Effects of Calcination Holding Time on Properties of Wide Band Gap Willemite Semiconductor Nanoparticles by the Polymer Thermal Treatment Method
Abstract
1. Introduction
2. Results and Discussion
2.1. Thermogravimetric Analysis
2.2. Raman Analysis
2.3. FT–IR Analysis
2.4. X-ray Diffraction Analysis
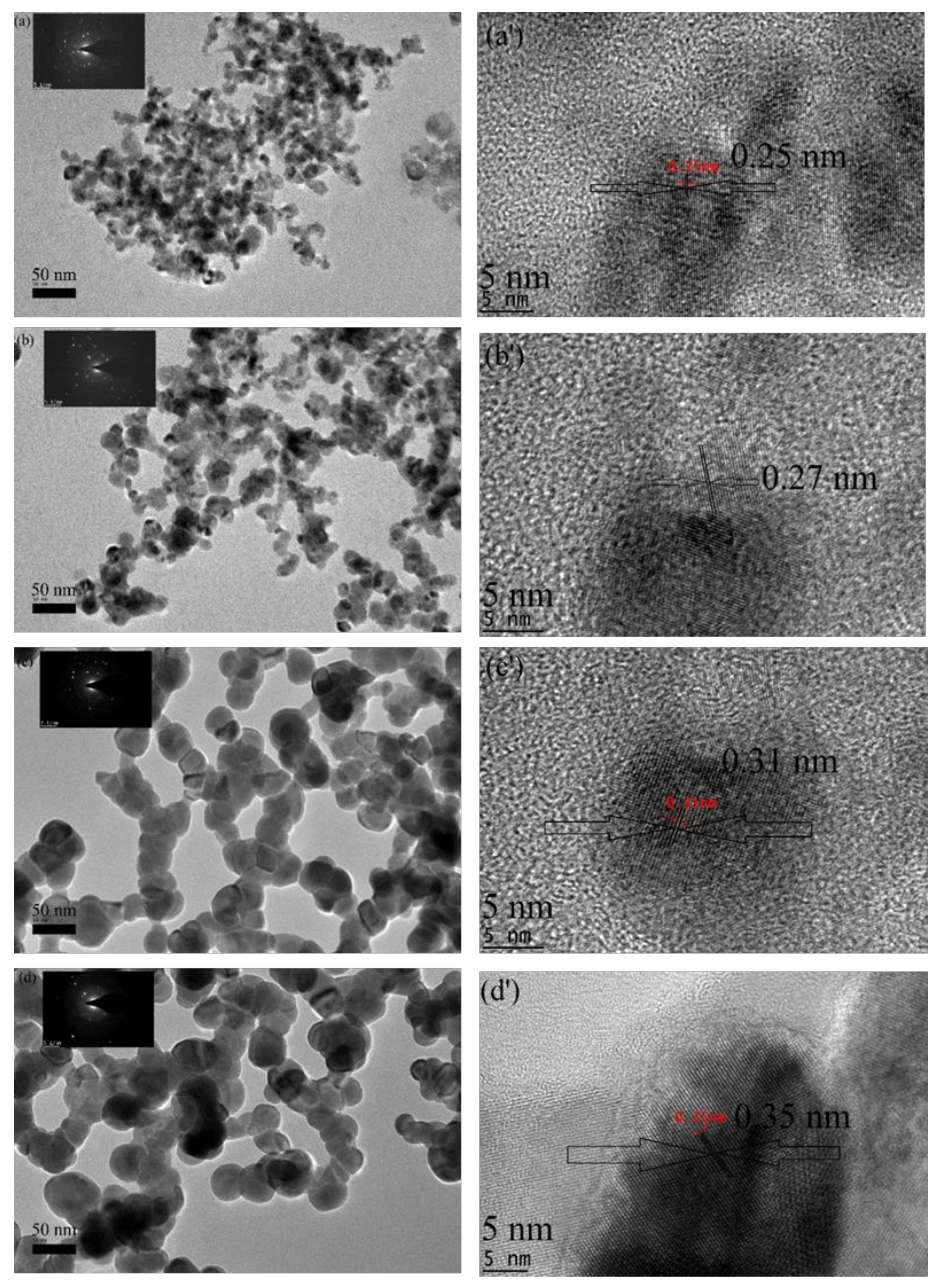
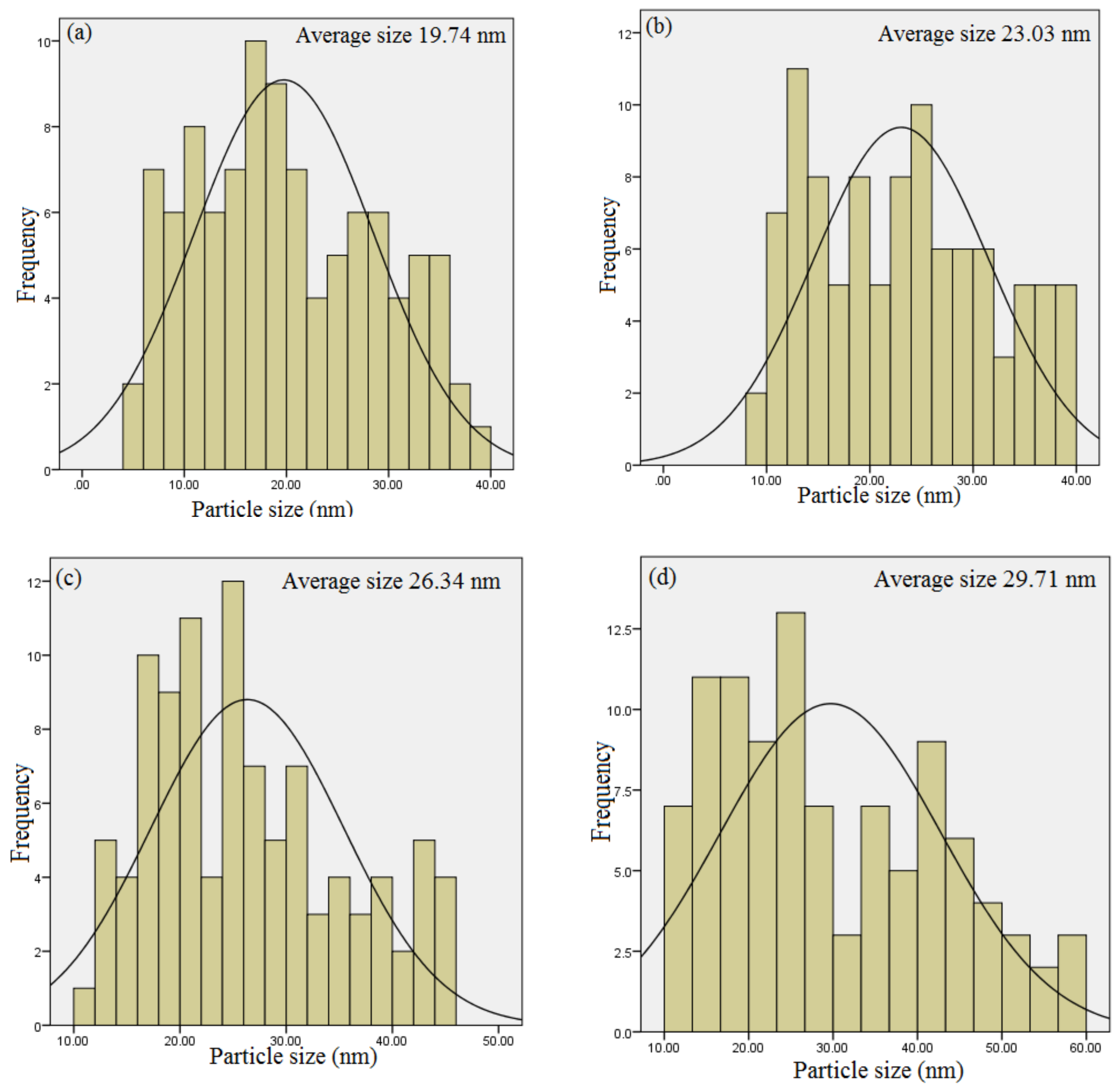
2.5. HR-TEM Analysis
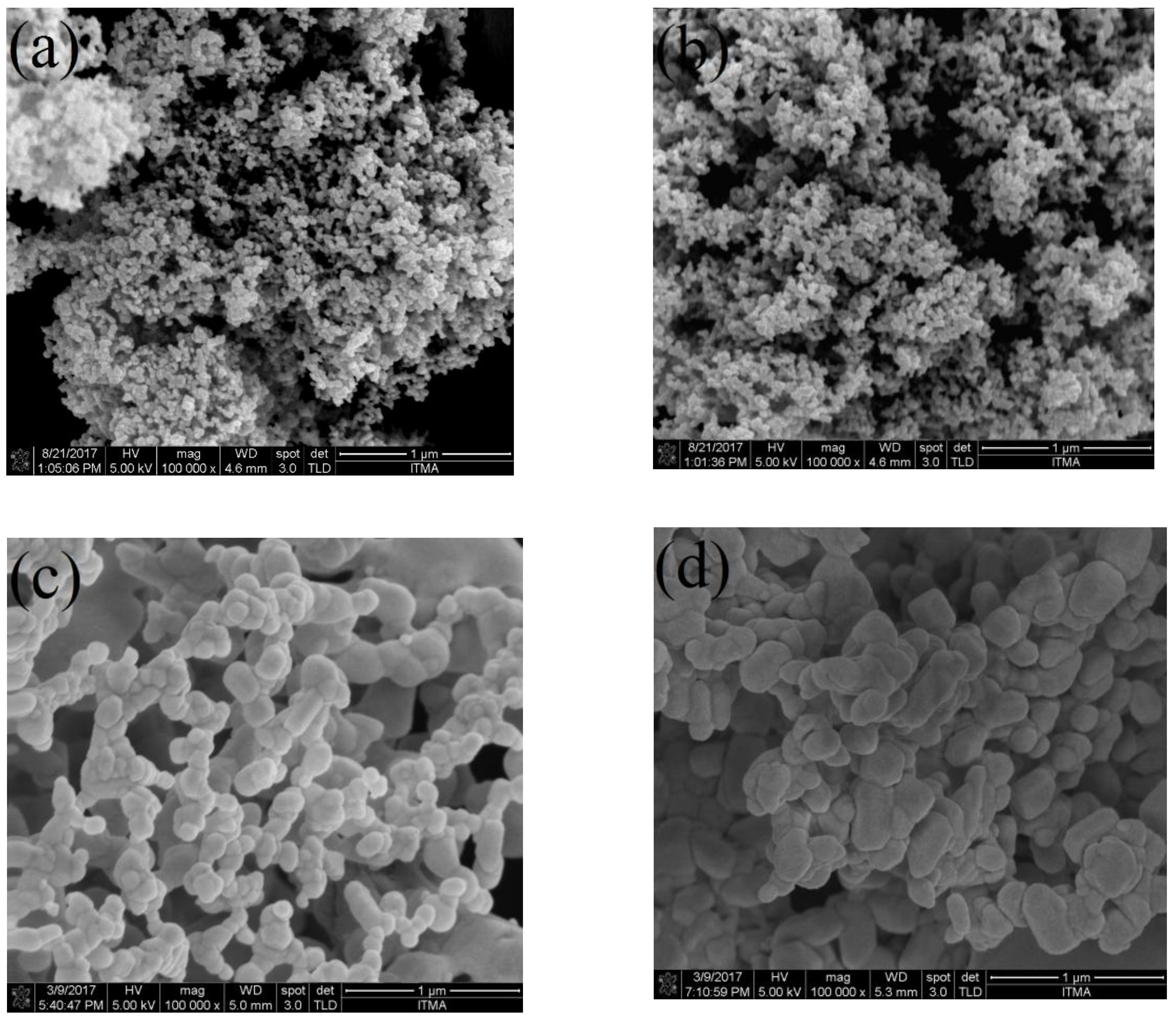
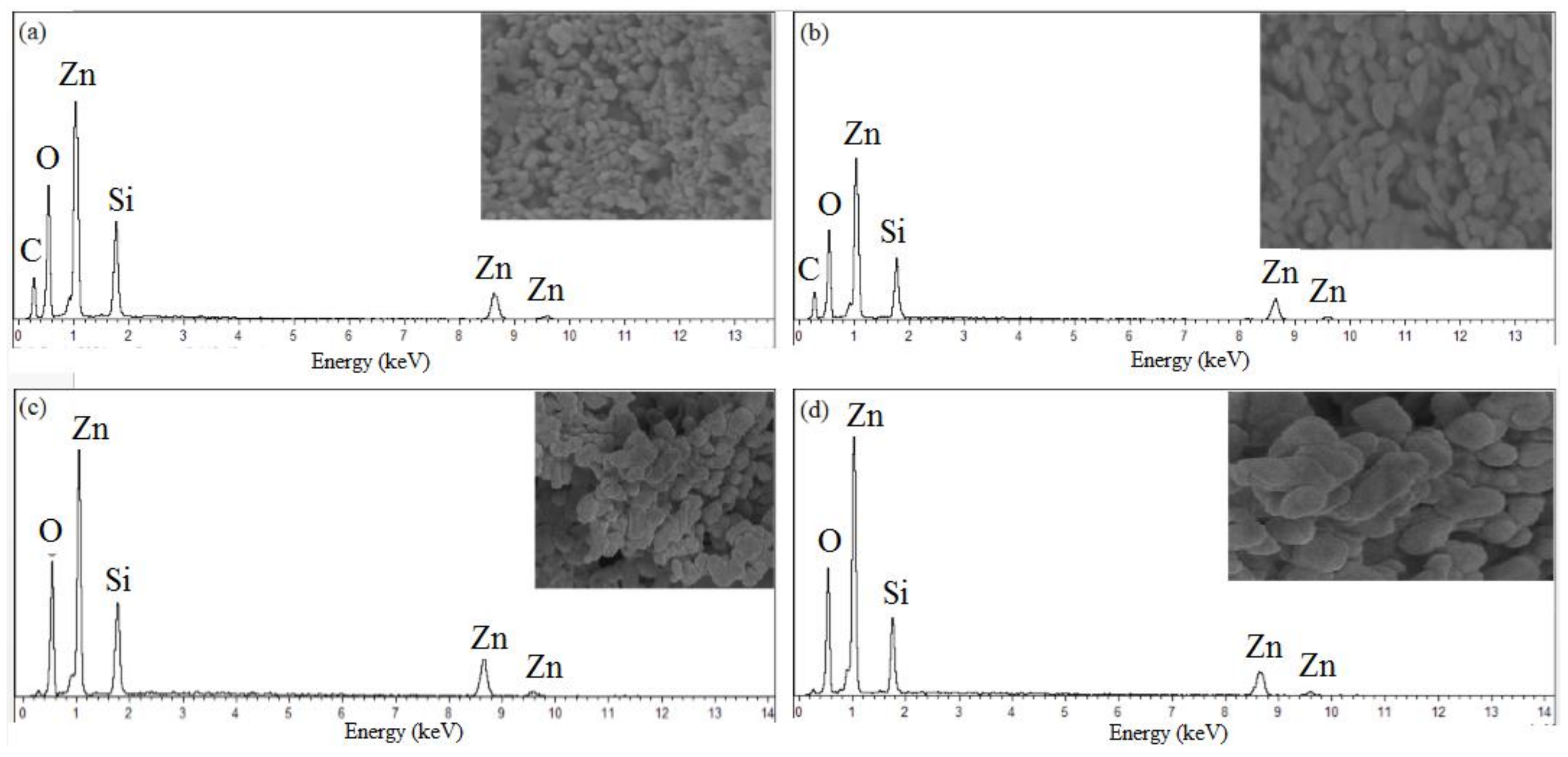
2.6. FESEM-EDX Analysis
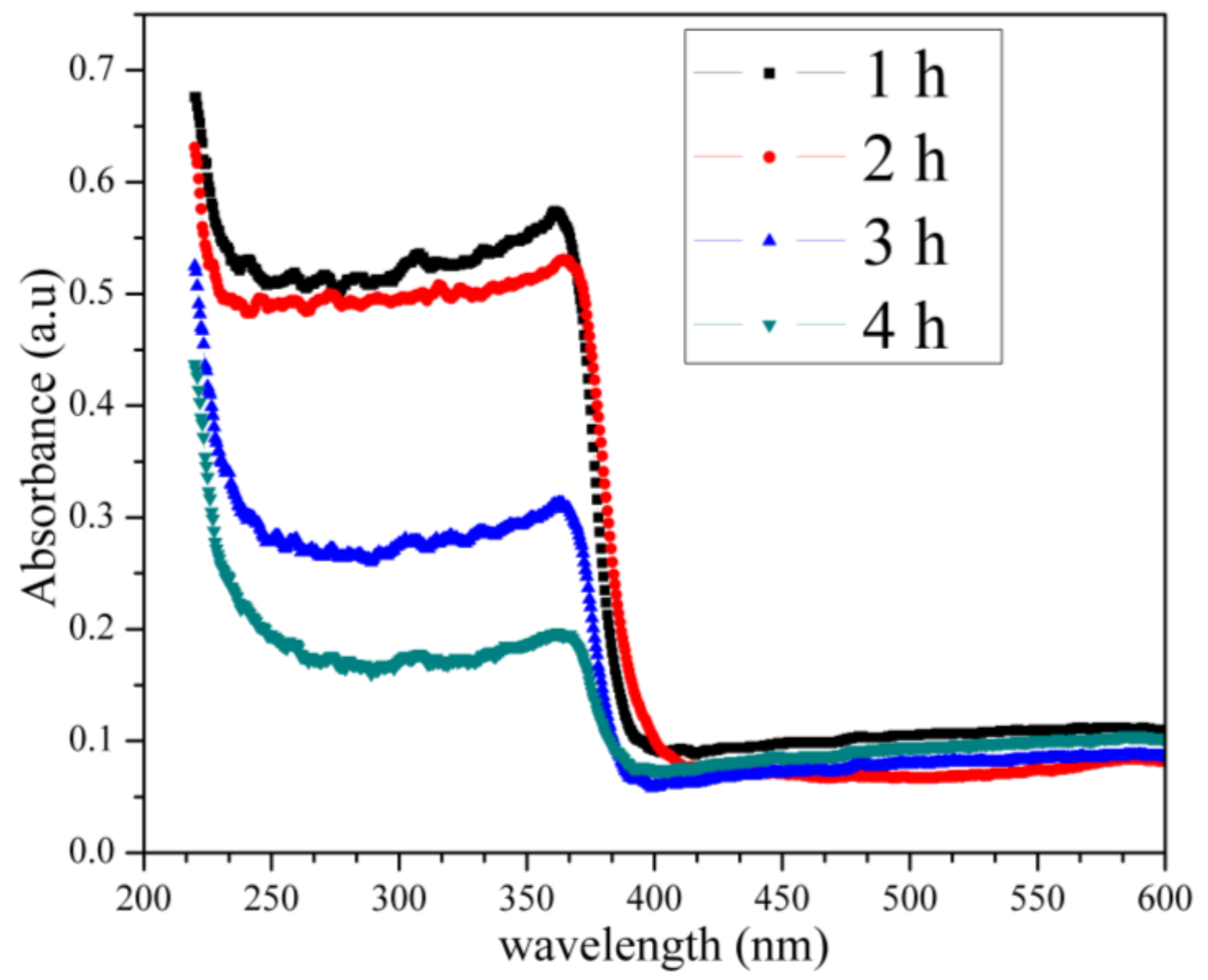
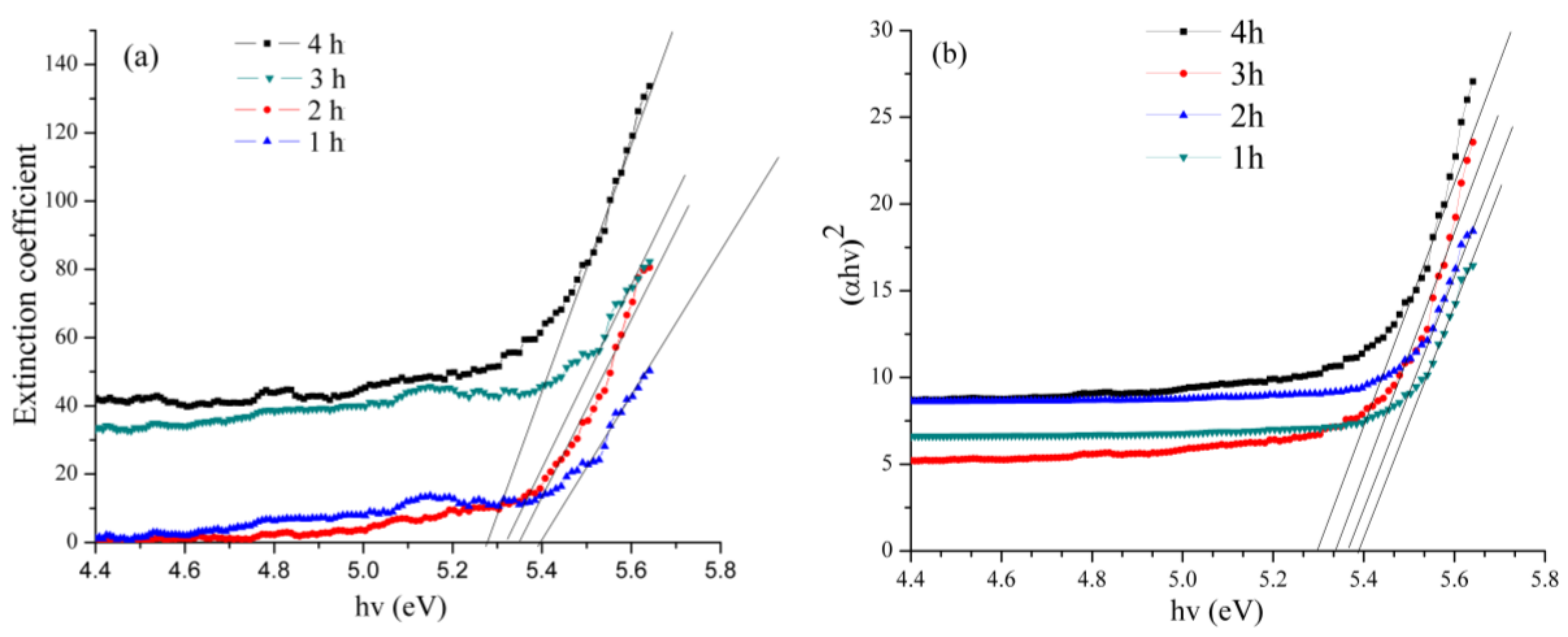
2.7. UV–Vis Analysis
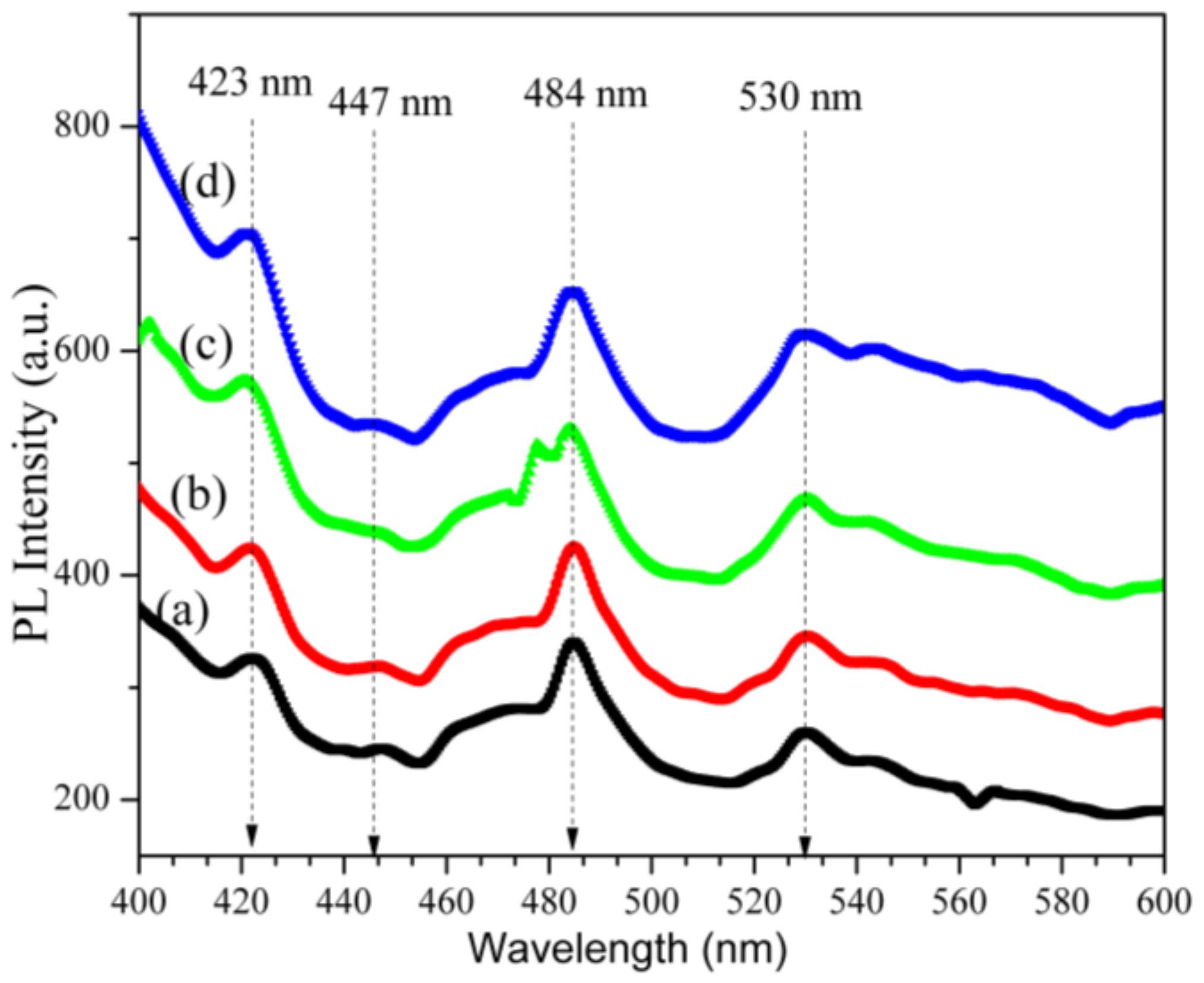
2.8. Photoluminescence Analysis
3. Material and Methods
Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, A.M.; Nie, S. Semiconductor nanocrystals: Structure, properties, and band gap engineering. Acc. Chem. Res. 2009, 43, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, J.; Benfield, R.E.; Ding, Y.; Grandjean, D.; Zhang, Z.; Ju, X. Structure and chemical transformation in cerium oxide nanoparticles coated by surfactant cetyltrimethylammonium bromide (CTAB): An X-ray absorption spectroscopic study. J. Phys. Chem. B 2002, 106, 4569–4577. [Google Scholar] [CrossRef][Green Version]
- Klaska, K.-H.; Eck, J.C.; Pohl, D. New investigation of willemite. Acta Crystallogr. 1978, 34, 3324–3325. [Google Scholar] [CrossRef]
- Chang, H.J.; Park, H.D.K.; Sohn, S.; Lee, J.D. Electronic structure of Zn2SiO4 and Zn2SiO4: Mn. J. Korean Phys. Soc. 1999, 34, 545–548. [Google Scholar]
- Wan, J.; Chen, X.; Wang, Z.; Mu, L.; Qian, Y. One-dimensional rice-like Mn-doped Zn2SiO4: Preparation, characterization, luminescent properties and its stability. J. Cryst. Growth 2005, 280, 239–243. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y. Synthesis and VUV spectral properties of nanoscaled Zn2SiO4:Mn2+ green phosphor. J. Phys. Chem. Solids 2009, 70, 1146–1149. [Google Scholar] [CrossRef]
- Zaid, M.H.M.; Matori, K.A.; Abdul Aziz, S.H.; Kamari, H.M.; Yunus, M.; Mahmood, W.; Abdul Wahab, Z.; Samsudin, N.F. Fabrication and crystallization of ZnO-SLS glass derived willemite glass-ceramics as a potential material for optics applications. J. Spectrosc. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Veremeichik, T.F.; Zharikov, E.V.; Subbotin, K.A. New laser crystals of complex oxides doped with ions of d elements with variable valence and different structural localization. Crystallogr. Rep. 2003, 48, 974–988. [Google Scholar] [CrossRef]
- Vitkin, V.V.; Dymshits, O.S.; Polyakov, V.M.; Zhilin, A.A.; Shemchuk, D.V.; Krylov, A.A.; Uskov, A.V.; Mak, A.A.; Kovalev, A.V. Pulse-burst Er: Glass laser. Proc. SPIE 2017, 10082, 1008224. [Google Scholar] [CrossRef]
