Complexes of CO2 with the Azoles: Tetrel Bonds, Hydrogen Bonds and Other Secondary Interactions
Abstract
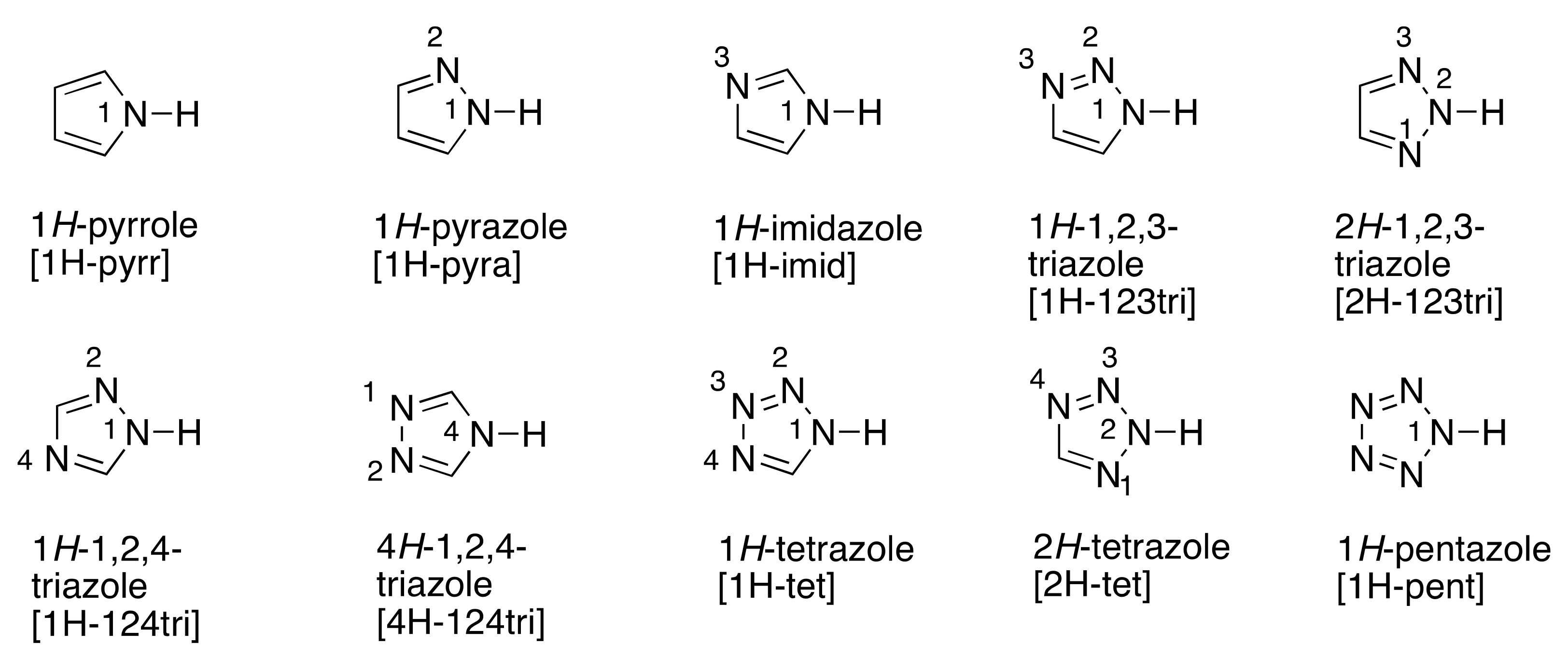
:1. Introduction
2. Methods
3. Results and Discussion
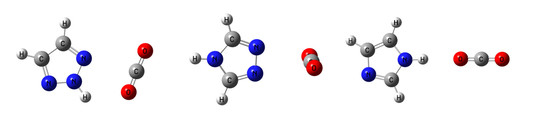
3.1. Overview of the CO2:Azole Complexes
3.2. Planar Complexes Stabilized by Tetrel Bonds
3.3. Perpendicular Complexes Stabilized by Tetrel Bonds
3.4. Complexes Stabilized by Hydrogen Bonds
4. Conclusions
