones and Nitronates; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Soldaini, G.; Cardona, F.; Goti, A. Catalytic Oxidation of Imines Based on Methyltrioxorhenium/Urea Hydrogen Peroxide: A Mild and Easy Chemo- and Regioselective Entry to Nitrones. Org. Lett. 2007, 9, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Thiel, W.R. Transition metal mediated oxygen transfer to organo nitrogen compounds. Coord. Chem. Rev. 2003, 245, 95–106. [Google Scholar] [CrossRef]
- Imada, Y.; Iida, H.; Ono, S.; Murahashi, S. Flavin catalyzed oxidations of sulfides and amines with molecular oxygen. J. Am. Chem. Soc. 2003, 125, 2868–2869. [Google Scholar] [CrossRef] [PubMed]
- Murahashi, S. Synthetic Aspects of Metal-Catalyzed Oxidations of Amines and Related Reactions. Angew. Chem. Int. Ed. 1995, 34, 2443–2465. [Google Scholar] [CrossRef]
- Chang, Z.Y.; Coates, R.M. Diastereoselectivity of organometallic additions to nitrones bearing stereogenic N-substituents. J. Org. Chem. 1990, 55, 3464–3474. [Google Scholar] [CrossRef]
- Boyd, D.R.; Campbell, P.B.; Grimshaw, J.; Neill, D.C.; Jennings, W.B. Dynamic stereochemistry of imines and derivatives. Part 18. Photosynthesis and photoracemization of optically active oxaziridines. J. Chem. Soc. Perkin Trans. 1985, 1, 849–855. [Google Scholar] [CrossRef]
- Cik, G.; Sersen, F. A study of photolysis of N-(4-azidobenzylidene)aniline-N-oxide. J. Imaging Sci. 1991, 35, 14–19. [Google Scholar]
- Cyr, D.R.; Mathew, T.; Ashok, K.; Das, P.K.; George, M.V. A laser flash photolysis study of aromatic nitrones. Triplet state properties and reactivity with singlet oxygen. J. Photochem. Photobiol. A Chem. 1991, 60, 161–174. [Google Scholar] [CrossRef]
- Khoee, S.; Memarian, H.R. Fluorescence self-quenching of substituted N,α-diphenylnitrones in various solvents. J. Photochem. Photobiol. A Chem. 2006, 177, 276–285. [Google Scholar] [CrossRef]
- Koyano, K.; Tanaka, I. The Photochemical and Thermal Isomerization of trans- and cis-α-Cyano-α-phenyl-N-phenylnitrones. J. Phys. Chem. 1965, 69, 2545–2550. [Google Scholar] [CrossRef]
- Lamchen, M.; Mittag, T.W. Nitrones. Part VII. The photochemistry and cycloaddition of a monocyclic α-dinitrone. J. Chem. Soc. C 1968, 1917–1921. [Google Scholar] [CrossRef]
- Shinzawa, K.; Tanaka, I. The Photochemical Isomerization of α,N-Diphenylnitrone. J. Phys. Chem. 1964, 68, 1205–1213. [Google Scholar] [CrossRef]
- Splitter, J.S.; Calvin, M. Oxaziridines. I. The Irradiation Products of Several Nitrones1. J. Org. Chem. 1965, 30, 3427–3436. [Google Scholar] [CrossRef]
- Brehm-Stecher, B.F.; Johnson, E.A. Single-cell microbiology: Tools, technologies, and applications. Microbiol. Mol. Biol. Rev. 2004, 68, 538–559. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, P.S.; Manz, A. Lab-on-a-chip: Microfluidics in drug discovery. Nat. Rev. Drug Discov. 2006, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- El-Ali, J.; Sorger, P.K.; Jensen, K.F. Cells on chips. Nature 2006, 442, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Vilkner, T.; Janasek, D.; Manz, A. Micro Total Analysis Systems. Recent Developments. Anal. Chem. 2004, 76, 3373–3386. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.R.; Throndset, W.R.; Whelan, R.J.; Leach, A.M.; Zare, R.N.; Liao, Y.H.; Farrell, K.; Manger, I.D.; Daridon, A. Microfluidic device for single-cell analysis. Anal. Chem. 2003, 75, 3581–3586. [Google Scholar] [CrossRef] [PubMed]