- Wang, X.; Huang, H.; Liu, B.; Liang, B.; Zhang, C.; Ji, Q.; Chen, D.; Shen, G. Shape evolution and applications in water purification: The case of CVD-grown Zn2SiO4 straw-bundles. J. Mater. Chem. 2012, 22, 5330–5335. [Google Scholar] [CrossRef]
- Dai, P.; Sun, Y.-Q.; Bao, Z.-W.; Zhu, J.; Wu, M.-Z. Optical and adsorption properties of mesoporous SiO2/Zn2SiO4:Eu3+ hollow nanospheres. IET Micro Nano Lett. 2017, 12, 248–251. [Google Scholar] [CrossRef]
- Zaid, M.H.; Matori, K.A.; Ab Aziz, S.H.; Kamari, H.M.; Wahab, Z.A.; Effendy, N.; Alibe, I.M. Comprehensive study on compositional dependence of optical band gap in zinc soda lime silica glass system for optoelectronic applications. J. Non-Cryst. Solids 2016, 449, 107–112. [Google Scholar] [CrossRef]
- Effendy, N.; Wahab, Z.A.; Abdul Aziz, S.H.; Matori, K.A.; Zaid, M.H.M.; Rashid, S.S.A. Characterization and optical properties of erbium oxide doped ZnO-SLS glass for potential optical and optoelectronic materials. Mater. Express 2017, 7, 59–65. [Google Scholar] [CrossRef]
- Romanov, S.G.; Fokin, A.V.; De La Rue, R.M. Eu3+ emission in an anisotropic photonic band gap environment. Appl. Phys. Lett. 2000, 76, 1656–1658. [Google Scholar] [CrossRef]
- Tarafder, A.; Molla, A.R.; Karmakar, B. Advanced Glass-Ceramic Nanocomposites for Structural, Photonic, and Optoelectronic Applications. Glass Nanocompos. 2016, 299–338. [Google Scholar] [CrossRef]
- Omri, K.; Lemine, O.M.; El Mir, L. Mn doped zinc silicate nanophosphor with bifunctionality of green-yellow emission and magnetic properties. Ceram. Int. 2017, 43, 6585–6591. [Google Scholar] [CrossRef]
- Babu, B.C.; Wang, G.-G.; Yan, B.; Yang, Q.; Baker, A.P. Effects of Cr3+ addition on the structure and optical properties of α-Zn2SiO4 synthesized by sol-gel method. Ceram. Int. 2018, 938–946. [Google Scholar] [CrossRef]
- El Ghoul, J. Green and yellow luminescence properties of Willemite Zn2SiO4 nanocomposites by sol-gel method. J. Mater. Sci. Mater. Electron. 2018, 29, 2999–3005. [Google Scholar] [CrossRef]
- Zaid, M.H.M.; Matori, K.A.; Aziz, S.H.A.; Kamari, H.M.; Wahab, Z.A.; Fen, Y.W.; Alibe, I.M. Synthesis and characterization of low cost willemite based glass-ceramic for opto-electronic applications. J. Mater. Sci. Mater. Electron. 2016, 27, 11158–11167. [Google Scholar] [CrossRef]
- Onufrieva, T.A.; Krasnenko, T.I.; Zaitseva, N.A.; Samigullina, R.F.; Enyashin, A.N. Concentration growth of luminescence intensity of phosphor Zn2-2xMn2xSiO4 (x ≤ 0.13): Crystal-chemical and quantum-mechanical justification. Mater. Res. Bull. 2018, 97, 182–188. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, Z.; Tait, W.R.; Pokhrel, M.; Mao, Y.; Liu, J.; Zhang, L.; Wang, W.; Sun, L. Synthesis of green phosphors from highly active amorphous silica derived from rice husks. J. Mater. Sci. 2018, 53, 1824–1832. [Google Scholar] [CrossRef]