- Three types of complexes have been found on the potential surfaces. These include ten complexes stabilized by tetrel bonds in which the azole molecule lies in the symmetry plane of the complex and seven complexes also stabilized by tetrel bonds, but which have the azole molecule perpendicular to the symmetry plane. In addition, there are four hydrogen-bonded complexes.
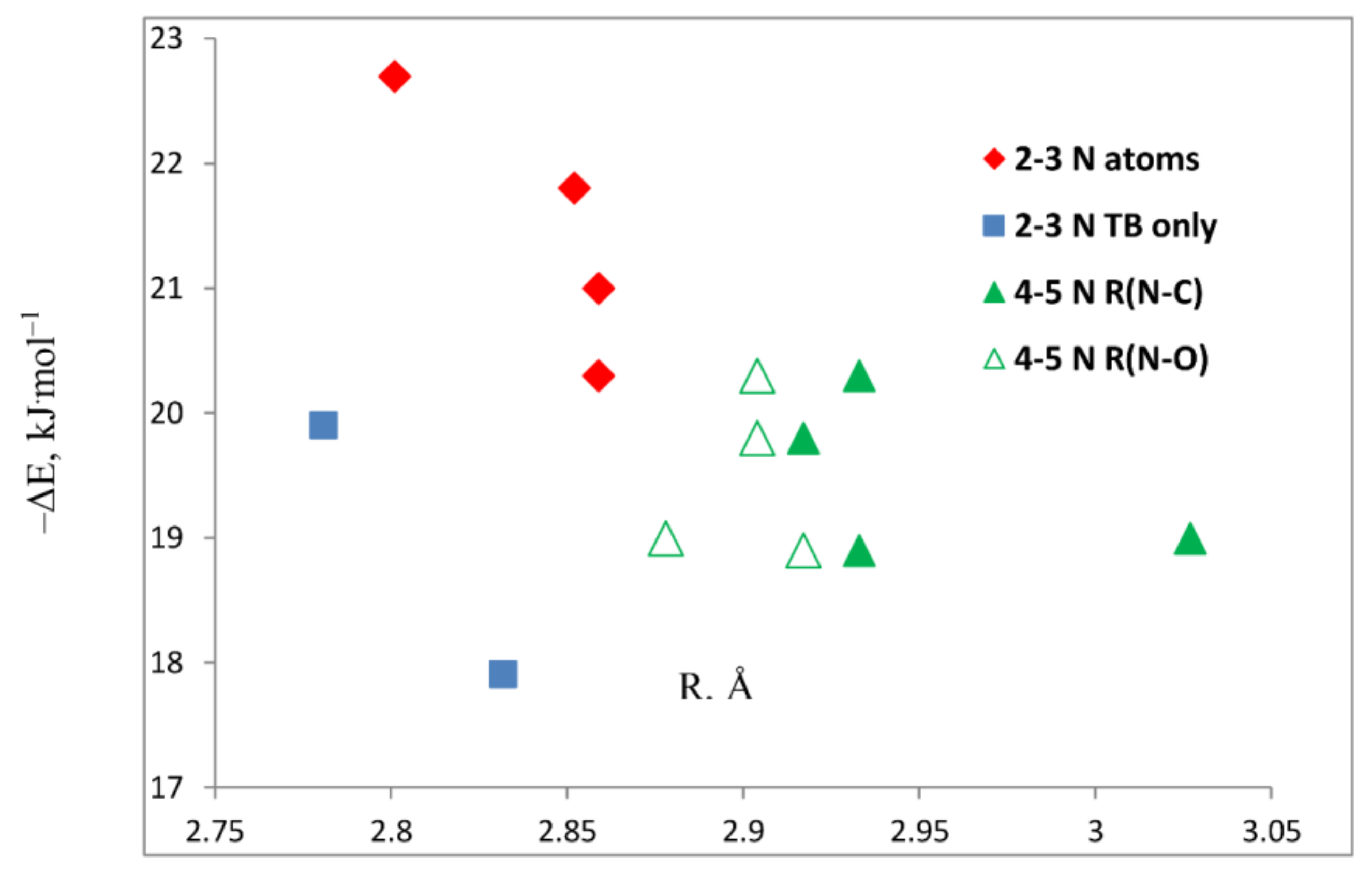
- The ten complexes stabilized by tetrel bonds that have the azole molecule in the symmetry plane of the complex have some common characteristics.
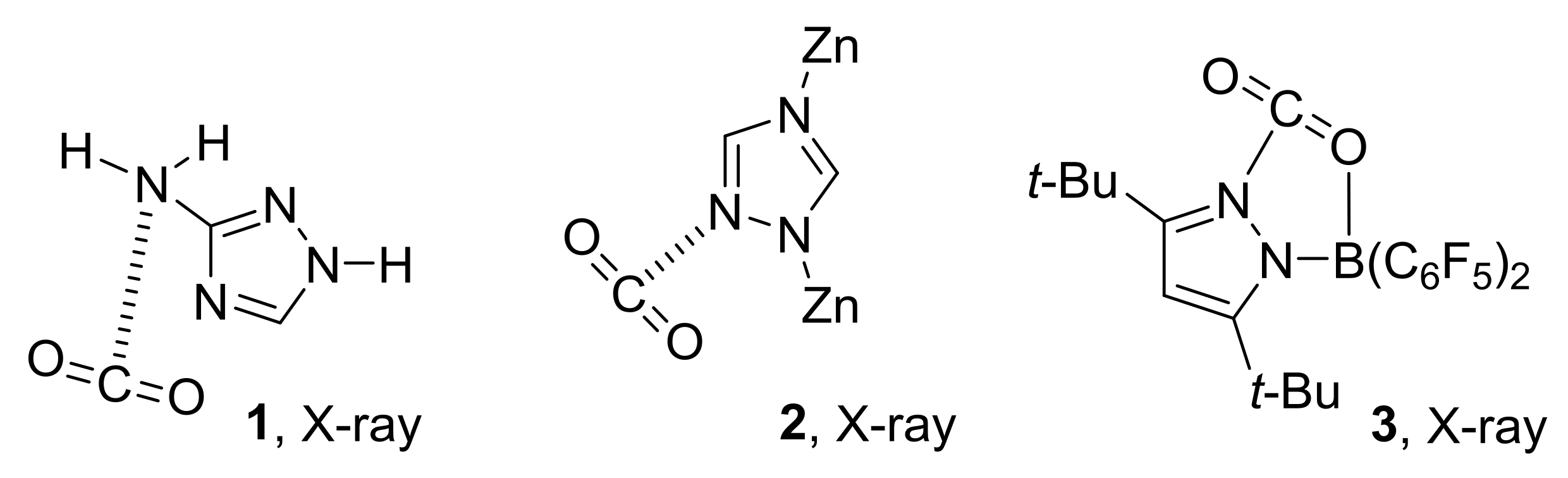