- Zare, R.N.; Kim, S. Microfluidic platforms for single-cell analysis. Annu. Rev. Biomed. Eng. 2010, 12, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.J.; Li, Q.; Lim, C.T. Manipulation and isolation of single cells and nuclei. Methods Cell Biol. 2010, 98, 79–96. [Google Scholar] [PubMed]
- Yershov, G.; Barsky, V.; Belgovskiy, A.; Kirillov, E.; Kreindlin, E.; Ivanov, I.; Parinov, S.; Guschin, D.; Drobyshev, A.; Dubiley, S.; et al. DNA analysis and diagnostics on oligonucleotide microchips. Proc. Natl. Acad. Sci. USA 1996, 93, 4913–4918. [Google Scholar] [CrossRef] [PubMed]
- Zlatanova, J.; Mirzabekov, A. Gel-Immobilized Microarrays of Nucleic Acids and Proteins. Production and Application for Macromolecular Research. Methods Mol. Biol. 2001, 170, 17–38. [Google Scholar] [PubMed]
- Beria, L.; Gevrek, T.N.; Erdog, A.; Sanyal, R.; Pasini, D.; Sanyal, A. Clickable Hydrogels for All: Facile Fabrication and Functionalization. Biomater. Sci. 2014, 2, 67–75. [Google Scholar] [CrossRef]
- Kemp, M.M.; Weïwer, M.; Koehler, A.N. Unbiased binding assays for discovering small-molecule probes and drugs. Bioorg. Med. Chem. 2012, 20, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. ImageJ. U. S.; National Institutes of Health: Bethesda, MD, USA, 2008. Available online: https://imagej.nih.gov/ij/ (accessed on 17 April 2018).
- Frigerio, M.; Santagostino, M.; Sputore, S. A User-Friendly Entry to 2-Iodoxybenzoic Acid (IBX). J. Org. Chem. 1999, 64, 4537–4538. [Google Scholar] [CrossRef]
- Wathier, M.; Johnson, M.S.; Carnahan, M.A.; Baer, C.; McCuen, B.W.; Kim, T.; Grinstaff, M.W. In situ polymerized hydrogels for repairing scleral incisions used in pars plana vitrectomy procedures. ChemMedChem 2006, 1, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Kainthan, R.K.; Mugabe, C.; Burt, H.M.; Brooks, D.E. Unimolecular micelles based on hydrophobically derivatized hyperbranched polyglycerols: Ligand binding properties. Biomacromolecules 2008, 9, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Murahashi, S.; Mitsui, H.; Shiota, T.; Tsuda, T.; Watanabe, S. Tungstate-catalyzed oxidation of secondary amines to nitrones. .alpha.-Substitution of secondary amines via nitrones. J. Org. Chem. 1990, 55, 1736–1744. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christadore, L.; Grinstaff, M.W.; Schaus, S.E. Fluorescent Dendritic Micro-Hydrogels: Synthesis, Analysis and Use in Single-Cell Detection. Molecules 2018, 23, 936. https://doi.org/10.3390/molecules23040936
Christadore L, Grinstaff MW, Schaus SE. Fluorescent Dendritic Micro-Hydrogels: Synthesis, Analysis and Use in Single-Cell Detection. Molecules. 2018; 23(4):936. https://doi.org/10.3390/molecules23040936
Chicago/Turabian StyleChristadore, Lisa, Mark W. Grinstaff, and Scott E. Schaus. 2018. "Fluorescent Dendritic Micro-Hydrogels: Synthesis, Analysis and Use in Single-Cell Detection" Molecules 23, no. 4: 936. https://doi.org/10.3390/molecules23040936
APA StyleChristadore, L., Grinstaff, M. W., & Schaus, S. E. (2018). Fluorescent Dendritic Micro-Hydrogels: Synthesis, Analysis and Use in Single-Cell Detection. Molecules, 23(4), 936. https://doi.org/10.3390/molecules23040936