- Zaitseva, N.A.; Onufrieva, T.A.; Barykina, J.A.; Krasnenko, T.I.; Zabolotskaya, E.V.; Samigullina, R.F. Magnetic properties and oxidation states of manganese ions in doped phosphor Zn2SiO4:Mn. Mater. Chem. Phys. 2018. [Google Scholar] [CrossRef]
- Dang, L.; Tian, C.; Zhao, S.; Lu, Q. Barium and manganese-doped zinc silicate rods prepared by mesoporous template route and their luminescence property. J. Cryst. Growth 2018, 419, 126–131. [Google Scholar] [CrossRef]
- Zaitseva, N.A.; Krasnenko, T.I.; Onufrieva, T.A.; Samigullina, R.F. Hydrothermal synthesis and microstructure of α-Zn2SiO4:V crystal phosphor. Russ. J. Inorg. Chem. 2017, 62, 168–171. [Google Scholar] [CrossRef]
- Rivera-Enríquez, C.E.; Fernández-Osorio, A.; Chávez-Fernández, J. Luminescence properties of α- and β-Zn2SiO4:Mn nanoparticles prepared by a co-precipitation method. J. Alloys Compd. 2016, 688, 775–782. [Google Scholar] [CrossRef]
- Masjedi-Arani, M.; Salavati-Niasari, M. A simple sonochemical approach for synthesis and characterization of Zn2SiO4 nanostructures. Ultrason. Sonochem. 2016, 29, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Lin, W.; Tian, H.; Xu, G.; Wang, L.; Xu, H. Study on Zn2SiO4 Formation Kinetics and Activity Stability of Desulfurization Sorbent. China Pet. Process. Petrochem. Technol. 2015, 17, 6–15. [Google Scholar]
- Kang, Y.C.; Park, S.B. Zn2SiO4:Mn phosphor particles prepared by spray pyrolysis using a filter expansion aerosol generator. Mater. Res. Bull. 2000, 1143–1151. [Google Scholar] [CrossRef]
- Nam, S.-H.; Kim, M.-H.; Lee, J.-Y.; Lee, S.D.; Boo, J.-H. Spray pyrolysis of manganese doped zinc silicate phosphor particles. Funct. Mater. Lett. 2010, 3, 7–100. [Google Scholar] [CrossRef]
- Takesue, M.; Suino, A.; Hakuta, Y.; Hayashi, H.; Smith, R.L., Jr. Formation mechanism and luminescence appearance of Mn-doped zinc silicate particles synthesized in supercritical water. J. Solid State Chem. 2008, 181, 1307–1313. [Google Scholar] [CrossRef]
- Shibuki, K.; Takesue, M.; Aida, T.M.; Watanabe, M.; Hayashi, H.; Smith, R.L. Continuous synthesis of Zn2SiO4:Mn2+ fine particles in supercritical water at temperatures of 400–500 °C and pressures of 30–35 MPa. J. Supercrit. Fluids 2010, 54, 266–271. [Google Scholar] [CrossRef]
- Takeshita, S.; Honda, J.; Isobe, T.; Sawayama, T.; Niikura, S. Size-tunable solvothermal synthesis of Zn2GeO4:Mn2+ nanophosphor in water/diethylene glycol system. Cryst. Growth Des. 2010, 10, 4494–4500. [Google Scholar] [CrossRef]
- Rasdi, N.M.; Fen, Y.W.; Omar, N.A.S.; Zaid, M.H.M. Effects of cobalt doping on structural, morphological, and optical properties of Zn2SiO4 nanophosphors prepared by sol-gel method. Results Phys. 2017, 7, 3820–3825. [Google Scholar] [CrossRef]