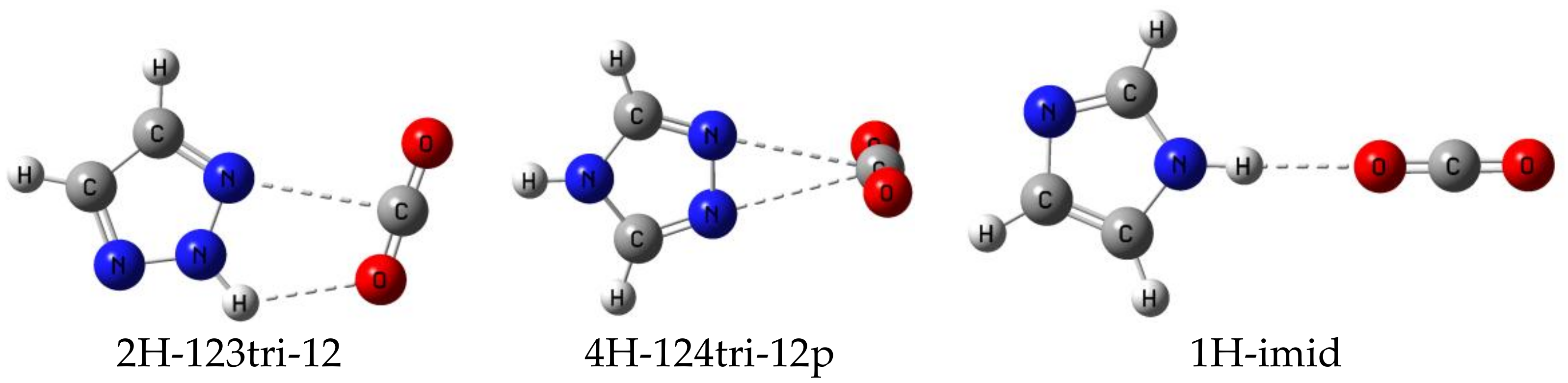
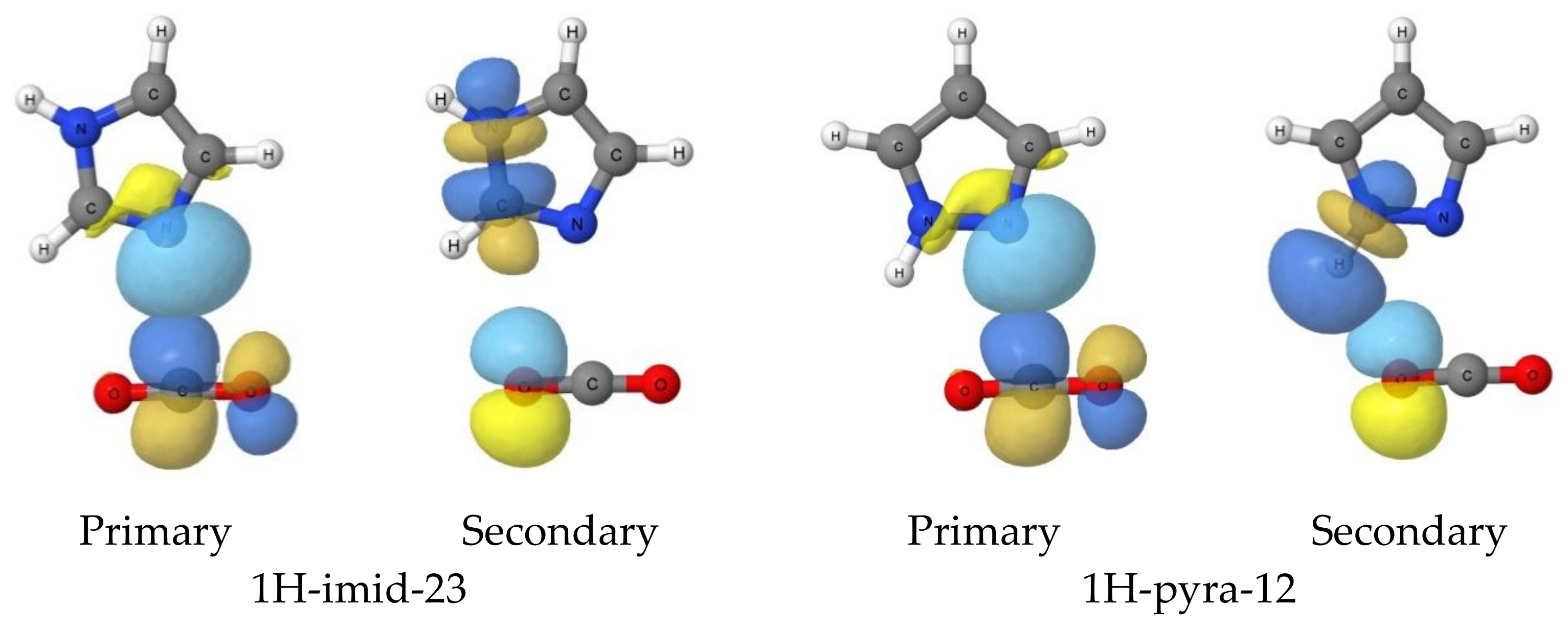
- Those complexes that have an Ny-H group bonded to Nx are stabilized primarily by Nx···C tetrel bonds and by a secondary interaction between Ny-H and O’, which assumes increased importance as the number of N atoms in the ring increases.
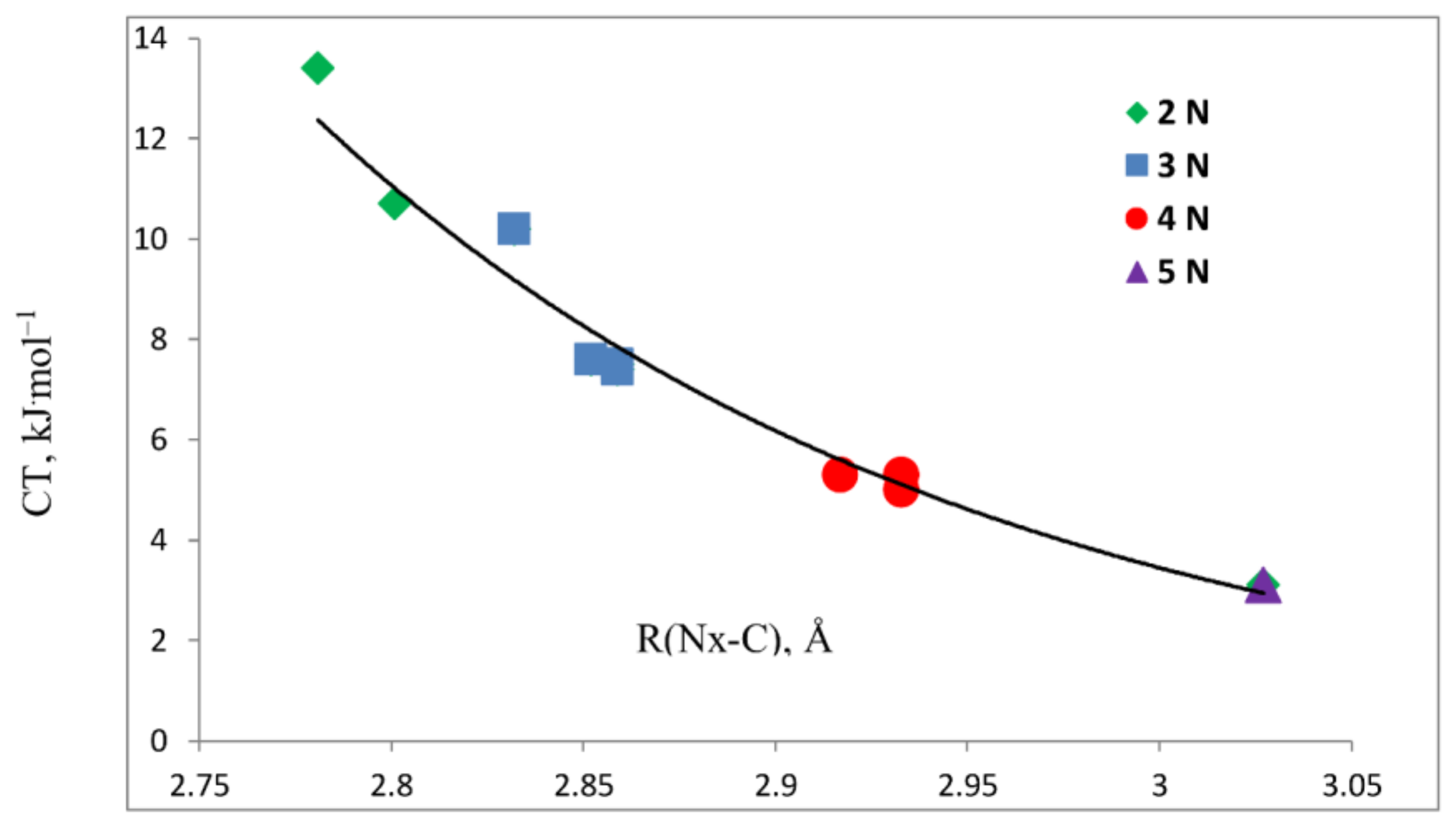
- The binding energies of the planar complexes do not correlate with the Nx-C distance, but the primary charge-transfer energies do.
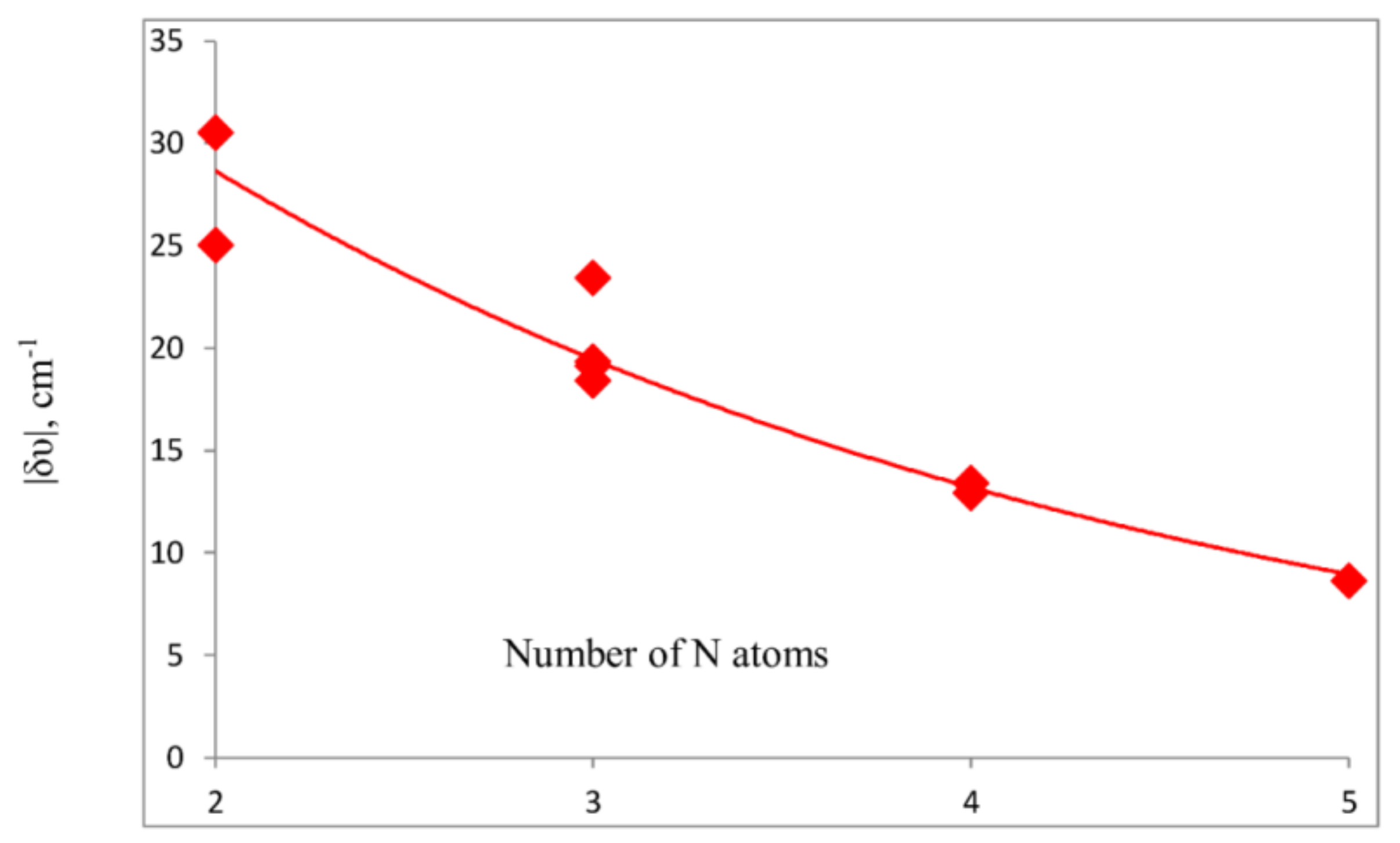
- The IR in-plane bending frequency of CO2 is most sensitive to complex formation. The change in this frequency decreases as the number of N atoms increases. NMR spin-spin coupling constants 1tJ(Nx-C), which are FC dominated, are less than 1 Hz, since there is little s-electron density at C in the direction of the tetrel bond.
- There are seven perpendicular tetrel-bonded complexes that arise when there are two adjacent N atoms in the ring, and each has a lone pair of electrons.
- The binding energies of perpendicular complexes decrease as the number of nitrogen atoms in the ring decreases.
- The IR bending mode of CO2 that moves the C atom toward and away from the Nx-Ny bond is most sensitive to complex formation. The change in its frequency upon complex formation decreases in the order triazole > tetrazole > pentazole.
- The NMR coupling constants 1tJ(Nx-C) and 1tJ(Ny-C) are negligibly small since there is a node at C in the complex symmetry plane.
- The four hydrogen-bonded complexes involve pyrrole, imidazole, 1,2,4-triazole and pentazole. Three of these form when the ring Nz-H is bonded to two C-H groups, thereby eliminating the possibility of tetrel-bond formation. The fourth forms with pentazole, which is the strongest acid.