- Babu, B.C; Buddhudu, S. Dielectric Properties of Willemite Zn2SiO4 Nano Powders by Sol-gel Method. Phys. Procedia 2013, 49, 128–136. [Google Scholar] [CrossRef]
- Diao, C.-C.; Yang, C.-F.; Wang, R.-L.; Lin, J.-J.; Fu, M.-Y. Prepare high efficiency Mn2+-doped Zn2SiO4 green phosphors in air using nano-particles. J. Lumin. 2011, 131, 915–920. [Google Scholar] [CrossRef]
- Al-Nidawi, A.J.A.; Matori, K.A.; Zakaria, A.; Zaid, M.H.M. Effect of MnO2 doped on physical, structure and optical properties of zinc silicate glasses from waste rice husk ash. Results Phys. 2017, 7, 955–961. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, M.; Li, Y.; Sun, F.; Yang, J.; Wang, S. Synthesis and electrochemical properties of Zn2SiO4 nano/mesorods. Mater. Lett. 2013, 100, 89–92. [Google Scholar] [CrossRef]
- An, J.S.; Noh, J.H.; Cho, I.S.; Roh, H.S.; Kim, J.Y.; Han, H.S.; Hong, K.S. Tailoring the morphology and structure of nanosized Zn2SiO4:Mn2+ phosphors using the hydrothermal method and their luminescence properties. J. Phys. Chem. C 2010, 114, 10330–10335. [Google Scholar] [CrossRef]
- Samigullina, R.F.; Tyutyunnik, A.P.; Gracheva, I.N.; Krasnenko, T.I.; Zaitseva, N.A.; Onufrieva, T.A. Hydrothermal synthesis of α-Zn2SiO4:V phosphor, determination of oxidation states and structural localization of vanadium ions. Mater. Res. Bull. 2017, 87, 27–33. [Google Scholar] [CrossRef]
- Xu, G.-Q.; Liu, J.-Q.; Zheng, Z.-X.; Wu, Y.-C. Low-temperature synthesis and luminescence properties of Zn2SiO4:Mn2+ phosphors. Chin. J. Lumin. 2011, 32, 550–554. [Google Scholar]
- Al-Hada, N.M.; Saion, E.B.; Shaari, A.H.; Kamarudin, M.A.; Flaifel, M.H.; Ahmad, S.H.; Gene, S.A. A facile thermal-treatment route to synthesize ZnO nanosheets and effect of calcination temperature. PLoS ONE 2014, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alibe, I.M.; Matori, K.A.; Saion, E.; Alibe, A.M.; Zaid, M.H.M.; Engku, E.G. A facile synthesis of amorphous silica nanoparticles by simple thermal treatment route. Dig. J. Nanomater. Biostruct. 2016, 11, 1155–1164. [Google Scholar]
- Alibe, I.M.; Matori, K.A.; Saion, E.; Ali, A.M.; Zaid, M.H.M. The Influence of Calcination Temperature on Structural and Optical Properties of ZnO Nanoparticles via Simple Polymer Synthesis Route. Sci. Sinter. 2017, 49, 263–275. [Google Scholar] [CrossRef]
- Naseri, M.G.; Kamari, H.M.; Dehzangi, A.; Kamalianfar, A.; Saion, E.B. Fabrication of a novel chromium-iron oxide (Cr2Fe6O12) nanoparticles by thermal treatment method. J. Magn. Magn. Mater. 2015, 389, 113–119. [Google Scholar] [CrossRef]