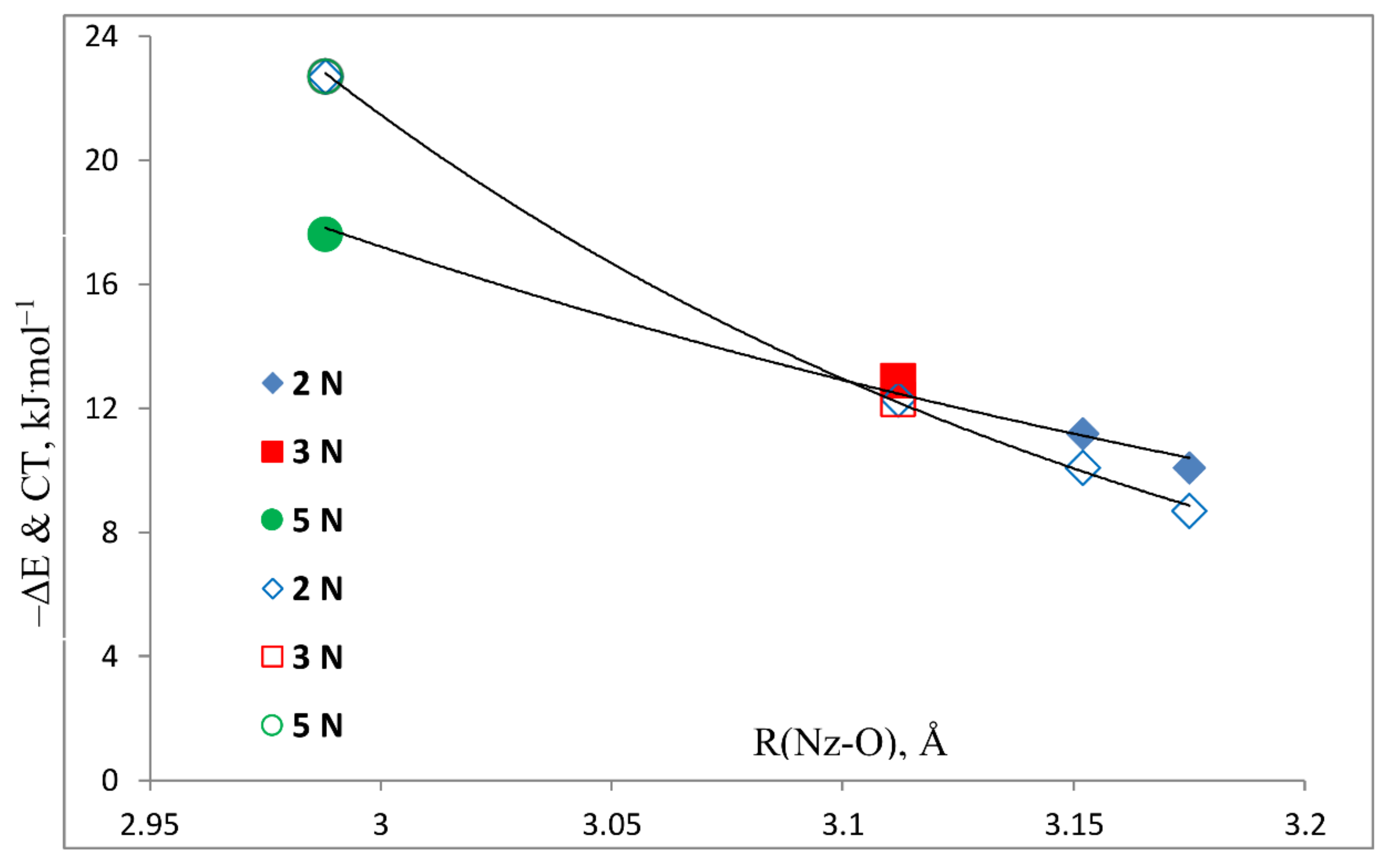
- The binding energies of these complexes and their charge-transfer energies increase as the number of N atoms in the ring increases.
- Hydrogen bonding produces a red-shift of the IR Nz-H stretching band. The magnitude of the red shift increases as the number of N atoms in the ring increases.
- The NMR coupling constant 2hJ(Nz-O) across the hydrogen bond increases as the number of nitrogen atoms in the ring increases.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Supuran, C.T.; Scozzafava, A.; Conway, J. (Eds.) Carbonic Anhydrase: Its Inhibitors and Activators; CRC Press Book: Boca Raton, FL, USA, 2004. [Google Scholar]
- Frost, S.C.; McKenna, R. (Eds.) Carbonic Anhydrase: Mechanism, Regulation, Links to Disease, and Industrial Applications; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Vedani, A.; Dunitz, J.D. Lone-Pair Directionality in Hydrogen Bond Potential Functions for Molecular Mechanics Calculations: The Inhibition of Human Carbonic Anhydrase II by Sulfonamides. J. Am. Chem. Soc. 1985, 107, 7653–7658. [Google Scholar] [CrossRef]
- Eriksoon, A.E.; Jones, T.A.; Liljas, A. Refined Structure of Human Carbonic Anhydrase II at 2.0 Å Resolution. Proteins 1988, 4, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Liljas, A.; Hakansoon, K.; Jonsson, B.H.; Xue, Y. Inhibition and Catalysis of Carbonic Anhydrase. Recent Crystallographic Analyses. Eur. J. Biochem. 1994, 219, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, L.L.; Paterno, S.A.; Fierke, C.A. Hydrogen Bond Network in the Metal Binding Site of Carbonic Anhydrase Enhances Zinc Affinity and Catalytic Efficiency. J. Am. Chem. Soc. 1995, 117, 6831–6837. [Google Scholar] [CrossRef]
- Thoms, S. Hydrogen Bonds and the Catalytic Mechanism of Human Carbonic Anhydrase II. J. Theor. Biol. 2002, 215, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Vullo, D.; Manole, G.; Casini, A.; Scozzafava, A. Designing of Novel Carbonic Anhydrase Inhibitors and Activators. Curr. Med. Chem. 2004, 2, 49–68. [Google Scholar] [CrossRef]
- Domsic, J.F.; Avvaru, B.S.; Kim, C.U.; Gruner, S.M.; Agbandje-McKenna, M.; Silverman, D.N.; McKenna, R. Entrapment of Carbon Dioxide in the Active Site of Carbonic Anhydrase II. J. Biol. Chem. 2008, 283, 30766–30771. [Google Scholar] [CrossRef] [PubMed]
- Sjöblom, B.; Polentarutti, M.; Djinović-Carugo, K. Structural Study of X-ray Induced Activation of Carbonic Anhydrase. PNAS 2009, 106, 10609–10613. [Google Scholar] [CrossRef] [PubMed]
- Avvaru, B.S.; Kim, C.U.; Sippel, K.H.; Gruner, S.M.; Agbandje-McKenna, M.; Silverman, D.N.; McKenna, R. A Short, Strong Hydrogen Bond in the Active Site of Human Carbonic Anhydrase II. Biochemistry 2010, 49, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Alberti, G.; Bertini, I.; Luchinat, C.; Scozzafava, A. A New Class of Inhibitors Capable of Binding Both the Acidic and Alkaline Forms of Carbonic Anhydrase. Biochim. Biophys. Acta Protein Struct. Mol. 1981, 668, 16–26. [Google Scholar] [CrossRef]
- Ilies, M.; Banciu, M.D.; Ilies, M.A.; Scozzafava, A.; Caproiu, M.T.; Supuran, C.T. Carbonic Anhydrase Activators: Design of High Affinity Isozymes I, II, and IV Activators, Incorporating Tri-/Tetrasubstituted-Pyridinium-Azole Moieties. J. Med. Chem. 2002, 45, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Oezguen, N.; Urvil, P.; Ferguson, C.; Dann, S.M.; Savidge, T.C. Regulation of Protein-Ligand Binding Affinity by Hydrogen Bond Pairing. Sci. Adv. 2016, 2, e1501240. [Google Scholar] [CrossRef] [PubMed]
- Schofield, K.; Grimmett, M.R.; Keene, B.R.T. The Azoles; Cambridge University Press: Cambridge, UK, 1976. [Google Scholar]