- Taylor, L.S.; Langkilde, F.W.; Zografi, G. Fourier transform Raman spectroscopic study of the interaction of water vapor with amorphous polymers. J. Pharm. Sci. 2001, 90, 888–901. [Google Scholar] [CrossRef] [PubMed]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Academic Press: New York, NY, USA, 1991. [Google Scholar]
- Lewis, I.R.; Edwards, H. Handbook of Raman Spectroscopy: From the Research Laboratory to the Process Line; Marcel Deker Inc.: New York, NY, USA, 2001. [Google Scholar]
- Babu, B.C.; Buddhudu, S. Analysis of structural and electrical properties of Ni2+:Zn2SiO4 ceramic powders by sol-gel method. J. Sol-Gel Sci. Technol. 2014, 70, 405–415. [Google Scholar] [CrossRef]
- Babu, B.C.; Rao, B.V.; Ravi, M.; Babu, S. Structural, microstructural, optical, and dielectric properties of Mn2+:Willemite Zn2SiO4 nanocomposites obtained by a sol-gel method. J. Mol. Struct. 2017, 1127, 6–14. [Google Scholar] [CrossRef]
- Al-Hada, N.M.; Saion, E.B.; Shaari, A.H.; Kamarudin, M.A.; Flaifel, M.H.; Ahmad, S.H. A facile thermal-treatment route to synthesize the semiconductor CdO nanoparticles and effect of calcination. Mater. Sci. Semicond. Process. 2017, 26, 460–466. [Google Scholar] [CrossRef]
- Kamari, H.M.; Al-Hada, N.M.; Saion, E.; Shaari, A.H.; Talib, Z.A.; Flaifel, M.H; Ahmed, A.A.A. Calcined solution-based PVP influence on ZnO semiconductor nanoparticle properties. Crystals 2017, 7, 2. [Google Scholar] [CrossRef]
- Baqer, A.A.; Matori, K.A.; Al-Hada, N.M.; Shaari, A.H.; Saion, E.; Chyi, J.L.Y. Effect of polyvinylpyrrolidone on cerium oxide nanoparticle characteristics prepared by a facile heat treatment technique. Results Phys. 2017, 7, 611–619. [Google Scholar] [CrossRef]
- Babu, K.S.; Reddy, A.R.; Reddy, K.V.; Mallika, A.N. High thermal annealing effect on structural and optical properties of ZnO-SiO2 nanocomposite. Mater. Sci. Semicond. Process. 2014, 27, 643–648. [Google Scholar] [CrossRef]
- Babu, K.S.; Reddy, A.R.; Reddy, K.V. Controlling the size and optical properties of ZnO nanoparticles by capping with SiO2. Mater. Res. Bull. 2014, 49, 537–543. [Google Scholar] [CrossRef]
- Toyama, S.; Takesue, M.; Aida, T.M.; Watanabe, M.; Smith, R.L., Jr. Easy emission-color-control of Mn-doped zinc silicate phosphor by use of pH and supercritical water conditions. J. Supercrit. Fluids 2015, 98, 65–69. [Google Scholar] [CrossRef]
- Shevchenko, E.V.; Talapin, D.V.; Rogach, A.L.; Kornowski, A.; Haase, M.; Weller, H. Colloidal synthesis and self-assembly of CoPt3 nanocrystals. J. Am. Chem. Soc. 2002, 124, 11480–11485. [Google Scholar] [CrossRef] [PubMed]
- Pearton, S.J.; Norton, D.P.; Ip, K.; Heo, Y.W.; Steiner, T. Recent progress in processing and properties of ZnO. Prog. Mater. Sci. 2005, 50, 293–340. [Google Scholar] [CrossRef]
- Ramakrishna, P.V.; Murthy, D.B.R.K.; Sastry, D.L.; Samatha, K. Synthesis, structural and luminescence properties of Mn doped ZnO/Zn2SiO4 composite microphosphor. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 129, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.C.; Kaloumenos, M.; Heinschke, S.; Erdem, E.; Jakes, P.; Eichel, R.A.; Schneider, J.J. Molecular precursor derived and solution processed indium-zinc oxide as a semiconductor in a field-effect transistor device. Towards an improved understanding of semiconductor film composition. J. Mater. Chem. C 2013, 1, 2577–2584. [Google Scholar] [CrossRef]
- Repp, S.; Erdem, E. Controlling the exciton energy of zinc oxide (ZnO) quantum dots by changing the confinement conditions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 152, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.C.; Johnson, K.H.; DeBoer, B.G.; Berkowitz, J.K.; Olsen, J.; Dale, E.A. First principles investigation of electronic structure and associated properties of zinc orthosilicate phosphors. J. Lumin. 1991, 47, 197–206. [Google Scholar] [CrossRef]
- Karazhanov, S.Z.; Ravindran, P.; Fjellvåg, H.; Svensson, B.G. Electronic structure and optical properties of ZnSiO3 and Zn2SiO4. J. Appl. Phys. 2009, 106. [Google Scholar] [CrossRef]
- Lee, C.S.; Matori, K.A.; Ab Aziz, S.H.; Kamari, H.M.; Ismail, I.; Zaid, M.H.M. Influence of zinc oxide on the physical, structural and optical band gap of zinc silicate glass system from waste rice husk ash. Opt.-Int. J. Light Electron Opt. 2017, 136, 129–135. [Google Scholar] [CrossRef]
- Kaftelen, H.; Ocakoglu, K.; Thomann, R.; Tu, S.; Weber, S.; Erdem, E. EPR and photoluminescence spectroscopy studies on the defect structure of ZnO nanocrystals. Phys. Rev. B 2012, 86, 1–9. [Google Scholar] [CrossRef]
- Erdem, E. Microwave power, temperature, atmospheric and light dependence of intrinsic defects in ZnO nanoparticles: A study of electron paramagnetic resonance (EPR) spectroscopy. J. Alloys Compd. 2014, 605, 34–44. [Google Scholar] [CrossRef]
- Gandhi, V.; Ganesan, R.; Abdulrahman Syedahamed, H.H.; Thaiyan, M. Effect of cobalt doping on structural, optical, and magnetic properties of ZnO nanoparticles synthesized by coprecipitation method. J. Phys. Chem. C 2014, 118, 9715–9725. [Google Scholar] [CrossRef]
- Zeng, H.; Duan, G.; Li, Y.; Yang, S.; Xu, X.; Cai, W. Blue Luminescence of ZnO nanoparticles based on non-equilibrium processes: Defect origins and emission controls. Adv. Funct. Mater. 2010, 20, 561–572. [Google Scholar] [CrossRef]
- Wakefield, G.; Holland, E.; Dobson, P.J; Hutchison, J.L. Luminescence properties of nanocrystalline Y2O3:Eu. Adv. Mater. 2001, 13, 1557–1560. [Google Scholar] [CrossRef]