- Mangani, S.; Liljas, A. Crystal Structure of the Complex Between Human Carbonic Anhydrase II and the Aromatic Inhibitor 1,2,4-Triazole. J. Mol. Biol. 1993, 232, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Chandra, S.; Garai, B.; Banerjee, R. Carbon Dioxide Capture by Metal Organic Frameworks. Indian J. Chem. 2012, 51A, 1223–1230. [Google Scholar]
- Seth, S.; Savitha, G.; Moorthy, J.N. Carbon Dioxide Capture by a Metal–Organic Framework with Nitrogen-Rich Channels Based on Rationally Designed Triazole-Functionalized Tetraacid Organic Linker. Inorg. Chem. 2015, 54, 6829–6835. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-Z.; Wang, X.-J.; Liu, J.; Lim, J.S.; Zou, R.; Zhao, Y. A Triazole-Containing Metal–Organic Framework as a Highly Effective and Substrate Size-Dependent Catalyst for CO2 Conversion. J. Am. Chem. Soc. 2016, 138, 2142–2145. [Google Scholar] [CrossRef] [PubMed]
- Panda, T.; Pachfule, P.; Chen, Y.; Jiang, J.; Banerjee, R. Amino Functionalized Zeolitic Tetrazolate Framework (Ztf) with High Capacity for Storage of Carbon Dioxide. Chem. Commun. 2011, 47, 2011–2013. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Park, H.B.; Robertson, G.P.; Dal-Cin, M.M.; Visser, T.; Scoles, L.; Guiver, M.D. Polymer Nanosieve Membranes for CO2-Capture Applications. Nat. Mater. 2011, 10, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Kenarsari, S.D.; Yang, D.; Jiang, G.; Zhang, S.; Wang, J.; Russell, A.G.; Wei, Q.; Fan, M. Review of Recent Advances in Carbon Dioxide Separation and Capture. RSC Adv. 2013, 3, 22739–22773. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Del Bene, J.E. Azines as Electron-Pair Donors to CO2 for N···C Tetrel Bonds. J. Phys. Chem. A 2017, 121, 8017–8025. [Google Scholar] [CrossRef] [PubMed]
- Vaidhyanathan, R.; Iremonger, S.S.; Shimizu, G.K.H.; Boyd, P.G.; Alavi, S.; Woo, T.K. Direct Observation and Quantification of CO2 Binding within an Amine-Functionalized Nanoporous Solid. Science 2010, 330, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.-Q.; Zhang, W.-X.; Zhang, J.-P.; Chen, X.-M. Efficient Purification of Ethene by an Ethane-Trapping Metal-Organic Framework. Nat. Commun. 2015, 6, 8697. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.-Q.; Zhou, D.-D.; Zhu, A.-X.; Jiang, L.; Lin, R.-B.; Zhang, J.-P.; Chen, X.-M. Strong and Dynamic CO2 Sorption in a Flexible Porous Framework Possessing Guest Chelating Claws. J. Am. Chem. Soc. 2012, 134, 17380–17383. [Google Scholar] [CrossRef] [PubMed]
- Theuergarten, E.; Schlosser, J.; Schluns, D.; Freytag, M.; Daniliuc, C.G.; Jones, P.G.; Tamm, M. Fixation of Carbon Dioxide and Related Small Molecules by a Bifunctional Frustrated Pyrazolylborane Lewis Pair. Dalton Trans. 2012, 41, 9101–9110. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzis, K.D.; Mavrandonakis, A.; Klopper, W.; Froudakis, G.E. Ab Initio Study of the Interactions between CO2 and N-Containing Organic Heterocycles. ChemPhysChem 2009, 10, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Prakash, M.; Mathivon, K.; Benoit, D.M.; Chambaud, G.; Hochlaf, M. Carbon Dioxide Interaction with Isolated Imidazole or Attached on Gold Clusters and Surface: Competition between σ H-Bond and π-Stacking Interaction. PCCP 2014, 16, 12503–12509. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Marín, E.; Lemus-Santana, A.A. Theoretical Study of the Formation of Complexes between CO2 and Nitrogen Heterocycles. J. Mex. Chem. Soc. 2015, 59, 36–42. [Google Scholar]
- Vidal-Vidal, Á.; Faza, O.N.; Silva López, C. CO2 Complexes with Five-Membered Heterocycles: Structure, Topology, and Spectroscopic Characterization. J. Phys. Chem. A 2017, 121, 9118–9130. [Google Scholar] [CrossRef] [PubMed]
- Pople, J.A.; Binkley, J.S.; Seeger, R. Theoretical Models Incorporating Electron Correlation. Int. J. Quantum Chem. Quantum Chem. Symp. 1976, 10, 1–19. [Google Scholar] [CrossRef]
- Krishnan, R.; Pople, J.A. Approximate Fourth-Order Perturbation Theory of the Electron Correlation Energy. Int. J. Quantum Chem. 1978, 14, 91–100. [Google Scholar] [CrossRef]
- Bartlett, R.J.; Silver, D.M. Many−Body Perturbation Theory Applied to Electron Pair Correlation Energies. I. Closed-Shell First Row Diatomic Hydrides. J. Chem. Phys. 1975, 62, 3258–3268. [Google Scholar] [CrossRef]
- Bartlett, R.J.; Purvis, G.D. Many-Body Perturbation Theory, Coupled-Pair Many-Electron Theory, and the Importance of Quadruple Excitations for the Correlation Problem. Int. J. Quantum Chem. 1978, 14, 561–581. [Google Scholar] [CrossRef]
- Del Bene, J.E. Proton Affinities of Ammonia, Water, and Hydrogen Fluoride and Their Anions: A Quest for the Basis-Set Limit Using the Dunning Augmented Correlation-Consistent Basis Sets. J. Phys. Chem. 1993, 97, 107–110. [Google Scholar] [CrossRef]
- Dunning, T.H. Gaussian Basis Sets for Use in Correlated Molecular Calculations. I. The Atoms Boron through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H. Gaussian Basis Sets for Use in Correlated Molecular Calculations. V. Core-Valence Basis Sets for Boron Through Neon. J. Chem. Phys. 1995, 103, 4572–4585. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian09; revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Bader, R.F.W. A Quantum Theory of Molecular Structure and its Applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Popelier, P.L.A. Atoms in Molecules: An Introduction; Prentice Hall: Harlow, UK, 2000. [Google Scholar]
- Matta, C.F.; Boyd, R.J. The Quantum Theory of Atoms in Molecules: From Solid State to DNA and Drug Design; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Keith, T.A. AIMAll; Version 17.11.14; TK Gristmill Software: Overland Park, KS, USA, 2017; Available online: aim.tkgristmill.com (accessed on 1 March 2018).
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular Interactions from a Natural Bond Orbital, Donor–Acceptor Viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Landis, C.R.; Weinhold, F. NBO 6.0; University of Wisconsin: Madison, WI, USA, 2013. [Google Scholar]
- Perera, S.A.; Nooijen, M.; Bartlett, R.J. Electron Correlation Effects on the Theoretical Calculation of Nuclear Magnetic Resonance Spin-Spin Coupling Constants. J. Chem. Phys. 1996, 104, 3290–3305. [Google Scholar] [CrossRef]
- Perera, S.A.; Sekino, H.; Bartlett, R.J. Coupled-Cluster Calculations of Indirect Nuclear Coupling Constants: The Importance of Non-Fermi Contact Contributions. J. Chem. Phys. 1994, 101, 2186–2196. [Google Scholar] [CrossRef]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully Optimized Contracted Gaussian Basis Sets for Atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Stanton, J.F.; Gauss, J.; Watts, J.D.; Nooijen, M.; Oliphant, N.; Perera, S.A.; Szalay, P.S.; Lauderdale, W.J.; Gwaltney, S.R.; Beck, S.; et al. ACES II; University of Florida: Gainesville, FL, USA, 1991. [Google Scholar]
- Catalan, J.; Elguero, J. Basicity and Acidity of Azoles. Adv. Heterocycl. Chem. 1987, 41, 187–274. [Google Scholar]

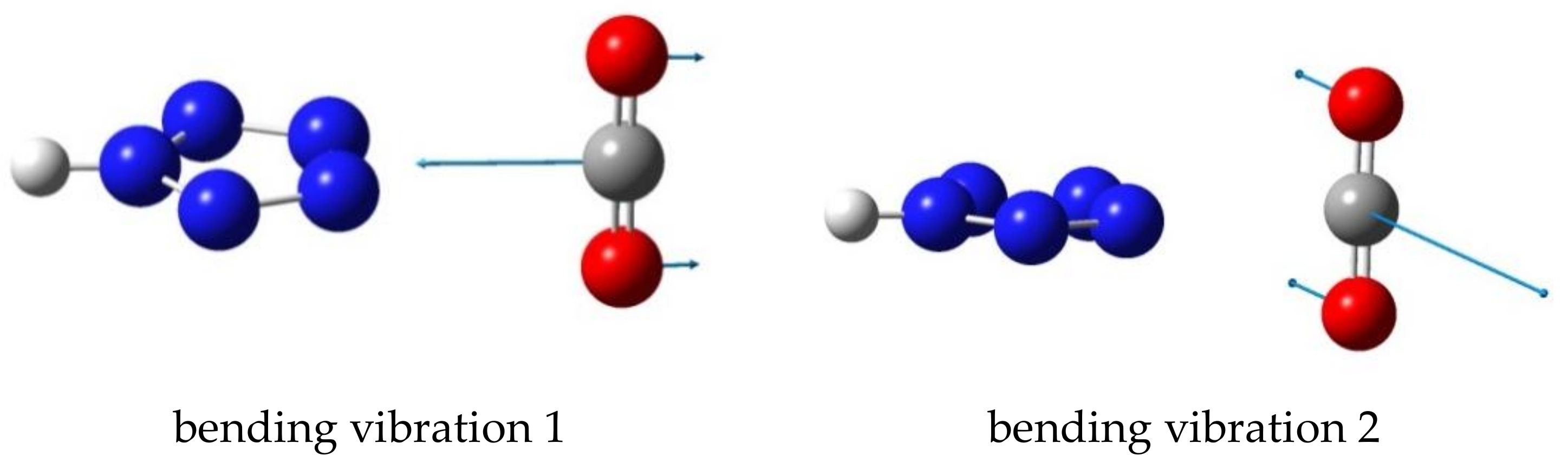
| Azole | Complex | −ΔE | Sym |
|---|---|---|---|
| pyrrole | 1H-pyrr | 10.1 | C2v |
| pyrazole | 1H-pyra-12 | 22.7 | Cs |
| imidazole | 1H-imid-23 | 19.9 | Cs |
| 1H-imid | 11.2 | Cs | |
| triazoles | 1H-123tri-12 | 21.8 | Cs |
| 2H-123tri-12 | 20.3 | Cs | |
| 1H-123tri-23p | 15.8 | Cs | |
| 1H-124tri-12 | 21.0 | Cs | |
| 4H-124tri-12p | 18.1 | C2v | |