- Lee, C.H.; Kang, Y.C.; Jung, K.Y.; Choi, J.G. Phosphor layer formed from the Zn2SiO4:Mn phosphor particles with spherical shape and fine size. Mater. Sci. Eng. B 2005, 117, 210–215. [Google Scholar] [CrossRef]
- Nazarov, M.V.; Kang, J.H.; Jeon, D.Y.; Popovici, E.J.; Muresan, L.; Tsukerblat, B.S. Lattice parameter and luminescence properties of europium activated yttrium oxide. Solid State Commun. 2005, 133, 183–186. [Google Scholar] [CrossRef]
- Jung, K.Y.; Han, K.H. Densification and photoluminescence improvement of Y2O3 phosphor particles prepared by spray pyrolysis. Electrochem. Solid-State Lett. 2005, 8, H17–H20. [Google Scholar] [CrossRef]
- Fu, Z.; Yang, B.; Li, L.; Dong, W.; Jia, C.; Wu, W. An intense ultraviolet photoluminescence in sol-gel ZnO-SiO2 nanocomposites. J. Phys. Condens. Matter 2003, 15, 2867–2873. [Google Scholar] [CrossRef]
- Kenny, N.; Kannewurf, C.R.; Whitmore, D.H. Optical absorption coefficients of vanadium pentoxide single crystals. J. Phys. Chem. Solids 1996, 27, 1237–1246. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
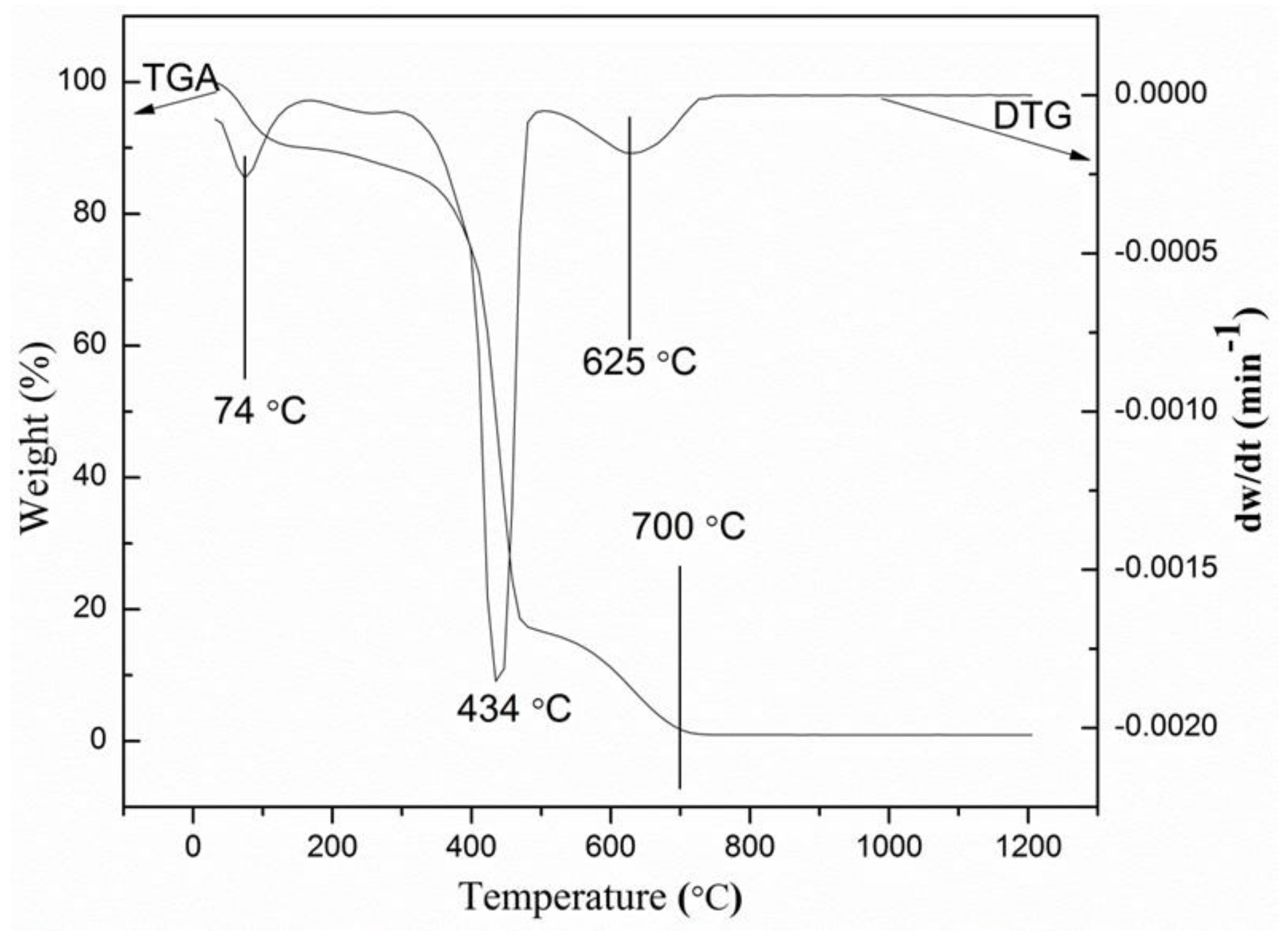
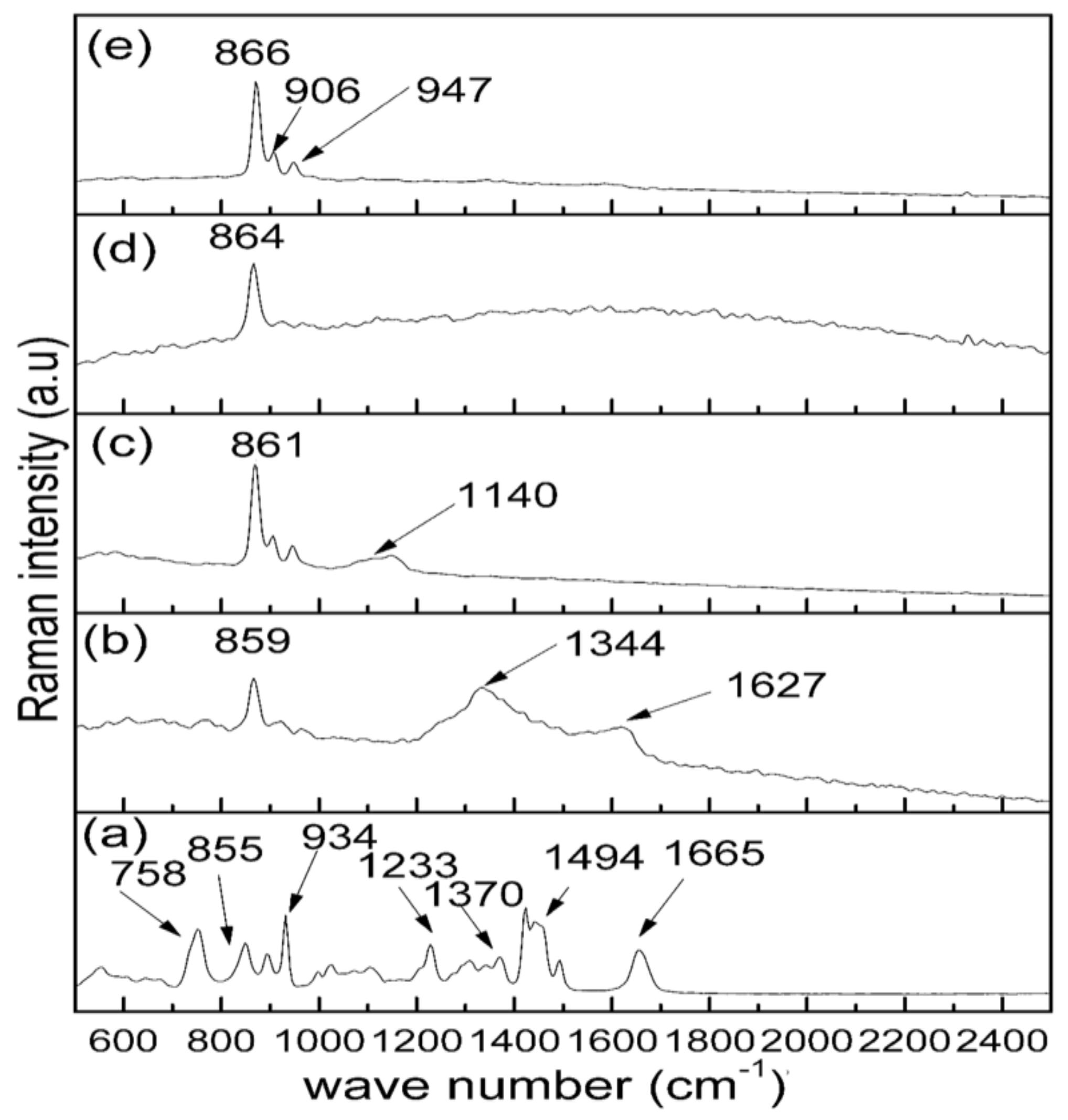

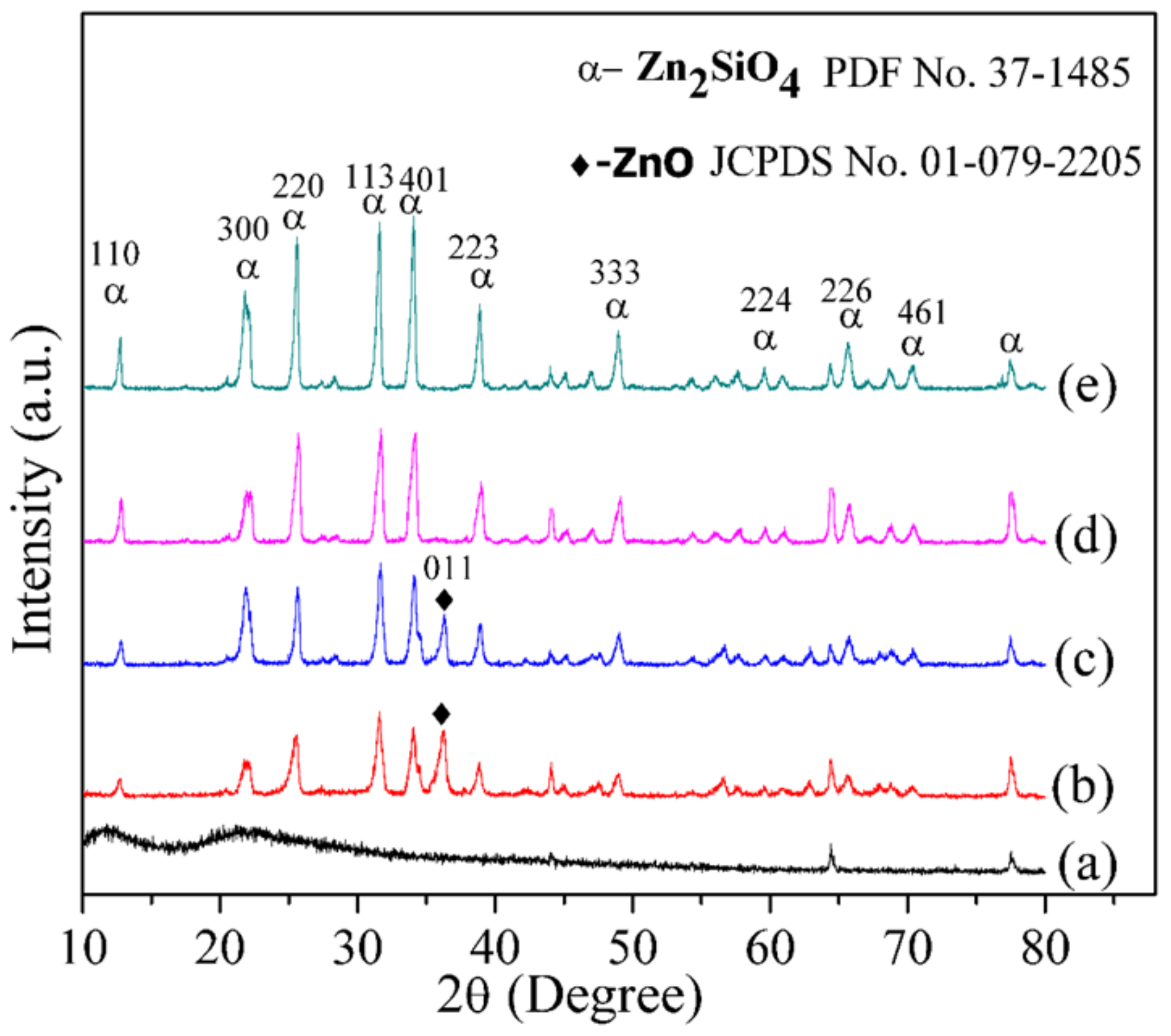
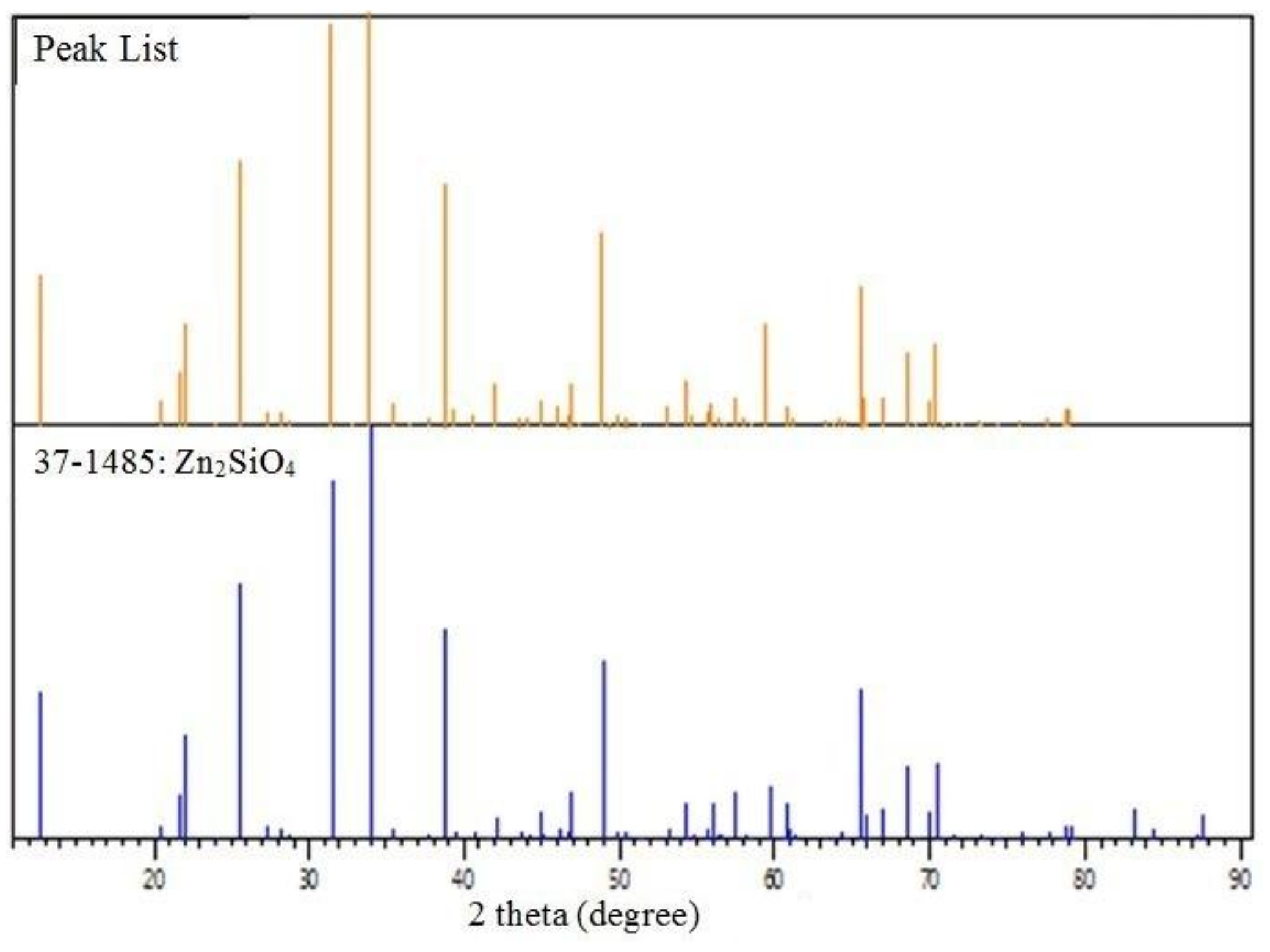
| Wave Number (cm–1) | Assignment of Vibration Mode | Reference |
|---|---|---|
| 800–1100 | Stretching vibration of SiO4 group | [47,48] |
| 859–866 | Crystalline Zn2SiO4 vibration peak | [48,49] |
| 758 | Ring C–C vibration | [45] |
| 885 | C–C stretching vibration | [45] |
| 934 | C–C Ring breathing | [45,46] |
| 1233 | C–C Back bone | [45] |
| 1370 | C–H Deformation | [45,46] |
| 1494 | CH2 Scissors | [45] |
| 1665 | C=O (amide) | [45] |
| Wave Number (cm–1) | Assignment of Vibration Mode | Reference |
|---|---|---|
| 379–441 | Zn–O asymmetric stretching vibration | [26] |
| 580 | Zn–O symmetric stretching vibration | [26] |
| 884–884 | Si–O stretching vibration | [26] |
| 639–641 | C–N=O bending vibration | [52] |
| 838 | C–C ring vibration | [51,52] |
| 1277 | C–N stretching vibration | [41,50,51] |
| 1402–1429 | –C–H– bending vibration of methylene group | [42] |
| 1648 | N–H stretching vibration | [50] |
| 2945 | C–H vibration | [41,50,51] |
| 3414 | N–H bending vibration | [41,50,51] |
| Calcination Holding Time (h) | Peak Position (2θ) | FWHM | DXRD (nm) | DTEM (nm) | Experimental Eg (eV) |
|---|---|---|---|---|---|
| 1 | 33.86 | 0.476 | 18.23 | 19.74 | 5.39 ± 0.04 |
| 2 | 33.90 | 0.388 | 21.70 | 23.03 | 5.35 ± 0.04 |
| 3 | 33.96 | 0.351 | 22.29 | 26.34 | 5.34 ± 0.04 |
| 4 | 33.98 | 0.308 | 27.40 | 29.71 | 5.27 ± 0.04 |
| Method | Precursors | Synthesis Conditions | Particle Size (nm) | Morphology | References |
|---|---|---|---|---|---|
| Solid–state | Zinc oxide, silicon oxide powder | calcination: 1330 °C, 4 h | 1000–5000 | Larger grain size | [22] |