| 1H-124tri-45 | 17.9 | Cs | |
| 4H-124tri | 12.9 | C2v | |
| tetrazoles | 1H-tet-12 | 20.3 | Cs |
| 2H-tet-23 | 19.8 | Cs | |
| 2H-tet-12 | 18.9 | Cs | |
| 1H-tet-34p | 15.1 | Cs | |
| 2H-tet-34p | 13.7 | Cs | |
| 1H-tet-23p | 13.3 | Cs | |
| pentazole | 1H-pent-12 | 19.0 | Cs |
| 1H-pent | 17.6 | C2v | |
| 1H-pent-34p | 12.2 | C2v | |
| 1H-pent-23p | 11.2 | Cs |
| Azole | Complex | −ΔE | R(Nx-C) | R(Ny-O’); R(Cy-O’) a | R(NyH-O’); R(CyH-O’) a,b | Primary CT c | Secondary CT d |
|---|---|---|---|---|---|---|---|
| pyrazole | 1H-pyra-12 | 22.7 | N2: 2.801 | N1: 2.939 | 2.285 | 10.7 | 2.0 |
| imidazole | 1H-imid-23 | 19.9 | N3: 2.781 | C2: 3.171 | 2.732 | 13.4 | 1.0 d |
| triazoles | 1H-123tri-12 | 21.8 | N2: 2.852 | N1: 2.918 | 2.250 | 7.6 | 6.1 |
| 2H-123tri-12 | 20.3 | N1: 2.859 | N2: 2.936 | 2.298 | 7.5 | 2.1 | |
| 1H-124tri-12 | 21.0 | N2: 2.859 | N1: 2.933 | 2.275 | 7.4 | 2.3 | |
| 1H-124tri-45 | 17.9 | N4: 2.832 | C5: 3.156 | 2.707 | 10.2 | 1.1 e | |
| tetrazoles | 1H-tet-12 | 20.3 | N2: 2.933 | N1: 2.094 | 2.222 | 5.0 | 4.5 |
| 2H-tet-23 | 19.8 | N3: 2.917 | N2: 2.904 | 2.252 | 5.3 | 6.3 | |
| 2H-tet-12 | 18.9 | N1: 2.933 | N2: 2.917 | 2.264 | 5.3 | 2.6 | |
| pentazole | 1H-pent-12 | 19.0 | N2: 3.027 | N1: 2.878 | 2.197 | 3.1 | 4.7 |
| Azole | Complex | ν a | δν | 1tJ(Nx-C) | J(Ny-O’) |
|---|---|---|---|---|---|
| pyrazole | 1H-pyra-12 | 634 | −25.0 | 0.5 | 0.8 |
| imidazole | 1H-imid-23 | 628 | −30.5 | 0.6 | |
| triazoles | 1H-123tri-12 | 640 | −19.1 | 0.4 | 0.9 |
| 2H-123tri-12 | 641 | −18.4 | 0.3 | 1.0 | |
| 1H-124tri-12 | 640 | −19.3 | 0.3 | 0.9 | |
| 1H-124tri-45 | 636 | −23.4 | 0.4 | ||
| tetrazoles | 1H-tet-12 | 646 | −13.4 | 0.2 | 1.1 |
| 2H-tet-23 | 646 | −13.4 | 0.2 | 1.2 | |
| 2H-tet-12 | 646 | −12.9 | 0.1 | 1.1 | |
| pentazoles | 1H-pent-12 | 650 | −8.6 | 0.0 | 1.5 |
| Azole | Complex | −ΔE | R(Nx-C); R(Ny-C) a | CT b | υ c,d |
|---|---|---|---|---|---|
| triazoles | 1H-123tri-23p | 15.8 | N2: 2.994; N3: 2.938 | 4.4 | 645 |
| 4H-124tri-12p | 18.1 | N1: 2.922; N2: 2.922 | 5.6 | 644 | |
| tetrazoles | 1H-tet-34p | 15.1 | N3: 2.959; N4: 2.981 | 4.9 | 648 |
| 2H-tet-34p | 13.7 | N3: 3.021; N4: 2.967 | 4.2 | 647 | |
| 1H-tet-23p | 13.3 | N2: 3.088; N3:2.954 | 4.1 | 647 | |
| pentazoles | 1H-pent-34p | 12.2 | N3: 3.016; N4: 3.016 | 3.0 | 651 |
| 1H-pent-23p | 11.2 | N2: 3.113; N3: 2.939 | 2.5 | 650 |
| Azole | Complex | −ΔE | R(Nz-O) | Olp→σ*Nz-H | 2hJ(Nz-O) | R(Nz-H) | Δν(Nz-H) |
|---|---|---|---|---|---|---|---|
| pyrrole | 1H-pyrr | 10.1 | 3.175 | 8.7 | 2.1 | 1.006 | −5.1 |
| imidazole | 1H-imid | 11.2 | 3.152 | 10.1 | 2.2 | 1.007 | −8.8 |
| 124-triazole | 4H-124tri | 12.9 | 3.112 | 12.3 | 2.6 | 1.008 | −17.1 |
| pentazole | 1H-pent | 17.6 | 2.988 | 22.7 | 4.6 | 1.014 | −47.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Bene, J.E.; Elguero, J.; Alkorta, I. Complexes of CO2 with the Azoles: Tetrel Bonds, Hydrogen Bonds and Other Secondary Interactions. Molecules 2018, 23, 906. https://doi.org/10.3390/molecules23040906
Del Bene JE, Elguero J, Alkorta I. Complexes of CO2 with the Azoles: Tetrel Bonds, Hydrogen Bonds and Other Secondary Interactions. Molecules. 2018; 23(4):906. https://doi.org/10.3390/molecules23040906
Chicago/Turabian StyleDel Bene, Janet E., José Elguero, and Ibon Alkorta. 2018. "Complexes of CO2 with the Azoles: Tetrel Bonds, Hydrogen Bonds and Other Secondary Interactions" Molecules 23, no. 4: 906. https://doi.org/10.3390/molecules23040906
APA StyleDel Bene, J. E., Elguero, J., & Alkorta, I. (2018). Complexes of CO2 with the Azoles: Tetrel Bonds, Hydrogen Bonds and Other Secondary Interactions. Molecules, 23(4), 906. https://doi.org/10.3390/molecules23040906