| Hydrothermal | zinc nitrate, silicon oxide | 700 °C, 10 h | 300–1000 | Non–uniform distribution | [39] |
| Super critical water | zinc hydroxide, Amorphous silica | 30 MPa, 1 h, 400 °C | 1000–2000 | Non–uniform nanorods, distribution | [55] |
| Sol–gel method | Tetraethylorthosilicate, and zinc acetate dihydrate | 1200 °C, 2 h, | 70–80 | Non–uniform particle distribution | [18] |
| Spray pyrolysis | zinc acetate, tetraethyl orthosilicate, sulpuric acid | 1000 °C, 10 h | 200–1000 | Spherical particles, aggregate morphology | [29] |
| Sonochemical | Tetraethylorthosilicate, and zinc acetate dihydrate | 950, 2h | 50 | Uniform distribution | [26] |
| Polymer thermal treatment | zinc acetate dihydrate Silicon tetraacetate | 900 °C 3 h | 26 | Uniform Nanoparticle | This Paper |
| Optical Bandmgap (eV) | Eg (Experimental) | Direct Allowed Transition n = 1/2 | Direct Forbidden Transition n = 3/2 | Indirect Allowed Transition n = 2 | Indirect Forbidden Transition n = 3 |
|---|---|---|---|---|---|
| 1 h | 5.39 ± 0.04 | 5.38 ± 0.04 | 5.32 ± 0.04 | 5.23 ± 0.04 | 5.43 ± 0.04 |
| 2 h | 5.36 ± 0.04 | 5.35 ± 0.04 | 5.27 ± 0.04 | 5.17 ± 0.04 | 5.38 ± 0.04 |
| 3 h | 5.34 ± 0.04 | 5.33 ± 0.04 | 5.21 ± 0.04 | 5.07 ± 0.04 | 5.27 ± 0.04 |
| 4 h | 5.27 ± 0.04 | 5.25 ± 0.04 | 5.16 ± 0.04 | 4.72 ± 0.04 | 5.10 ± 0.04 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alibe, I.M.; Matori, K.A.; Sidek, H.A.A.; Yaakob, Y.; Rashid, U.; Alibe, A.M.; Mohd Zaid, M.H.; Ahmad Khiri, M.Z. Effects of Calcination Holding Time on Properties of Wide Band Gap Willemite Semiconductor Nanoparticles by the Polymer Thermal Treatment Method. Molecules 2018, 23, 873. https://doi.org/10.3390/molecules23040873
Alibe IM, Matori KA, Sidek HAA, Yaakob Y, Rashid U, Alibe AM, Mohd Zaid MH, Ahmad Khiri MZ. Effects of Calcination Holding Time on Properties of Wide Band Gap Willemite Semiconductor Nanoparticles by the Polymer Thermal Treatment Method. Molecules. 2018; 23(4):873. https://doi.org/10.3390/molecules23040873
Chicago/Turabian StyleAlibe, Ibrahim Mustapha, Khamirul Amin Matori, Hj Abdul Aziz Sidek, Yazid Yaakob, Umer Rashid, Ali Mustapha Alibe, Mohd Hafiz Mohd Zaid, and Mohammad Zulhasif Ahmad Khiri. 2018. "Effects of Calcination Holding Time on Properties of Wide Band Gap Willemite Semiconductor Nanoparticles by the Polymer Thermal Treatment Method" Molecules 23, no. 4: 873. https://doi.org/10.3390/molecules23040873
APA StyleAlibe, I. M., Matori, K. A., Sidek, H. A. A., Yaakob, Y., Rashid, U., Alibe, A. M., Mohd Zaid, M. H., & Ahmad Khiri, M. Z. (2018). Effects of Calcination Holding Time on Properties of Wide Band Gap Willemite Semiconductor Nanoparticles by the Polymer Thermal Treatment Method. Molecules, 23(4), 873. https://doi.org/10.3390/molecules23040873






